Abstract
AIMS
To assess the steady-state pharmacokinetic and QTc effects of domperidone and ketoconazole, given alone and together.
METHODS
A randomized, placebo-controlled, double-blind, crossover study was carried out. Healthy subjects (14 men, 10 women; age 18–39 years; mean weight 73.5 kg, range 53.8–98.8 kg; 23 Europid, 1 Afro-Caribbean) received orally, for 7 days each, placebo, domperidone 10 mg, four doses daily, at 4 h intervals, ketoconazole 200 mg 12-hourly and domperidone and ketoconazole together. The washout period was 15 days. Pharmacokinetics and serial 12-lead ECGs were assessed on day 7, and serial ECGs on day −1 and at follow-up. Two subjects withdrew before the third treatment period, so data were available for 22–24 subjects.
RESULTS
Ketoconazole tripled domperidone concentrations at steady-state. Domperidone, ketoconazole and their combination significantly increased QTcF in men. Overall adjusted mean differences from placebo were 4.20 (95% CI 0.77, 7.63), 9.24 (95% CI 5.85, 12.63) and 15.90 (95% CI 12.47, 19.33) ms, respectively. In women, QTcF was not significantly different from placebo on either domperidone or ketoconazole alone, or in combination. However, QTc was positively correlated with plasma drug concentrations, in both men and women. ΔQTcF increased by about 2 ms per 10 ng ml–1 rise in domperidone concentration, and per 1 µg ml–1 rise in ketoconazole concentration.
CONCLUSIONS
Ketoconazole tripled the plasma concentrations of domperidone. Domperidone and ketoconazole increased QTcF in men, whether given together or separately. The effect of domperidone alone was below the level of clinical importance. The negative result in women is unexplained.
Keywords: domperidone, drug interaction, ketoconazole, thorough QT
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Domperidone, a dopamine antagonist metabolized mainly by CYP3A, has been used as an anti-emetic and prokinetic agent by mouth for over 30 years.
Domperidone has a good safety record. However, early studies of high dose intravenous domperidone were associated with QTc prolongation and arrhythmias, including torsade de pointes.
There have been no thorough QTc studies of domperidone.
WHAT THIS STUDY ADDS
We assessed the effect on QTc of oral domperidone and ketoconazole, alone and in combination.
Ketoconazole, a CYP3A inhibitor, tripled domperidone concentrations, whilst domperidone did not affect ketoconazole concentrations.
Domperidone and ketoconazole, alone and in combination, increased QTc significantly in men, and there was a positive correlation between increased QTc and concentrations of domperidone and ketoconazole in both sexes.
The effect of domperidone alone on QTc was small and not clinically important, but domperidone should not be co-administered with ketoconazole.
Introduction
Domperidone, a dopamine antagonist, is an orally active anti-emetic and prokinetic agent [1] with a long safety record. It has been widely used for >30 years. Since 1998 domperidone has been available over-the-counter (OTC) in the UK with a maximum recommended single dose of 10 mg and total daily dose of 40 mg. However, early studies of high dose intravenous domperidone were associated with QT prolongation, arrhythmias [2] including torsade de pointes (TdP) [3], cardiac arrest [4, 5] and sudden death [6, 7]. Some of these patients had cancer and were on cardiotoxic chemotherapy, so there were contributing factors. Domperidone has been reported to prolong QT interval by a mean of 14 ms in neonates of gestational age <32 weeks [8], but a more recent study, also in neonates, yielded a negative result [9].
Domperidone is rapidly and almost completely absorbed after oral administration, but its bioavailability is only 13–17% because of extensive first pass metabolism in intestine and liver [10]. The main route of metabolism of domperidone is hydroxylation and oxidative N-dealkylation by CYP3A. Studies in vitro suggest that other CYP isoforms contribute little [11, 12]. Domperidone is also a substrate of P-glycoprotein [13, 14].
Ketoconazole, a commonly-used antifungal agent, is a powerful inhibitor of CYP3A and P-glycoprotein, so a pharmacokinetic interaction between ketoconazole and domperidone would be predicted to occur. Ketoconazole interacts with many other drugs. For example, QT interval prolongation and TdP have been reported after co-administration of ketoconazole with terfenadine [15], astemizole [16] and cisapride [17], all of which were subsequently withdrawn from the market.
No data have been published on the possible effects of inhibition of CYP3A or P-glycoprotein on the pharmacokinetics or safety of domperidone, although several authors have cautioned that such combinations might increase the risk of arrhythmias [11, 18].
We assessed the pharmacokinetics and effects on QT interval of domperidone and ketoconazole, alone and in combination, in healthy volunteers, mainly to obtain evidence to support the transfer of oral domperidone from prescription only to OTC status in many more countries.
Methods
We did the study in 2001 in accordance with ICH Guideline for Good Clinical Practice. The Brent Medical Ethics Committee (now designated as North London REC 1) approved the study and all subjects gave fully informed, written consent.
Subject population
We studied 24 healthy volunteers (14 men, 10 women), aged 18–39 years (mean, SD = 26.6, 5.8 years) and with body mass index 18–28 kg m–2 (mean, SD = 23.8, 2.9 kg m–2) and weight 53.8–98.8 kg (mean, SD = 73.5, 13.1 kg). Twenty-three were Europid and one was Afro-Caribbean. All were non-smokers and taking no medicines. We deemed them healthy on the basis of a medical history and examination, 12-lead ECG, 24 h ambulatory ECG and safety tests of blood (including electrolytes) and urine. We excluded any subject with corrected QT (QTc, using Bazett's correction for heart rate) interval ≥450 ms (women) or ≥430 ms (men) on 12-lead ECG, resting heart rate outside the range 50–100 beats min−1 and ≥50 premature ventricular contractions, or any significant arrhythmia, on the 24 h ECG.
Study design
The study was randomized, placebo-controlled, double-blind, double-dummy and crossover, using a Williams' balanced design. Each subject received four treatments: placebo, domperidone 10 mg, four doses daily, at 4 h intervals, ketoconazole 200 mg every 12 h and the combination of domperidone and ketoconazole. The active drugs and matching placebo were all supplied, as tablets, by Janssen-Cilag. Each treatment period was 7 days, with a 15 day washout between periods. Subjects were resident from 36 h before dosing until 24 h after the last dose in each period. While resident, subjects received standard meals at scheduled times, and were not allowed alcohol, grapefruit juice, quinine or caffeine-containing beverages. Subjects returned for a follow-up assessment 7 days after the final dose.
Assay of domperidone and ketoconazole
In each period, we took blood samples for assay of domperidone and ketoconazole before the morning dose on days 5, 6 and 7, and at 0.5, 1, 2, 4, 5, 8, 9, 12, 12.5, 13, 14, 16, 24 and 36 h after the morning dose on day 7.
We separated plasma within 1 h of sampling, and stored it at −18°C or below until assay for domperidone by Janssen Research Foundation, Beerse, Belgium, using a validated HPLC method. PPD Development, Richmond, Virginia, USA assayed ketoconazole using a validated LC-MS/MS method. Limits of reliable quantitation were 1.0 ng ml–1 and 0.05 µg ml–1, respectively. Intra- and inter-assay coefficients of variation were ≤7%.
Pharmacokinetic analysis
We used standard non-compartmental methods and actual elapsed sampling times to calculate pharmacokinetic parameters of domperidone and ketoconazole. We determined the maximum and minimum observed concentrations at steady-state (Cmax,ss and Cmin,ss) and the time of maximum concentration (tmax), directly from the observed data on day 7. We used the linear trapezoidal method to calculate the area under the plasma concentration–time curve from 0–24 h at steady-state on day 7 (AUC(0,24 hss)). We defined the average steady-state plasma concentration (Cavg,ss) over 24 h as AUC(0,24 hss)/24. We calculated the elimination half-life at steady-state (t1/2,z,ss) from the terminal portion of the log plasma concentration–time curve on day 7.
Pharmacodynamics
We recorded 12-lead ECGs (GE/Marquette MAC 1200) on day −1 and at follow-up, at the following times relative to the scheduled time of the day 1 morning dose: 0, 0.5, 1, 2, 4, 5, 8, 9, 12, 12.5, 13, 14 and 16 h. We also recorded 12-lead ECGs before the morning dose on days 5, 6 and 7 and at 0.5, 1, 2, 4, 5, 8, 9, 12, 12.5, 13, 14, 16, 24 and 36 h after the morning dose on day 7.
From the 12-lead ECGs, Quintiles Cardiac Alert (UK) calculated average RR and QT interval from measurements in 3–5 consecutive beats, nearly always in lead II, using calibrated electronic calipers with resolution of 0.4 ms. The same analyst measured all ECG intervals for an individual subject.
We corrected QT interval for heart rate using Bazett's (QTcB = QT/(RR/1000)0.5), Fridericia's (QTcF = QT/(RR/1000)0.33) and Sagie's (QTLc = QT + (1000–RR) × 0.154) formulae.
Safety and tolerability assessments
We did continuous cardiac monitoring via telemetry from 1 h before dosing on day 1 until 24 h after the last dose on day 7, vital signs at screening, on day −1, before and at 1 h after each dose on days 1–7 and at follow-up, laboratory safety tests at screening, on days −1 and 8 and at follow-up, medical examination at screening, on days −1 and 8 and at follow-up. We recorded three-lead, 24 h ambulatory ECGs on days −1 and 7 and at follow-up, using ACS model 9000 Holter recorders (Laguna Hills, USA). We monitored adverse events throughout the study.
Statistical analysis
All analyses were done on an intention to treat basis, in that data were included from all subjects who completed at least one treatment period, even if those subjects did not complete the entire study. To test for bioequivalence, we did an analysis of variance (anova) of log transformed Cmax,ss, Cmin,ss, AUC(0,24 hss), Cavg,ss. and t1/2,ss. Initial models contained fixed effects for period, treatment and carryover, and random effects for subject. If carryover effects were not significant at the 10% level, we dropped them from the model. For each pharmacokinetic parameter, we calculated the mean treatment ratio (combined therapy)/(single therapy), and the associated 90% confidence interval, using the mean square error from the anova.
To examine the relationship between pharmacokinetics and pharmacodynamics at steady-state, we did linear regression of the difference from placebo on day 7 in QTcB, QTcF and QTLc (ΔQTcB, ΔQTcF and ΔQTLc) on plasma concentrations of domperidone and ketoconazole.
To test for differences between treatments in heart rate, QTcB, QTcF and QTLc, at baseline (day −1) and at steady-state, we used a linear mixed effects regression model for repeated measurements. Initial models had fixed effects for treatment group, treatment sequence, period, gender, timing post dose, treatment-timing interaction and treatment-gender interaction. Day −1 ECG results were included as a covariate in the analysis at steady-state. Subject and subject-time were included as random effects. We compared covariance structures using likelihood ratio tests, to determine whether more complex structures led to significant improvement in model fit. If the treatment-gender interaction was significant, we stratified the analyses by gender. All P values were two-sided.
Results
Subjects
Of 14 men and 10 women who entered the study, we withdrew two men, both of them before the third treatment period. One had mildly elevated liver enzymes. He had been dosed with ketoconazole alone and domperidone alone. The other had a 1 min episode of asymptomatic ventricular tachycardia. He had been dosed with combination treatment and then ketoconazole alone, in the two previous treatment periods. The timing of the tachycardia (2 weeks after the last dose of ketoconazole) makes it unlikely that it was related to treatment.
In all, we dosed 23 subjects with domperidone, 24 with ketoconazole, 23 with combination and 22 with placebo, each treatment for 7 days.
Pharmacokinetics of domperidone
On both regimens, domperidone was rapidly absorbed. Plasma concentrations peaked within 2 h after each dose (Figure 1). Over the 24 h period, median tmax was 9.0 h after the morning dose on domperidone alone, and 5.0 h after the morning dose of the combination. Thus in both cases the peak concentrations were at 1 h after the immediately preceding dose.
Figure 1.
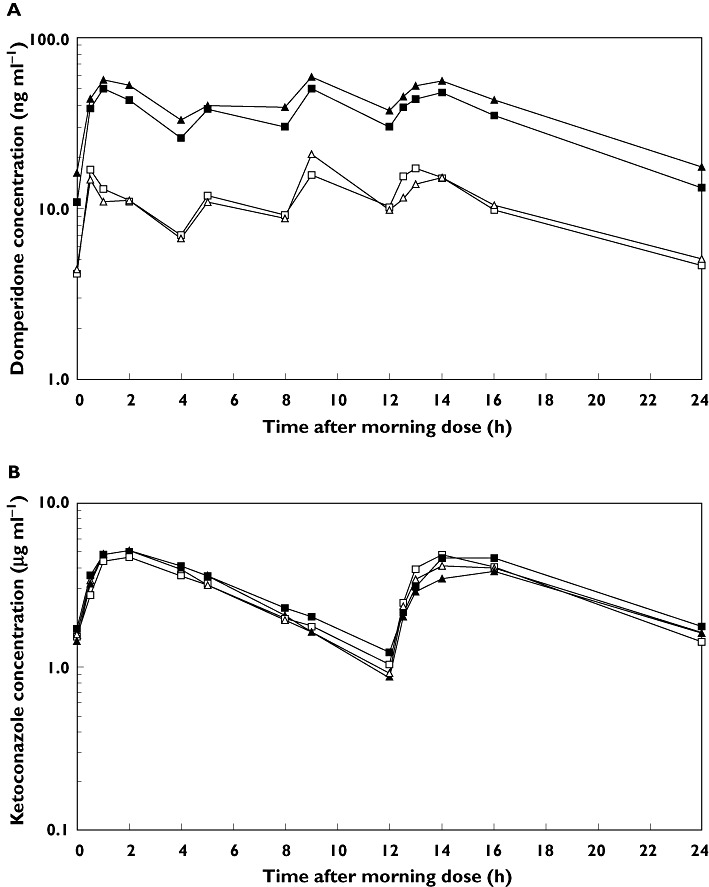
Plasma concentrations of domperidone (A) and ketoconazole (B) over 24 h in men and women, at steady-state (day 7 of dosing). Single therapy: men (□) and women (▵); combinatiion therapy: men ( ) and women (▴)
) and women (▴)
After the final dose, domperidone concentrations declined in a biphasic manner, on both treatments. Mean domperidone t1/2,z,ss was significantly shorter on combination treatment than when given alone (6.8 h vs. 9.0 h, respectively). t1/2,z,ss decreased in all but two subjects, in whom it increased marginally (≤4.5%).
Ketoconazole increased the plasma concentrations of domperidone in all subjects. Mean AUC(0,24 hss), Cmax,ss, Cmin,ss and Cavg,ss of domperidone were significantly higher when domperidone was given with ketoconazole than when given alone (Table 1, Figure 1). Mean (90% CI) treatment ratios for each parameter were 3.57 (3.31, 3.86), 2.93 (2.65, 3.25), 3.12 (2.83, 3.43) and 3.57 (3.31,3.86), respectively. Within-subject standard deviations (SD) estimated from the anova models ranged from from 0.14 to 0.18.
Table 1.
Arithmetic mean (SD) of pharmacokinetic parameters of domperidone and ketoconazole at steady-state (day 7 of dosing), given as single therapy and in combination
| Parameter | Single | Combination | Ratio (90% CI)1 |
|---|---|---|---|
| Domperidone | |||
| AUC(0,24 hss) (ng ml−1 h) | 249.0 (65.33) | 878.1 (267.7) | 3.57 (3.31, 3.86)* |
| Cmax,ss (ng ml−1) | 23.48 (7.35) | 67.85 (21.11) | 2.93 (2.65, 3.25)* |
| Cmin,ss (ng ml−1) | 4.23 (1.12) | 13.23 (4.80) | 3.12 (2.83, 3.43)* |
| Cavg,ss (ng ml−1) | 10.38 (2.73) | 36.60 (11.16) | 3.57 (3.31, 3.86)* |
| Vd/Fss (l) | 788.1 (336.0) | 191.6 (85.0) | 0.24 (0.22, 0.26)* |
| CL/F,sss (ml min−1) | 1005.7 (314.8) | 321.9 (91.5) | 0.32 (0.29, 0.34)* |
| t1/2,z,ss (h) | 8.99 (1.24) | 6.81 (1.38) | 0.75 (0.71, 0.79)* |
| tmax† (h) | 9.0 (0.5–14.0) | 5.0 (0.5–14.1) | |
| Ketoconazole | |||
| AUC(0,24 hss) (µg ml−1 h) | 70.4 (28.1) | 73.9 (34.8) | 1.03 (0.95, 1.12) |
| Cmax,ss (µg ml−1) | 5.69 (1.76) | 5.73 (2.11) | 0.99 (0.92, 1.07) |
| Cmin,ss (µg ml−1) | 1.53 (0.94) | 1.58 (1.01) | 1.07 (0.93, 1.22) |
| Cavg,ss (µg ml−1) | 2.94 (1.17) | 3.08 (1.45) | 1.03 (0.95, 1.12) |
| Vd/Fss (l) | 28.14 (7.94) | 27.53 (12.68) | 1.02 (0.90, 1.15) |
| CL/F,sss (ml min−1) | 71.48 (29.41) | 66.52 (14.09) | 0.94 (0.80, 1.11) |
| t1/2,z,ss (h) | 4.20 (1.62) | 4.56 (2.08) | 0.93 (0.84, 1.04) |
| tmax† (h) | 2.1 (0.5–24.0) | 2.0 (0.5–24.2) |
Ratio computed on log transformed data and back-transformed; 90% CI calculated using within-subject SD from anova models: Domperidone: AUC(0,24 h) = 0.14; Cmax = 0.18; Cmin = 0.17; Cavg = 0.14; Vd/F = 0.20; CL/F = 0.15; t1/2,z = 0.10. Ketoconazole AUC(0,24 h) = 0.15; Cmax = 0.14; Cmin = 0.24; Cavg = 0.15; Vd/F = 0.11; CL/F = 0.08; t1/2,z = 0.19.
Statistically significant difference (P < 0.05).
Median and range.
Mean pre dose concentrations of domperidone did not differ between the mornings of days 5, 6 and 7 on either regimen, but were about three times higher on combination treatment. Thus, domperidone had reached steady-state by day 5 on both treatments.
Pharmacokinetic parameters of domperidone did not differ significantly between genders on either regimen.
Pharmacokinetics of ketoconazole
At steady-state, plasma concentrations of ketoconazole were similar during combination treatment and monotherapy (Figure 1). On both treatments, ketoconazole was variably absorbed. tmax was 0.5–24 h after each dose. Over the 24 h period, median tmax was 2 h after the morning dose in both treatment groups. After tmax, ketoconazole concentrations declined exponentially in a monophasic manner. Mean t1/2,z.ss of ketoconazole on monotherapy did not differ significantly from that during combination treatment (4.6 h vs. 4.2 h, respectively).
Mean AUC(0,24 hss), Cmax,ss, Cmin,ss and Cavg,ss during ketoconazole-domperidone combination treatment did not differ from those during monotherapy. Mean (90% CI) treatment ratios are shown in Table 1. Within-subject SD estimated from the anova models ranged from from 0.14 to 0.24.
Mean pre dose concentrations of ketoconazole, whether given alone or in combination, did not differ among the mornings of days 5, 6 and 7. Thus, ketoconazole had reached steady-state by day 5 of twice daily dosing, during both regimens.
Pharmacokinetic parameters of ketoconazole did not differ significantly between genders on either regimen.
Pharmacodynamics: 12-lead ECG
We report results based on QTcF as our primary method of correction. Results based on QTcB and QTLc were similar (Table 2).
Table 2.
Overall mean QTc (ms), and mean (95% confidence interval) difference from placebo, on 12-lead ECG from 0–16 h after dosing, in men and women at steady-state (day 7 of dosing)
| Placebo | Domperidone | Ketoconazole | Combination | ||||
|---|---|---|---|---|---|---|---|
| Mean QTc | Mean QTc | Adjusted mean difference from placebo1(95% CI); P value | Mean QTc | Adjusted mean difference from placebo1(95% CI); P value | Mean QTc | Adjusted mean difference from placebo1(95% CI); P value | |
| Men | |||||||
| QTcB2 | 396.5 | 399.0 | 2.94 (–1.02, 6.91); P = 0.14 | 400.9 | 7.00 (3.06, 10.93); P = 0.001 | 406.5 | 12.29 (8.32, 16.26); P < 0.001 |
| QTcF3 | 394.0 | 397.7 | 4.20 (0.77, 7.63); P = 0.017 | 401.3 | 9.24 (5.85, 12.63); P < 0.001 | 408.3 | 15.90 (12.47, 19.33); P < 0.001 |
| QTLc4 | 393.7 | 397.0 | 3.75 (0.43, 7.08); P = 0.028 | 400.2 | 8.68 (5.39, 11.96); P < 0.001 | 406.9 | 15.06 (11.74, 18.39); P < 0.001 |
| Women | |||||||
| QTcB2 | 412.3 | 409.7 | –2.70 (–7.85, 2.45); P = 0.29 | 407.9 | –4.37 (-9.54, 0.80); P = 0.09 | 414.2 | 2.01 (–3.14, 7.16); P = 0.43 |
| QTcF3 | 407.9 | 406.4 | –1.52 (–6.72, 3.69); P = 0.55 | 405.6 | –2.58 (-7.80, 2.64); P = 0.32 | 410.7 | 2.71 (–2.49, 7.91); P = 0.29 |
| QTLc4 | 407.7 | 406.2 | –1.55 (–6.56, 3.46); P = 0.53 | 404.6 | –3.42 (-8.45, 1.61); P = 0.17 | 410.5 | 2.59 (–2.42, 7.59); P = 0.30 |
Means are model-based means from repeated measures analysis, adjusted for day −1 as a covariate. Within subject SD estimated from model: Men: QTcB = 13.0; QTcF = 10.1; QTLc = 10.5. Women: QTcB = 11.7; QTcF = 8.4; QTLc = 8.8.
QT corrected for heart rate using Bazett's formula: QTcB = QT/(RR/1000)0.5.
QT corrected for heart rate using Fridericia's formula: QTcF = QT/(RR/1000)0.33.
QT corrected for heart rate using Sagie's formula: QTLc = QT + (1000–RR) × 0.154.
The effect of treatment on mean QTcF on day 7 depended strongly on gender (P for interaction ≤0.002), so we stratified all analyses by gender.
At steady-state, mean QTcF in men on domperidone or ketoconazole alone was significantly higher than on placebo. On domperidone alone, the overall mean (95% CI) increase in QTcF from placebo was 4.20 ms (0.77, 7.63), and the maximum increase was 9.11 ms at 13 h post dose. On ketoconazole alone, the overall mean (95% CI) increase in QTcF from placebo was 9.24 ms (5.85, 12.63) and the maximum increase was 13.60 ms at 4 h post dose (Figure 2, Table 3). During the 7 days of dosing, QTcF prolongation was observed in three men (four events) on domperidone and two men (two events) on ketoconazole. The maximum observed QTcF was 450 ms on domperidone and 461 ms on ketoconazole. No QTc values >500 ms were recorded on either treatment, using any of the three corrections for heart rate.
Figure 2.
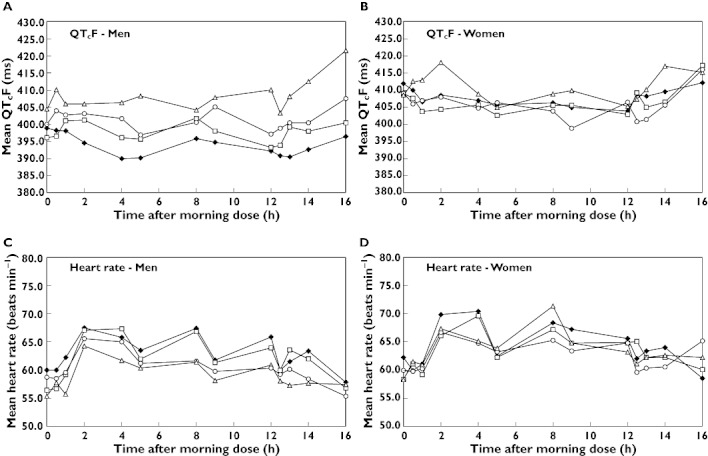
Mean QTcF and heart rate in men (A and C) and women (B and D) on placebo (♦), domperidone (□), ketoconazole (○) and combination therapy (▵), from 0–16 h after the morning dose on day 7
Table 3.
Mean1 (95% confidence interval) difference from placebo in QTcF (ms) in men and women at steady-state (day 7 of dosing). Differences in QTcB and QTLc showed the same pattern (see Figure 2)
| Domperidone | Ketoconazole | Combination | ||||
|---|---|---|---|---|---|---|
| Time | Mean difference (95% CI) | P value | Mean difference (95% CI) | P value | Mean difference (95% CI) | P value |
| Men | ||||||
| 0 h | –2.32 (–10.63, 5.99) | 0.58 | 3.06 (–5.11, 11.24) | 0.46 | 7.17 (–1.14, 15.48) | 0.09 |
| 0.5 h | –1.15 (–9.46, 7.16) | 0.79 | 7.53 (–0.67, 15.74) | 0.07 | 13.43 (5.12, 21.74) | 0.002 |
| 1 h | 3.32 (–4.99, 11.63) | 0.43 | 6.58 (–1.60, 14.76) | 0.11 | 9.34 (1.04, 17.65) | 0.03 |
| 2 h | 7.23 (–1.07, 15.54) | 0.09 | 10.52 (2.34, 18.70) | 0.01 | 12.96 (4.65, 21.27) | 0.002 |
| 4 h | 6.73 (–1.57, 15.04) | 0.11 | 13.60 (5.43, 21.78) | 0.001 | 18.10 (9.77, 26.44) | <0.001 |
| 5 h | 5.94 (–2.37, 14.25) | 0.16 | 8.56 (0.38, 16.73) | 0.04 | 19.58 (11.27, 27.88) | <0.001 |
| 8 h | 6.27 (–2.03, 14.58) | 0.14 | 6.57 (–1.60, 14.75) | 0.11 | 10.05 (1.73, 18.37) | 0.02 |
| 9 h | 3.76 (–4.55, 12.07) | 0.37 | 12.22 (4.05, 20.39) | 0.003 | 14.72 (6.41, 23.03) | <0.001 |
| 12 h | 1.59 (–6.72, 9.90) | 0.71 | 6.82 (–1.36, 14.99) | 0.10 | 19.60 (11.29, 27.91) | <0.001 |
| 12.5 h | 3.65 (–4.66, 11.96) | 0.39 | 10.25 (2.08, 18.43) | 0.01 | 14.41 (6.10, 22.72) | <0.001 |
| 13 h | 9.11 (0.80, 17.42) | 0.03 | 11.72 (3.54, 19.89) | 0.005 | 19.08 (10.77, 27.39) | <0.001 |
| 14 h | 5.86 (–2.45, 14.17) | 0.17 | 9.67 (1.49, 17.84) | 0.02 | 21.41 (13.08, 29.73) | <0.001 |
| 16 h | 4.61 (–3.70, 12.92) | 0.28 | 13.05 (4.88, 21.22) | 0.002 | 26.89 (18.58, 35.20) | <0.001 |
| Women | ||||||
| 0 h | –3.10 (–11.77. 5.56) | 0.48 | –3.62 (–12.30, 5.07) | 0.41 | –3.48 (–12.14, 5.19) | 0.43 |
| 0.5 h | –2.16 (–10.85, 6.54) | 0.63 | –4.25 (–12.93, 4.44) | 0.34 | 1.39 (–7.46, 10.23) | 0.76 |
| 1 h | –2.61 (–11.29, 6.06) | 0.55 | 0.07 (–8.61, 8.74) | 0.99 | 6.36 (–2.30, 15.02) | 0.15 |
| 2 h | –4.24 (–12.90, 4.43) | 0.34 | –0.51 (–9.19, 8.17) | 0.91 | 9.74 (1.07, 18.40) | 0.03 |
| 4 h | –1.08 (–9.76, 7.60) | 0.81 | –2.22 (–10.92, 6.47) | 0.61 | 2.33 (–6.36, 11.03) | 0.60 |
| 5 h | –3.22 (–11.89, 5.44) | 0.46 | 0.34 (–8.34, 9.01) | 0.94 | –0.72 (–9.39, 7.94) | 0.87 |
| 8 h | –0.56 (–9.24, 8.13) | 0.90 | –2.24 (–10.94, 6.47) | 0.61 | 3.17 (–5.52, 11.86) | 0.47 |
| 9 h | 0.19 (–8.47, 8.86) | 0.97 | –6.82 (–15.50, 1.86) | 0.12 | 4.61 (–4.05, 13.27) | 0.30 |
| 12 h | –0.79 (–9.48, 7.90) | 0.86 | 2.11 (–6.57, 10.79) | 0.63 | 1.57 (–7.10, 10.23) | 0.72 |
| 12.5 h | 0.14 (–8.52, 8.80) | 0.97 | –8.49 (–17.17, 0.19) | 0.06 | –1.67 (–10.34, 7.00) | 0.70 |
| 13 h | –3.33 (–11.99, 5.33) | 0.45 | –7.29 (–15.97, 1.39) | 0.10 | 1.57 (–7.10, 10.23) | 0.72 |
| 14 h | –3.72 (–12.39, 4.95) | 0.40 | –4.19 (–12.87, 4.49) | 0.34 | 7.27 (–1.40, 15.93) | 0.10 |
| 16 h | 4.79 (–3.88, 13.45) | 0.28 | 3.59 (–5.09, 12.27) | 0.42 | 3.15 (–5.52, 11.81) | 0.47 |
Means are model-based means from repeated measures analysis, adjusted for day −1 as a covariate.
Men on combination therapy had significantly higher mean QTcF than on placebo, overall (Table 2) and at most timepoints up to 16 h after dosing (Figure 2, Table 3). The overall mean (95% CI) increase in QTcF from placebo was 15.90 ms (12.47, 19.33) and the maximum increase was 26.89 ms at 16 h post dose. During the 7 days of dosing, QTcF prolongation (>450 ms) was observed in two men (four events). The maximum recorded QTcF was 459 ms. No QTc values >500 ms were recorded, using any of the three corrections for heart rate. The within subject SD estimated from the mixed effects regression model was 10.1 ms.
Among women, the overall effect of treatment on QTc, using any of the three corrections for heart rate, was not statistically significant (Table 2). With all three corrections, mean QTc in women on domperidone or ketoconazole alone tended to be lower than on placebo, but tended to be higher on the combined treatment. The mean differences from placebo in QTcF were mostly negative on domperidone and ketoconazole alone, and mostly positive on combination treatment (Figure 2, Table 3). QTc prolongation (>470 ms) was not observed at any timepoint in women on domperidone, ketoconazole, or combination treatment, using any of the three corrections for heart rate. The maximum observed QTcF was 440 ms on domperidone, 442 ms on ketoconazole, and 453 ms on combination. The within subject SD estimated from the mixed effects regression model was 8.4 ms.
The effect of treatment on mean heart rate on day 7 was not gender-dependent, so we did not stratify the analysis by gender. There was no statistically significant effect of treatment on mean heart rate, nor any significant treatment-timing interaction (Figure 2). The maximum mean change in heart rate from placebo was −3.8 beats min−1 on domperidone, −4.4 beats min−1 on ketoconazole and −4.3 beats min−1 on the combination.
At baseline (day −1) neither mean QTc, using any of the three corrections, nor mean heart rate, differed significantly among treatments or between genders. Mean baseline QTcF in men was 400.3 ms (range 346–459) and in women was 405.8 ms (range 350–463) (P = 0.30, from mixed effect repeated measures model)
Pharmacokinetic–pharmacodynamic relationships
In both men and women, plasma concentrations of domperidone had a weak, but statistically significant, positive linear association with ΔQTcF accounting for 8% of the variability in those measurements (Figure 3). In both sexes, the slope of the regression line was shallow, corresponding to ≍ 2 ms increase in ΔQTcF per 10 ng ml–1 rise in plasma concentration.
Figure 3.
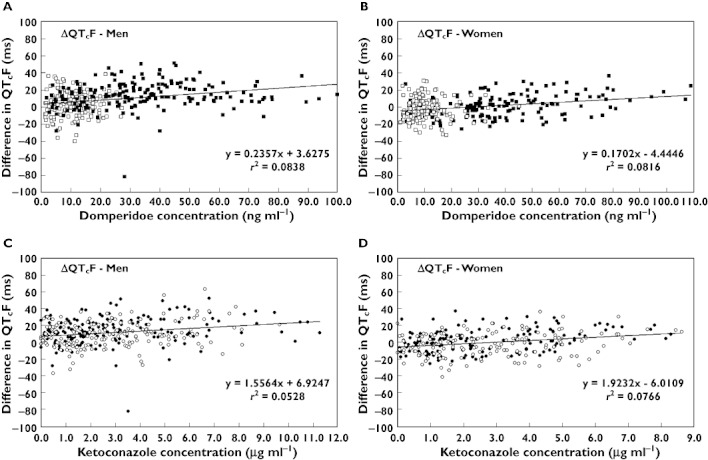
Relationship between plasma domperidone (A and B) and ketoconazole (C and D) concentration and difference in QTcF from placebo (ΔQTcF), in men (A and C) and women (B and D) on single and combination therapy at steady-state (day 7 of dosing). (Domperidone alone □; domperidone in combination  ; ketoconazole alone ○; ketoconazole in combination •)
; ketoconazole alone ○; ketoconazole in combination •)
Plasma concentration of ketoconazole had a weak, but statistically significant, positive linear association with ΔQTcF in men and women, accounting for <8% of the variability in those measurements (Figure 3). The slope of the regression line was shallow, corresponding to <2 ms increase in ΔQTcF per 1 µg ml–1 increase in ketoconazole concentration.
Safety and tolerability
There were no clinically significant changes in vital signs, physical examination, 12-lead ECG, or laboratory safety tests. Telemetric and 24 h ambulatory ECG showed no untoward events other than the above-mentioned 1 min episode of asymptomatic ventricular tachycardia, which occurred at 2 weeks after the end of ketoconazole treatment.
The most common treatment-related adverse events were abdominal discomfort reported by three subjects and breast discomfort and nipple discharge reported by three women.
Discussion
Ketoconazole increased plasma concentrations of domperidone at steady-state in all subjects. Mean Cmax and AUC were about three-fold higher. In contrast, domperidone did not affect plasma concentrations of ketoconazole. Contrary to our expectation, ketoconazole decreased domperidone t1/2,z,ss significantly (P < 0.01), so the increase in Cmax and AUC must have been due to reduced first-pass extraction, rather than to reduced clearance.
The half-life of drugs with a high hepatic extraction ratio (EH) reflects liver blood flow more than enzyme activity [19]. Domperidone has an intermediate EH (≍ 0.60), so CYP3A activity might be expected to affect its t1/2,z. However, the relative contribution of intestinal and hepatic CYP3A to the oral first-pass effect differs among drugs, so EH may be an unreliable predictor of the effect of CYP3A inhibition on clearance. In studies of oral midazolam (EH = 0.44), grapefruit juice increased AUC and Cmax of the drug but did not change its t1/2,z, reflecting inhibition of intestinal, but not hepatic, CYP3A4 [20, 21]. First-pass intestinal metabolism appears to be an important contributor to the low oral bioavailability of domperidone [10].
Domperidone appears to be a substrate for P-glycoprotein (P-gp) as well as CYP3A [13, 14]. P-gp is a transmembrane efflux pump expressed in normal cells, including the intestinal epithelium and the liver. It plays a key role in the absorption, distribution and elimination of many drugs. Ketoconazole inhibits both CYP3A and P-gp [22]. Thus, the increase in plasma concentrations of domperidone when given with ketoconazole was predictable. In contrast, the reduction of t1/2,βof domperidone during ketoconazole treatment is hard to explain, but might reflect a complex interplay between CYP3A and P-gp inhibition in both the intestine and the liver.
The QTcF results were remarkable. In men, all three treatment regimens significantly increased QTcF, whereas in women neither single therapy nor the combination had any significant effect. That difference between the men and women was the reverse of what we expected. Other drugs, such as quinidine and erythromycin, cause larger increases in QT in women than in men, and there are physiological reasons why women may be more susceptible to QT prolongation [23]. As expected, we found that mean QTcF at baseline was slightly longer (406 vs. 400 ms) in women than in men, albeit not significantly so.
Although the susceptibility of women to drug-induced prolongation of QT interval varies with phase of the menstrual cycle, Rodriguez et al. [24] showed that ibutilide prolongs QT interval in women more than in men, irrespective of phase of menstrual cycle. We found no pharmacokinetic differences between men and women, for either domperidone or ketoconazole. Fewer women (n = 10) than men (n = 13) took part in our study, and the sample size was small, so our QT result in women might be a false negative. In support of that notion, we found a similarly positive correlation between QTcF and plasma concentrations of domperidone or ketoconazole in both men and women (Figure 3). It is possible, therefore, that the QT interval was systematically prolonged during placebo treatment, thus obscuring any possible effects of the test drugs. There is no obvious explanation for such a systematic difference among treatments, given that the study was of an balanced crossover design, both male and female subjects were managed similarly, as regards days of admission to the research ward, all ECGs were recorded using the same type of machine and the same technicians and ECGs were analyzed ‘blind’ by an external laboratory. However, only 10 women took part in the study, so the group size was small and spuriously higher values of QT could have arisen by chance alone. Further studies would be needed to show whether women are truly less susceptible than men to QTc prolongation induced by domperidone and ketoconazole.
Men on combination therapy at steady-state had significantly longer QTc than on placebo, irrespective of which of three different formulae was used to correct QT for heart rate. The highest mean increase in QTcF from placebo was 15.90 ms (Figure 2 and Table 2, 95% CI 12.47, 19.33; P < 0.001). Ketoconazole given alone also increased QTcF significantly (Figure 2 and Table 2: mean difference from placebo 9.24 ms, 95% CI 5.85, 12.63; P < 0.001). Domperidone given alone increased QTcF, but the difference from placebo was smaller than that seen with ketoconazole alone (Figure 2 and Table 2, mean difference domperidone–placebo 4.20 ms, 95% CI 0.77, 7.63; P = 0.017). The effect of domperidone on QTcF was less than half that of ketoconazole, and the sum of the effects of domperidone and ketoconazole was slightly less than the effect of the combined treatments. Thus the interaction between the two drugs was additive.
The potential for ketoconazole monotherapy to affect cardiac repolarization has been noted by others [25, 26]. Studies in healthy men have shown that ketoconazole prolongs QTc by an average of about 6 ms [27, 28]. For those reasons, Sarapa et al. [28] proposed ritonavir instead of ketoconazole as a CYP3A inhibitor for thorough QT studies, because they found that ritonavir did not prolong QT.
Most drugs that prolong QT do so by blocking the rapid component (IKr) of the delayed rectifier potassium current in cardiac conduction pathways, or by altering trafficking of proteins forming the ion channel. Domperidone blocks IKr in vitro, and, at concentrations of 100 nmol l–1 (43 ng ml–1), prolongs cardiac repolarization by 25–30% [18]. That concentration is below the mean Cmax of domperidone (67.8 ng ml–1) in subjects on combination therapy in our study. However, the in vitro data are misleading. Domperidone is 93% bound to plasma protein at concentrations up to 100 ng ml–1in vivo, so the free concentration of the drug is only about 7% of total [10]. The concentrations quoted in studies in vitro usually represent unbound drug, so a free concentration of 43 ng ml–1 corresponds to a total plasma concentration of 860 ng ml–1, much higher than the maximum concentrations in our study. However, we cannot exclude the possibility that ketoconazole affected the protein binding of domperidone, yielding higher free concentrations of the drug. An increase in free domperidone could also account for the apparent reduction of t1/2,z of domperidone by ketoconazole.
Increases in QTcF correlated positively with plasma concentrations of domperidone or ketoconazole in both men and women, albeit weakly (Figure 3). However, the correlation analysis was complicated by the fact that ketoconazole substantially increased plasma concentrations of domperidone. As Figure 3 shows, plasma concentrations of domperidone >30 ng ml–1 occurred almost exclusively during combination therapy with ketoconazole. Correlation analysis cannot tell us whether the increase in QTcF was caused by the addition of ketoconazole or by the increased concentrations of domperidone. Although the model based means from the repeated measures ancova (Table 2) suggest that the sum of the separate effects of domperidone and ketoconazole on QT in men was less than the effect of combination treatment, that difference was too small to allow a reliable conclusion.
In 2005, guidelines (ICH E14) were issued for assessing a drug's potential to prolong QT interval [29]. They recommended that a ‘thorough QT study’ be done, preferably in healthy volunteers, not only for new drugs, but also for marketed drugs in the same pharmacologic class as drugs associated with QT prolongation. Furthermore, if drug plasma concentrations are increased by interactions involving metabolizing enzymes, the effect on QT should be assessed. Features of a thorough QT study include investigation of the effects on QT of concentrations higher than those after therapeutic doses, robust methods for measuring QT interval and its correction for heart rate and emphasis on analysis of drug effect throughout the dosing interval by use of mean time-matched, baseline-corrected differences from placebo. Our study, which was done before ICH E14, meets the criteria of a thorough QT study.
ICH E14 defines a ‘negative thorough QT study’ as one in which the largest baseline-subtracted, time-matched, mean difference between drug and placebo is ≤5 ms, with an upper bound of the 95% CI of ≤10 ms [29]. We found that, in both men and women, the maximum mean QTc increase on domperidone alone compared with placebo was >5 ms at some time points, but never exceeded 12 ms. Therefore, according to ICH E14, ours is a borderline ‘positive’ QT study for domperidone monotherapy. However, there have been remarkably few reports of serious cardiac arrhythmias that might have been caused by domperidone, in spite of its wide use for more than 30 years in many countries, often in high risk patient populations [30]. In the UK, pharmacovigilance data have raised no concern about the cardiovascular safety of domperidone (MHRA, personal communication). That is in sharp contrast with the unfavourable pharmacovigilance data that led to the withdrawal of other drugs that prolong QT, such as cisapride [17].
According to ICH E14, ours is definitely a ‘positive’ QT study for the combination of ketoconazole and domperidone. However, there have been no reports of an interaction between ketoconazole and domperidone. Furthermore, pharmacovigilance data have not identified any cardiovascular concerns (MHRA, personal communication). Nevertheless, since we completed our study, the datasheets for domperidone and ketoconazole have both been amended to contraindicate their co-administration [31]. Our results support that action.
In conclusion, the results of this study show that co-administration of ketoconazole increased domperidone plasma concentrations about three-fold, probably by inhibition of first-pass metabolism by CYP3A in the intestine and the liver. The higher concentrations of domperidone increased QT interval, at least in men. Ketoconazole probably also contributed to the QT prolongation. Thus, domperidone and ketoconazole should not be co-administered.
Acknowledgments
We thank Paul Soons (Johnson and Johnson, Beerse, Belgium) for his contribution to the study design and analysis and Dr William Carey for his careful supervision of the subjects.
Competing Interests
Johnson and Johnson, Beerse, Belgium paid Hammersmith Medicines Research (HMR) to do the study. Malcolm J Boyce owns HMR. Steven J Warrington is employed by HMR. Kathy J. Baisley was formerly employed by HMR.
REFERENCES
- 1.Reynolds JC. Prokinetic agents: a key in the future of gastroenterology. Gastroenterol Clin North Am. 1989;18:437–57. [PubMed] [Google Scholar]
- 2.Osborne RJ, Slevin ML, Hunter RW, Hamer J. Cardiac arrhythmias during cytotoxic chemotherapy: role of domperidone. Hum Toxicol. 1985;4:617–26. doi: 10.1177/096032718500400608. [DOI] [PubMed] [Google Scholar]
- 3.Bruera E, Villamayor R, Roca E, Barugel M, Tronge J, Chacon R. Q-T interval prolongation and ventricular fibrillation with i.v. domperidone. Cancer Treat Rep. 1986;70:545–6. [PubMed] [Google Scholar]
- 4.Roussak JB, Carey P, Parry H. Cardiac arrest after treatment with intravenous domperidone. BMJ (Clin Res Ed) 1984;289:1579. doi: 10.1136/bmj.289.6458.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron HA, Reyntjens AJ, Lake-Bakaar G. Cardiac arrest after treatment with intravenous domperidone. BMJ (Clin Res Ed) 1985;290:160. doi: 10.1136/bmj.290.6462.160-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joss RA, Goldhirsch A, Brunner KW, Galeazzi RL. Sudden death in cancer patient on high-dose domperidone. Lancet. 1982;1:1019. doi: 10.1016/s0140-6736(82)92016-5. [DOI] [PubMed] [Google Scholar]
- 7.Giaccone G, Bertetto O, Calciati A. Two sudden deaths during prophylactic antiemetic treatment with high doses of domperidone and methylprednisolone. Lancet. 1984;2:1336–7. doi: 10.1016/s0140-6736(84)90841-9. [DOI] [PubMed] [Google Scholar]
- 8.Djeddi D, Kongolo G, Lefaix C, Mounard J, Léké A. Effect of domperidone on QT interval in neonates. J Pediatr. 2008;153:663–6. doi: 10.1016/j.jpeds.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Günlemez A, Babaoğlu A, Arisoy AE, Türker G, Gökalp AS. Effect of domperidone on the QTc interval in premature infants. J Perinatol. 2010;30:50–3. doi: 10.1038/jp.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heykants J, Hendriks R, Meuldermans W, Michiels M, Scheygrond H, Reyntjens H. On the pharmacokinetics of domperidone in animals and man. IV. The pharmacokinetics of intravenous domperidone and its bioavailability in man following intramuscular, oral and rectal administration. Eur J Drug Metab Pharmacokinet. 1981;6:61–70. doi: 10.1007/BF03189516. [DOI] [PubMed] [Google Scholar]
- 11.Ward BA, Morocho A, Kandil A, Galinsky RE, Flockhart DA, Desta Z. Characterization of human cytochrome P450 enzymes catalyzing domperidone N-dealkylation and hydroxylation in vitro. Br J Clin Pharmacol. 2004;58:277–87. doi: 10.1111/j.1365-2125.2004.02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meuldermans W, Hurkmans R, Swysen E, Hendrickx J, Michiels M, Lauwers W, Heykants J. On the pharmacokinetics of domperidone in animals and man III. Comparative study on the excretion and metabolism of domperidone in rats, dogs and man. Eur J Drug Metab Pharmacokinet. 1981;6:49–60. doi: 10.1007/BF03189515. [DOI] [PubMed] [Google Scholar]
- 13.Tsujikawa K, Dan Y, Nogawa K, Sato H, Yamada Y, Murakami H, Ohtani H, Sawada Y, Iga T. Potentiation of domperidone-induced catalepsy by a P-glycoprotein inhibitor, cyclosporin A. Biopharm Drug Dispos. 2003;24:105–14. doi: 10.1002/bdd.343. [DOI] [PubMed] [Google Scholar]
- 14.Schinkel AH, Wagenaar E, Mol CA, van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–24. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmermann M, Duruz H, Guinand O, Broccard O, Levy P, Lacatis D, Bloch A. Torsades de Pointes after treatment with terfenadine and ketoconazole. Eur Heart J. 1992;13:1002–3. doi: 10.1093/oxfordjournals.eurheartj.a060277. [DOI] [PubMed] [Google Scholar]
- 16.Tsai WC, Tsai LM, Chen JH. Combined use of astemizole and ketoconazole resulting in torsade de pointes. J Formos Med Assoc. 1997;96:144–6. [PubMed] [Google Scholar]
- 17.Wysowski DK, Corken A, Gallo-Torres H, Talarico L, Rodriguez EM. Postmarketing reports of QT prolongation and ventricular arrhythmia in association with cisapride and Food and Drug Administration regulatory actions. Am J Gastroenterol. 2001;96:1698–703. doi: 10.1111/j.1572-0241.2001.03927.x. [DOI] [PubMed] [Google Scholar]
- 18.Drolet B, Rousseau G, Daleau P, Cardinal R, Turgeon J. Domperidone should not be considered a no-risk alternative to cisapride in the treatment of gastrointestinal motility disorders. Circulation. 2000;102:1883–5. doi: 10.1161/01.cir.102.16.1883. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson GR. Clearance approaches in pharmacology. Pharmacol Rev. 1987;39:1–47. [PubMed] [Google Scholar]
- 20.Veronese ML, Gillen LP, Burke JP, Dorval EP, Hauck WW, Pequignot E, Waldman SA, Greenberg HE. Exposure-dependent inhibition of intestinal and hepatic CYP3A4 in vivo by grapefruit juice. J Clin Pharmacol. 2003;43:831–9. doi: 10.1177/0091270003256059. [DOI] [PubMed] [Google Scholar]
- 21.Greenblatt DJ, von Moltke LL, Harmatz JS, Chen G, Weemhoff JL, Jen C, Kelley CJ, LeDuc BW, Zinny MA. Time course of recovery of cytochrome p450 3A function after single doses of grapefruit juice. Clin Pharmacol Ther. 2003;74:121–9. doi: 10.1016/S0009-9236(03)00118-8. [DOI] [PubMed] [Google Scholar]
- 22.Yasuda K, Lan LB, Sanglard D, Furuya K, Schuetz JD, Schuetz EG. Interaction of cytochrome P450 3A inhibitors with P-glycoprotein. J Pharmacol Exp Ther. 2002;303:323–32. doi: 10.1124/jpet.102.037549. [DOI] [PubMed] [Google Scholar]
- 23.Benton RE, Sale M, Flockhart DA, Woosley RL. Greater quinidine-induced QTc interval prolongation in women. Clin Pharmacol Ther. 2000;67:413–8. doi: 10.1067/mcp.2000.105761. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA. 2001;14:1322–6. doi: 10.1001/jama.285.10.1322. [DOI] [PubMed] [Google Scholar]
- 25.Dumaine R, Roy ML, Brown AM. Blockade of HERG and Kv1.5 by ketoconazole. J Pharmacol Exp Ther. 1998;286:727–35. [PubMed] [Google Scholar]
- 26.Mok NS, Lo YK, Tsui PT, Lam CW. Ketoconazole induced torsades de pointes without concomitant use of QT interval-prolonging drug. J Cardiovasc Electrophysiol. 2005;16:1375–7. doi: 10.1111/j.1540-8167.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 27.Chaikin P, Gillen MS, Malik M, Pentikis H, Rhodes GR, Roberts DJ. Co-administration of ketoconazole with H1-antagonists ebastine and loratadine in healthy subjects: pharmacokinetic and pharmacodynamic effects. Br J Clin Pharmacol. 2005;59:346–54. doi: 10.1111/j.1365-2125.2005.02348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarapa N, Nickens DJ, Raber SR, Reynolds RR, Amantea MA. Ritonavir 100 mg does not cause QTc prolongation in healthy subjects: a possible role as CYP3A inhibitor in thorough QTc studies. Clin Pharmacol Ther. 2008;83:153–9. doi: 10.1038/sj.clpt.6100263. [DOI] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration. 2005. International Conference on Harmonisation (ICH) guidance document Topic E14. The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugsCHMP/ICH/2/04.
- 30.Rocha CM, Barbosa MM. QT interval prolongation associated with the oral use of domperidone in an infant. Pediatr Cardiol. 2005;26:720–3. doi: 10.1007/s00246-004-0922-z. [DOI] [PubMed] [Google Scholar]
- 31.Medicines Control Council. Interaction between ketoconazole and domperidone and the risk of QT prolongation – important safety information. S Afr Med J. 2006;96:596. [PubMed] [Google Scholar]


