Abstract
AIMS
The UK Medicines and Healthcare products Regulatory Agency (MHRA) runs a national spontaneous reporting system (Yellow Card Scheme) to collect ‘suspected’ adverse drug reaction (ADR) data. MHRA advice is to report all suspected ADRs in paediatric (<17 years) patients.
METHODS
Data on all ADRs reported to the MHRA in patients <17 years from the years 2000–9 were supplied in two datasets, inclusive and exclusive of vaccines.
RESULTS
Of 222 755 ADR reports received by the MHRA from 2000–9, 31 726 (14.2%) were in children <17 years. The number of reports in 2000 was greater than in subsequent years (12 035) due to a national vaccination programme (Meningococcal Serogroup C conjugate vaccine). The median number of ADR reports per annum (2001–2009) for children was 2146 (95% CI 1801, 2575). Vaccines were included in 22 102 (66.5%) paediatric ADR reports, with Meningococcal Serogroup C conjugate vaccine reported most frequently (12 106 reports) and headache the commonest symptom (3163). Excluding vaccines, methylphenidate (653 reports) and atomoxetine (491) were the most commonly reported medications, and the most commonly reported symptom was vomiting (374). Reporting by nurses increased from 396 in 2001 to 1295 in 2009 (41.8% of all reports); reporting by doctors stayed constant. Reports from patients, parents or carers more than doubled but remained infrequent (1.5% in 2005, 4.0% in 2009).
CONCLUSIONS
Although under-reporting is probably common, the Yellow Card Scheme in the UK receives more than 2000 reports per year on patients <17 years. Nurses now report more suspected ADRs in children than any other healthcare professional.
Keywords: adverse drug reaction, paediatrics, pharmacology
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Use of off-label and unlicensed medicines in children is associated with a higher risk of adverse drug reactions (ADRs).
Spontaneous reporting systems such as the UK Yellow Card Scheme run by the Medicines and Healthcare Products Regulatory Agency (MHRA) are important in identifying signals of ADRs.
Up to 95% of all ADRs are not reported, despite current MHRA advice to report all suspected paediatric ADRs.
WHAT THIS STUDY ADDS
Despite under-reporting, the Yellow Card Scheme receives more than 2000 reports per year on patients <17 years.
Vaccines are the most commonly reported therapeutic class in children.
Nurses now report more suspected ADRs in children than any other healthcare professional.
Introduction
In the UK, children comprise around 19% of the population [1] and receive approximately 5% of the total medicines prescribed (based on data for England [2]). Until recently, clinical development of drugs potentially beneficial to children has not always included studies in this age group [3, 4], and drug dosing estimates, efficacy and safety data are often extrapolated from adult data. This has led to high levels of off-label and unlicensed drug use in children. Ninety percent of inpatient neonates receive off-label or unlicensed medicines, and approximately 36% of all medications prescribed in hospitalized children are either off-label or unlicensed [5, 6]. For new compounds, it is hoped that the provision of the European Union's Regulation on Medicines for Paediatric Use (2007), together with the US Best Pharmaceuticals for Children Act (2002) and Pediatric Research Equity Act (2003), will increase the available evidence for drug use in children [7].
The use of off-label and unlicensed medicines in children is associated with a higher risk of adverse drug reactions (ADRs) [5, 8]. A systematic review of ADRs in children examining 101 studies has shown ADRs cause between 0.4–10.3% confidence interval (CI) of admissions to hospital (pooled estimate 2.9%; 2.6–3.1% CI), and outpatient ADR rates of between 0–11% of all children exposed to a drug [9]. This review also found that anti-infectives and anti-epileptics were the most frequently reported therapeutic classes associated with admission to hospital [9].
Spontaneous reporting systems such as the UK Yellow Card Scheme run by the Medicines and Healthcare products Regulatory Agency (MHRA) are important in identifying signals of ADRs. For the paediatric population, the MHRA advise reporting of all ADRs regardless of the seriousness of the reaction. The main problem with spontaneous reporting systems, however, is the significant degree of under-reporting, with estimates that up to 95% of all ADRs are not reported [10, 11].
During the period of data collection, the Scheme was expanded to include nurses, patients, parents and carers to report suspected ADRs. In a study of suspected ADRs from a mixed population of adults and children, the proportion and quality of Yellow Card reports from nurses has been shown to be similar to those for doctors [12]. There are currently little published data on information provided by parents to spontaneous reporting schemes. Adult patients have been shown in some cases to identify ADRs faster than medical professionals [13], although those reported by health professionals are more likely to result in hospitalization [14]. For specific, very severe paediatric ADRs, parental reporting provided information of equivalent quality to that of health professionals [15].
Despite under-reporting, the Yellow Card Scheme is an important tool for pharmacovigilance, which can identify new signals and provide data that may be utilized for regulatory action. We have therefore undertaken a review of all suspected ADRs reported to the MHRA Yellow Card Scheme for children and young people under 17 years of age over a 10 year period, in order to identify patterns in reporting over time including the number of reports, type of drugs reported and the identity of the reporters.
Methods
Yellow Card data
Data on Yellow Card reports between 2000–2009 from health professionals, parents, patients, industry and other reporters were supplied by the MHRA. The data were supplied in an unlinked anonymized format, so correlation of an individual drug with a particular ADR or profession of the reporter was not possible. The Yellow Card reporting scheme was initially restricted to doctors. Hospital pharmacists were introduced to the scheme in 1997 and in 1999 all community pharmacists were included. As part of the National Meningitis C vaccine immunization campaign in 2000, nurses were also able to report ADRs via the Yellow Card Scheme, and from October 2002 the scheme was extended generally to all nurses, midwives and health visitors. Parents, carers and patients have been able to report ADRs since 2003, initially through the telephone helpline NHS direct. Following an independent review of access to the Yellow Card Scheme, a nationwide pilot scheme was launched for parents, carers and patients to report ADRs directly to the MHRA in January 2005, with formal implementation in February 2008.
The information was provided by the MHRA in two versions. Initially, a complete overview of ADR reports was supplied, divided into each calendar year for children and young people aged <17 years at the time of the reaction (including suspected drugs, age of patient, reporter qualification and type of reaction). The 20 most commonly implicated drugs and the types of reactions were supplied for each year. The 25 most common reactions (with numbers of reports linked to that reaction) were also supplied. After initial analysis it became clear that vaccines represented the largest category numerically and might be obscuring data from other types of drugs, and so a second dataset with vaccine data removed was also supplied. In this vaccine free dataset, the 20 most commonly reported reactions per year were supplied (if at least five Yellow Card reports for that ADR had been received by the MHRA), and the 20 most common reported reactions (provided at least 10 reports of that reaction had been received). The cut-off values in the data supplied were in accordance with policy guidelines applied by the MHRA to preserve confidentiality of reporters and patients. To determine if a medication was a black triangle (▾) drug, i.e. a newly licensed drug, in the year of reporting, the MHRA website was interrogated (http://www.MHRA.gov.uk).
Therapeutic class
For each medication-related ADR report (≥five separate ADR reports in a single year reported to MHRA), the drugs were grouped into approximate therapeutic classes. The therapeutic classes roughly correspond with paediatric sub-specialties (Cardiology, Anaesthesia and Intensive Care, Respiratory, etc.) but some medications (e.g. corticosteroids) are common to numerous sub-specialties and needed to be categorized separately. The total reports for all drugs listed by therapeutic class do not equal the total reports above, as drugs with less than five reports in a year were not supplied (resulting in some missing data), while on many Yellow Card reports more than one suspect drug and/or vaccine was listed. For mixed preparation products (e.g. inhalers containing salmeterol and fluticasone), the product was assigned to the category of the agent deemed most likely to have caused an ADR (as determined by the study team; in the previous example, fixed combination inhalers containing steroids were included with corticosteroids rather than anti-asthma medication). For drugs used in more than one therapeutic area (e.g. erythromycin, used as antibiotic and pro-kinetic), the most common usage was used to determine the therapeutic class (anti-infective in this case). The number of medications contributing to a therapeutic class per year was calculated. From this, the mean number of medications for a given therapeutic class was calculated over 3 year periods at the beginning and end of the epoch (2000–2 and 2007–9). Data analysis, categorization of the drugs into therapeutic classes and categorization of both mixed preparation products and medications used in more than one therapeutic area were undertaken independently by two authors (DH and PM), with any disagreements resolved by discussion.
Vaccinations
The UK childhood vaccination schedule varied over the time period 2000–9. Commencing November 1999, a national vaccination program using Meningococcal Serogroup C conjugate vaccine was undertaken for all children and young people <18 years in the UK [16]. The average number of births per year in the UK for the 18 years preceding the Meningococcal Serogroup C conjugate catch up vaccination campaign was 658 800 [17]. Meningococcal Serogroup C conjugate vaccine coverage of approximately 85% was achieved in the target population [18]. In November 2001 a pertussis booster was added at age 3–4 years [19]. The BCG vaccination manufacturer supplying the UK was changed to the Statens Serum Institut (SSI) BCG vaccine in November 2002 following withdrawal of the Evans BCG vaccine [20]. In September 2004 oral polio vaccine was replaced with inactivated polio vaccine and acellular pertussis vaccine replaced whole cell pertussis vaccine [21]. The routine administration of BCG vaccine to all schoolchildren was discontinued in July 2005 [22]. In June 2006 a seven-valent conjugate Pneumococcal vaccination was introduced for children less than 2 years of age and the administration schedule for Haemophilus influenzae type b and Meningococcal Serogroup C conjugate vaccinations were modified [23]. Finally, in October 2008, the Human Papilloma virus vaccination was introduced for girls aged 12–13 years [24].
Statistics
Data were described as frequencies, percentages, medians, or means with 95% confidence intervals. Statistical analysis was undertaken using Excel 2007 (Microsoft Corporation, USA) and StatsDirect (StatsDirect Ltd, Cheshire, UK).
Results
ADR reports over time
Over the 10 year period (2000–9), a total of 222 755 Yellow Card reports were received by the MHRA. Of these, 31 726 (14.2%) were for children and young people <17 years of age at the time of the reaction. Of these 31 726, 21 102 (69.7%) included vaccines in the list of possible causative agents. There were 170 different products included on the Yellow Cards, comprising 18 vaccines and 152 medicines. In 2000, the total number of reports and reports in children were larger than in all other years (33 145 and 12 035 respectively). The difference in total reports could be attributed to the excess paediatric reports in this year. For the years 2001–9, the median number of ADR reports per annum for children was 2146 (95% CI 1801, 2575), with the last 2 years (2008 and 2009) having the largest numbers of reports (2965 and 2903 respectively). These data are summarized in Figure 1A,B. In November 1999, the Meningococcal Serogroup C conjugate vaccine was introduced (including catch up vaccination of all under 18-year-olds [18]), which alone generated 10 788 individual reports. Over the 10 year period, the percentage of paediatric ADR reports related to vaccinations was 69.7% (95% CI 69.2, 70.2%). If 2000 is excluded, the percentage of paediatric ADR reports related to vaccination was 55.3% (95% CI 54.4, 56.2%).
Figure 1.
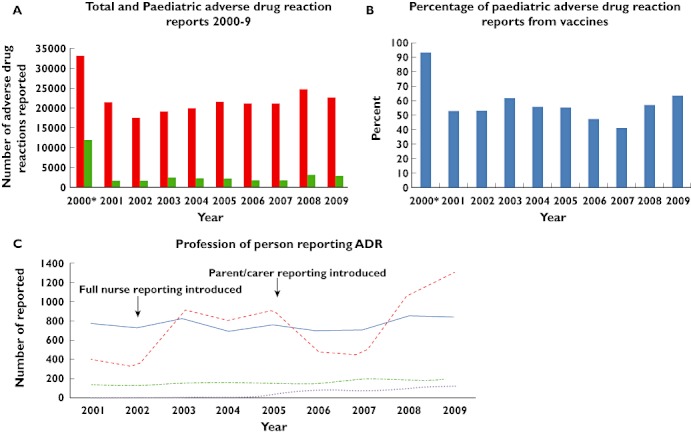
(A) Graph of the total (adult and paediatric) and paediatric (age < 17 years) adverse drug reactions (ADR) reported to the MHRA annually for the years 2000–2009 (including vaccines). (B) Graph of the percentage of paediatric ADR reports from vaccines for the years 2000–2009 (C) Graph of the number of Yellow Card reports per year grouped by profession of the reporter. Nurse reporting was initially introduced in 2000, but this was limited. Full nurse reporting was introduced in 2002, and parent and carer reporting introduced in 2005. *2000 contained a spike in reports related to Neisseria meningitidis Group C vaccine. (A) ( ) Total number; (
) Total number; ( ) Total number < 17 years; (C) (
) Total number < 17 years; (C) ( ) Doctors; (
) Doctors; ( ) Nurses; (
) Nurses; ( ) Pharmacist; (
) Pharmacist; ( ) Parent/Carer
) Parent/Carer
Reporter
In the year 2000, during the spike in reporting activity, there were 4227 doctor and 5421 nurse generated reports. Over the years 2001–9, the total number of reports from doctors remained stable over time. The proportion of ADRs reported by nurses increased from 23.5% in 2001 (n = 396) to 41.8% in 2009 (n = 1295). Reports by pharmacy teams remained at approximately 5–8%, and other sources (patients, parents, carers, lawyers, optometrists, coroners, pharmaceutical industry and literature reviews) comprised the remainder of the reports. Data regarding the profession of the reporter of suspected paediatric ADRs (2001–9) is shown in Figure 1C.
Patient, parent and carer reports were introduced in 2005; although the proportion of reports received from this group has increased over time, the absolute numbers remain low. In 2005, 34 reports were received and by 2009 this increased to 123 reports (Figure 1C).
ADR reports by age
ADR reports were received for all paediatric age groups, with the frequency of reports varying with different age groups and whether vaccine data were included. Peaks in reporting suspected ADRs were seen in children in the first year of life for both vaccine and non-vaccine related reports (Figure 2A,B). Subsequent peaks were seen in older children for vaccine related ADR reports at ages 4 and 12 years, corresponding to times of vaccination in childhood (Figure 2A). For non-vaccine related reactions, there was a gradual increase after the first year of life (Figure 2B).
Figure 2.
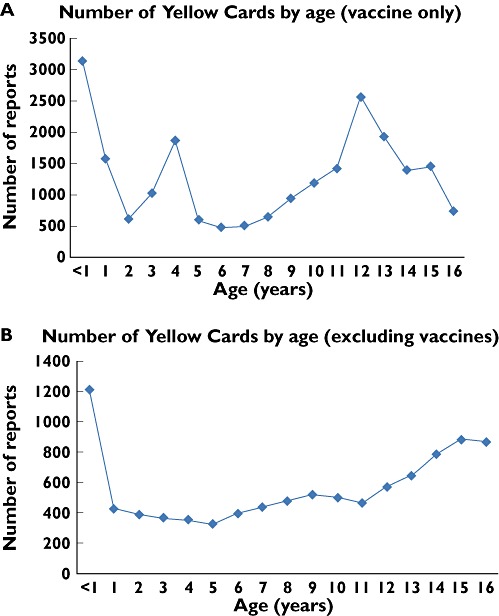
Graphs of number of reports received per year of age in the years 2000–9 for (A) vaccines only and (B) excluding vaccines
Vaccine related ADR reports
From the 18 vaccines listed on Yellow Cards as suspected of causing an ADR, the 10 most frequently reported are shown in Table 1. The most commonly listed vaccine on Yellow Cards was the Meningococcal Serogroup C conjugate vaccine (12 106 reports). Alterations in the vaccination schedule also affected the number of reports. Following the change in the BCG vaccine manufacturer (November 2002), reports increased from 110 (2002) to a 10 year peak of 449 reports (2003) and this was the most commonly suspected agent reported to the MHRA in paediatrics for 2003 and 2004. However, by 2006 (when the school BCG programme was halted), the number of reports for BCG had decreased to 31, and it was not reported in sufficient numbers to feature in the 2009 dataset (Figure 3).
Table 1.
The top 10 most commonly reported medications and vaccines on Yellow Cards for patients aged less than 17 years 2000–2009
| Number of reports 2000–9 | |
|---|---|
| Vaccines | |
| Meningococcal Serogroup C conjugate | 12 106 |
| Human papilloma virus | 2 470 |
| Bacillus Calmette Guerin (BCG) | 1 316 |
| MMR vaccine | 1 060 |
| DTwP and Hib | 955 |
| Streptococcus pneumoniae | 898 |
| DTaP IPV Hib | 877 |
| DTaP IPV | 828 |
| DTaP | 696 |
| Poliomyelitis virus (oral, OPV) | 516 |
| Medications | |
| Methylphenidate | 653 |
| Atomoxetine | 491 |
| Valproic acid | 254 |
| Montelukast | 243 |
| Mycobacterium* | 215 |
| Lamotrigine | 209 |
| Risperidone | 201 |
| Paracetamol | 170 |
| Topiramate | 158 |
| Carbamazepine | 157 |
Mycobacterium refers to the tuberculin skin test rather than the vaccination against tuberculosis (see BCG in vaccinations). MMR: Measles/mumps/rubella, DTwP: Diptheria, tetanus and (whole cell) pertussis, DTaP: Diptheria, tetanus and (acellular) pertussis, IPV, Inactivated polio vaccine; HIb, Haemophilus influenzae type b.
Figure 3.
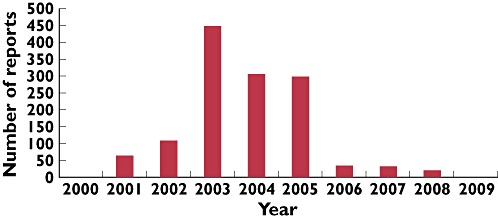
Graph of the number of Yellow Card reports listing Bacillus Calmette Guerin (BCG) vaccination as a possible causative agent per year. A new BCG vaccine was introduced in December 2002
Non-vaccine related ADR reports
For medications, 152 different drugs were listed on Yellow Cards, but the number of reports submitted per agent was much lower than for the vaccinations (Table 1). The drug most frequently associated with paediatric ADR reports was methylphenidate (653 reports), followed by atomoxetine (491). These are used to treat attention deficit hyperactivity disorder (ADHD). The drug which was third most frequently associated with paediatric ADR reports was valproic acid (254), with other drugs primarily used to treat epilepsy comprising another three of the 10 most frequently reported medications (lamotrigine [6th, 209 reports], topiramate [9th, 158], and carbamazepine [10th, 157]). Data on whether a drug was a black triangle (▾) medication were only available for the years 2007–9. In 2007, five of the 10 most commonly reported medications were black triangle (▾) medications. In both 2008 and 2009, four of the 10 most commonly reported medications were black triangle medications.
Therapeutic class
The Yellow Card reports are shown by therapeutic class in Table 2. The total number of reports in this table (30 252) differs from the total number of ADRs reported (31 726) as each Yellow Card report may contain more than one suspect drug. Vaccines and ADHD medications were the most frequently reported therapeutic classes. The number of reports in the class of ADHD medications increased markedly following the introduction of atomoxetine to the UK in 2004 [25]. The median number of paediatric ADR reports from this class in the 3 years prior to introduction of atomoxetine was 54 per year, but for the 3 years after its introduction, this increased to 164 per year. Anti-epileptic drugs were the third most frequently reported therapeutic class, with a consistent rate of reporting noted across the 10 year period (range 68–128 reports, Table 2), with no particular drug being prominent. Over the epoch there was an increase in the reporting of suspected ADRs due to immunosuppressives and chemotherapy. The median number of agents in this class reported as causing a paediatric ADR (2000–2) was 2 (95% CI 2, 3) and in 2007–9 it had increased to 9 (95% CI 7, 13). The median number of biological agents increased from 1 (95% CI 0, 2) for the years 2000–2 to 4 (95% CI 2, 4) in 2007–9. Other categories with lower total numbers of reports had spikes of reporting; for example, there was a large increase in reporting of analgesic-related ADRs in 2008 (n = 125).
Table 2.
Number of suspected adverse drug reaction (ADR) reports made to the MHRA through the Yellow Card Scheme by therapeutic class of agent
| Therapeutic class | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccines | 11 733 | 1031 | 1006 | 1782 | 1356 | 1329 | 950 | 831 | 1738 | 1852 | 23 608 |
| ADHD medication and melatonin | 37 | 45 | 54 | 88 | 123* | 213 | 164 | 158 | 155 | 132 | 1 169 |
| Anti-epileptics (including benzodiazepines) | 128 | 101 | 83 | 100 | 74 | 96 | 68 | 97 | 126 | 108 | 981 |
| Antibiotics, antivirals, antifungals | 45 | 51 | 61 | 98 | 104 | 83 | 68 | 136 | 65 | 87 | 798 |
| Immunosuppressants and chemotherapy (excluding steroids) | 18 | 18 | 13 | 57 | 53 | 54 | 61 | 76 | 161 | 225 | 736 |
| Psychiatry agents (antidepressants and antipshychotics) | 44 | 58 | 83 | 105 | 76 | 86 | 70 | 68 | 72 | 65 | 727 |
| Endocrine, hormones (including bone) | 22 | 14 | 40 | 25 | 55 | 57 | 57 | 31 | 70 | 38 | 409 |
| Pain relief (NSAID, paracetamol, migraine) | 17 | 7 | 13 | 26 | 23 | 17 | 26 | 32 | 125† | 50 | 336 |
| Anti-asthma and hayfever (excluding steroids) | 54 | 44 | 28 | 21 | 30 | 16 | 39 | 27 | 39 | 35 | 333 |
| Corticosteroids (including dual preparation items e.g. Seretide) | 21 | 20 | 21 | 18 | 10 | 6 | 5 | 31 | 62 | 40 | 234 |
| Local/general anaesthetics/intensive care | 56 | 41 | 11 | 49 | 11 | 5 | 13 | 0 | 6 | 7 | 199 |
| Vitamins and derivatives | 24 | 24 | 13 | 16 | 18 | 17 | 12 | 17 | 13 | 15 | 169 |
| Monoclonal antibodies and biologics (e.g. etanercept) | 5 | 0 | 13 | 0 | 0 | 10 | 13 | 19 | 33 | 58 | 151 |
| Gastrointestinal | 10 | 5 | 5 | 0 | 10 | 25 | 28 | 0 | 20 | 23 | 126 |
| Haematology/coagulation | 6 | 0 | 5 | 0 | 0 | 0 | 0 | 15 | 22 | 30 | 78 |
| Immunoglobulin | 5 | 9 | 7 | 0 | 16 | 5 | 9 | 8 | 6 | 7 | 72 |
| Topical drugs and preparations (excluding steroids) | 0 | 0 | 5 | 13 | 18 | 0 | 0 | 6 | 5 | 11 | 58 |
| Metabolic disease | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 5 | 8 | 23 |
| Opiates | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 10 | 5 | 0 | 20 |
| Anti-arrhythmics and antihypertensives | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 14 | 0 | 19 |
| Chelating agents | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Total | 12 231 | 1468 | 1461 | 2403 | 1977 | 2034 | 1583 | 1562 | 2742 | 2791 | 30 252 |
Year atomoxetine licensed.
Year that additional data from a publication about paracetamol and liver damage added to MHRA database.
These figures differ from the total number of ADR reports as each Yellow Card report may contain more than one suspect drug.
Reactions reported
A total of 2587 unique reaction terms were reported in the total paediatric dataset for 2000–2009. The 10 most frequently reported across the entire time period are shown in Table 3. Most frequently reported clinical effects were similar between vaccines and other medications, with five of the 10 reactions featuring in both lists (headache, pyrexia, vomiting, nausea and erythema). When examined on an annual basis, there were wide variations in the most commonly reported reactions. For example, from the non-vaccine dataset, the two most common suspected reactions reported in 2008 were overdose and liver injury. However, overdose was not in the top 10 reported for any other year in the dataset, and liver injury fell below the minimum number of suspected reports per year and did not feature in any other years' data (data not shown).
Table 3.
The 10 most commonly reported reactions for paediatric patients 2000–2009. Data supplied included reactions only if more than 10 reports of that reaction were received in a year
| Reaction (vaccines included) | Total number of reports 2000–9 | Reaction (vaccines excluded) | Total number of reports 2000–9 |
|---|---|---|---|
| Headache | 3163 | Vomiting | 374 |
| Dizziness | 2947 | Rash | 371 |
| Pyrexia | 2215 | Urticaria | 366 |
| Vomiting | 2013 | Headache | 316 |
| Nausea | 1923 | Aggression | 283 |
| Erythema | 1844 | Convulsion | 282 |
| Syncope | 1735 | Pyrexia | 253 |
| Oedema peripheral | 1643 | Nausea | 214 |
| Local reaction | 1554 | Pruritus | 198 |
| Malaise | 1535 | Erythema | 197 |
Discussion
Post marketing surveillance is an essential tool to enable detection of ADRs, particularly the less common, but sometimes very serious ADRs. For an ADR with an incidence of 1 in 10 000 exposed individuals, it has been estimated that at least 30 000 people need to be treated with a drug to detect at least one case [26]. Clearly this is more than would normally be tested during drug development. Post marketing surveillance is particularly important in children who have different (and developing) physiology, body proportions, tissue composition, organ function and ADR profile (e.g. growth suppression) from the adult population routinely tested during drug development.
The data presented here show that there are a substantial number (median of 2146 reports for the years 2001–9) of suspected ADRs in children reported in the UK. These ADRs occur at all ages, are noted to be from a large number of therapeutic classes (particularly vaccines) and cause a wide variety of reactions. In addition, the time period reviewed covered the introduction of a broader range of reporters for suspected ADRs (including parents/carers and patients themselves) and different means of reporting (online Yellow Card completion).
These data are derived entirely from the UK spontaneous ADR reporting scheme (MHRA Yellow Card Scheme), and are subject to the limitations of any such scheme. These include under-reporting of ADRs (secondary to lack of recognition or failure to carry out the reporting process) [27, 28], inability to calculate the true incidence of any ADRs reported [29, 30], variable quality in Yellow Card completion [29], assessment of causality between a drug and an ADR and difficulty in identifying ADRs with long latency periods following use of the drug [31]. Under-reporting in particular is believed to be very common, with some estimates that up to 95% of all ADRs are not reported [10, 11]. However, this kind of scheme also has many positive aspects, including the ability to identify previously unknown ADRs (for example vigabatrin and visual field defects [32]), and feedback to prescribers through MHRA publications such as Drug Safety Update (http://www.mhra.gov.uk/Publications/Safetyguidance/DrugSafetyUpdate/index.htm).
What is clear from these data are that administration of routine vaccinations is associated with a large number of reports of suspected ADRs, dwarfing the number reported for medications. This reflects the high usage of vaccines in paediatrics compared with medicines. The high level of vaccine-related reports of suspected ADRs peaked in the year 2000 (n = 11 216), where the number of reports was much higher than for all of the subsequent years (Figure 1A), as well as in previous years. This high volume of reports was attributed to a national Meningitis C immunization campaign which ran that year immunizing all children (0–18 years), and during which nursing staff were invited to report reactions to the vaccine using Yellow Cards. The estimated frequency of any ADR associated with the Meningococcal Serogroup C conjugate vaccination in the year 2000, based on the proportion of the target population vaccinated and the number of Yellow Cards completed, is at least 0.1% [33], with the proviso that not all ADRs will have been reported. This compares favourably with an estimated frequency of outpatient paediatric ADRs (1.5%) [34]. Previous reviews of ADRs associated with Meningococcal Serogroup C conjugate vaccination have found that in primary and secondary school age children there are very similar types and frequencies of ADRs between the Meningococcal Serogroup C conjugate vaccination and a combined diphtheria/tetanus booster vaccination [18], and across various populations severe reactions to the vaccine have been found to be very rare (1 : 200 000–500 000 doses) [33, 35]. A previous publication has focussed in detail on the increase in suspected ADRs following the 2002 change in BCG brand in the UK [36].
The high number of vaccination-related suspected ADRs may also have been influenced by any or all of the following: the large number of vaccine doses given compared with other drugs; the high level of anxiety associated with administration of vaccinations (for example following the unfounded autism/MMR link [37, 38]); the administration of vaccinations to healthy individuals (so any symptom could be related to the vaccination, whereas in a sick individual receiving a medication, the symptoms of the disease are taken into consideration); the person giving the vaccine and/or completing the Yellow Card (45% of ADRs reported to the Yellow Card Scheme by nurses in one study were from vaccinations [12]); and the setting where the vaccination takes place (a dedicated vaccination clinic may have better procedures for ensuring documentation like Yellow Cards are completed).
An expansion of nurse reporting to include any drug or reaction via the Yellow Card Scheme took place in 2002. It is not clear if there is a difference in the drugs reported (e.g. more vaccines, as these are often administered by nursing staff) or nature of the suspected ADR reported by nurses and doctors in paediatric patients, and it is not possible to discern this from our data as it was provided in an unlinked format. There has been an increase in nurse reporting to 43% of all reports in 2009, but no decline in doctor reporting, which suggests that nurses are reporting ADRs which were previously not reported at all and the impact of nurse reporting is likely to have improved overall detection of ADRs in children. There has been little published about the utility of parent reporting of ADRs, except in extreme circumstances (such as death of a child [15]), but with the increased reporting in this area, it may become a useful area to assess. The only direct comparison of healthcare professional and patient completion of Yellow Card reports used adult patients, and showed that the healthcare professionals were more likely to report severe reactions that led to hospitalization [14].
The categorization of medicines into therapeutic classes should aid clinicians examining these data by allowing them to see if ADRs from classes they prescribe are being reported, rather than scanning long lists of individual drugs, bearing in mind the assumptions detailed previously about how these categorizations were arrived at. For some medications, reporting has remained fairly stable over the decade (e.g. anti-epileptic medications, endocrine and hormonal medications, psychiatric medications). Increases in reporting were seen in some groups, notably ADHD medication (following the introduction of atomoxetine in 2004) and immunosuppressives and chemotherapy (with increases in the number of agents reported per year). The high frequency of reports for anti-infective and anti-epileptic medications has also been reflected in a recent systematic review [9], where these therapeutic classes were the most commonly noted. Spikes in reporting were also noted, such as for BCG vaccination (Figure 3) and pain relief (following inclusion in the Yellow Card database of a series of paracetamol related ADRs reported in a literature review published in 2008 detailing 89 children aged 12 years or younger who were reported to have suffered liver damage after the therapeutic use of paracetamol [39].)
Even when groups of medications are collected into therapeutic classes, there are still areas where reporting did not reach the threshold of five per year needed to be included in the datasets, so absence from this list does not mean that no reports were received (although numbers for each individual drug must have been <five reports). In particular, for the therapeutic class ‘anti-arrhythmics and antihypertensives’, there were low levels of reporting across the entire time period. We also acknowledge that omission of certain therapeutic classes from the list does not necessarily mean under-reporting or suggest that particular drugs have a good safety profile. A rare, serious ADR would not be picked up in this way.
The current advice from the MHRA is for all ADRs affecting children to be reported, not just severe reactions or those from drugs under intensive surveillance (black triangle ▾ medications). The UK data collected over a 10 year period has considerably fewer reports in certain therapeutic classes than would be expected. This could be due to a number (or combination) of factors including frontline ‘editing’ of which reactions to report (potentially secondary to time constraints, reluctance to report medically ‘mild’ ADRs, use of adult type reporting patterns with only new drugs or suspected serious ADRs reported) or a belief that the ADR in question is to be expected from the action of the drug and hence not worthy of reporting (for example first dose hypotension from antihypertensives). The fact that the 10 most commonly reported suspected paediatric ADRs feature serious reactions, such as convulsions, would suggest that reporters are more likely to report the serious reactions. Under-reporting is known to be common in spontaneous reporting schemes [10, 11], and we believe this to be present in this scheme as well, although it is not possible to know the scale of under-reporting present.
The increase in the number of reports in the last 2 years to the highest levels seen in the time period 2001–2009 is heartening. This increase is mostly attributable to increased nurse reporting; although doctor reporting has remained stable in terms of number of reports, the proportion has decreased. Parent and carer reporting, although numerically small, also showed an increase in the number of reports submitted. A variety of reporters with different roles and responsibilities in the lives of children and young people are likely to be able to recognize a greater number of possible ADRs, and may generate information in those therapeutic classes that currently receive few reports. We would therefore encourage everyone (health professionals of all types, as well as parents/carers and others) to continue to report suspected ADRs.
In conclusion, post marketing surveillance is essential to permit detection of rarer, but potentially serious ADRs. To help generate this information, the current MHRA advice is for all suspected paediatric ADRs in the UK to be reported. Although under-reporting is probably common, the Yellow Card Scheme in the UK receives more than 2000 reports per year on patients <17 years, half of which are related to vaccines. From 2001–2009 the identity of the reporter altered such that nurses now report more suspected ADRs in children than any other healthcare professional. Patient and parent reporting remains an infrequent source of reports, although this may become more important with time.
Acknowledgments
We thank Sarah Cumber, Mick Foy and Shelley Gandhi from the MHRA for collating and supplying the data. MP and RLS are NIHR Senior Investigators. MP and RLS are also supported by the NIHR Programme Grant on adverse drug reactions in children. MP also wishes to thank the DH (NHS Chair of Pharmacogenetics), MRC, Wellcome Trust and EU-FP7 programme for grant support.
Competing Interests
DH and PM are members of the MHRA Trainee Doctors Advisory Board.
AR is a member of the Joint Committee on Vaccination and Immunisation (JCVI).
RLS and MP are members of the Commission on Human Medicines. MP chairs the Pharmacovigilance Expert Advisory Group, while RLS chairs the Paediatric Medicines Expert Advisory Group. The views expressed in this article are solely those of the authors and not of any institutions that they represent.
REFERENCES
- 1.Office for National Statistics. Key Population and Vital Statistics. Key Population and Vital Statistics No 34, PPI No 30, 2007 Data: population and vital statistics by area of usual residence in the United Kingdom, 2007. 2009. Available at http://www.ons.gov.uk/ons/rel/kpvs/key-population-and-vital-statistics/no--34--2007-edition/index.html (last accessed 31 October 2011)
- 2.Office for National Statistics. Prescriptions dispensed in the community statistics for 1997 to 2007: England. 2008. Available at http://www.ic.nhs.uk/webfiles/publications/PCA%20publication/Final%20version%20210708.pdf (last accessed 17 May 2011)
- 3.Shirkey H. Editorial comment: therapeutic orphans. J Pediatr. 1968;72:119–20. doi: 10.1016/s0022-3476(68)80414-7. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson T. The medicines for children agenda in the UK. Br J Clin Pharmacol. 2006;61:716–9. doi: 10.1111/j.1365-2125.2006.02676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner S, Nunn AJ, Fielding K, Choonara I. Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: a prospective study. Acta Paediatr. 1999;88:965–8. doi: 10.1080/08035259950168469. [DOI] [PubMed] [Google Scholar]
- 6.Choonara I, Conroy S. Unlicensed and off-label drug use in children – implications for safety. Drug Saf. 2002;25:1–5. doi: 10.2165/00002018-200225010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Hawcutt DB, Smyth RL. The new European regulation on pediatric medicines: regulatory perspective. Pediatr Drugs. 2008;10:143–6. doi: 10.2165/00148581-200810030-00002. [DOI] [PubMed] [Google Scholar]
- 8.Horen B, Montastruc JL, Lapeyre-Mestre M. Adverse drug reactions and off-label drug use in paediatric outpatients. Br J Clin Pharmacol. 2002;54:665–70. doi: 10.1046/j.1365-2125.2002.t01-3-01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smyth RMD, Gargon E, Kirkham JJ, Cresswell L, Smyth RL, Williamson PR. Adverse drug reactions in children – a systematic review. PLoS Med. 2011 doi: 10.1371/journal.pone.0024061. (in press). Available at https://www.escholar.manchester.ac.uk/uk-ac-man-scw:126372 (last accessed 31 October 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittmann N, Knowles SR, Gomez M, Fish JS, Cartotto R, Shear NH. Evaluation of the extent of under-reporting of serious adverse drug reactions – the case of toxic epidermal necrolysis. Drug Saf. 2004;27:477–87. doi: 10.2165/00002018-200427070-00004. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher AP. Spontaneous adverse drug reaction reporting vs event monitoring – a comparion. J R Soc Med. 1991;84:341–4. doi: 10.1177/014107689108400612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison-Griffiths S, Walley TJ, Park BK, Breckenridge AM, Pirmohamed M. Reporting of adverse drug reactions by nurses. Lancet. 2003;361:1347–8. doi: 10.1016/S0140-6736(03)13043-7. [DOI] [PubMed] [Google Scholar]
- 13.Egberts TCG, Smulders M, deKoning FHP, Meyboom RHB, Leufkens HGM. Can adverse drug reactions be detected earlier? A comparison of reports by patients and professionals. BMJ. 1996;313:530–1. doi: 10.1136/bmj.313.7056.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLernon DJ, Bond CM, Hannaford PC, Watson MC, Lee AJ, Hazell L, Avery A. Adverse drug reaction reporting in the UK: a retrospective observational comparison of yellow card reports submitted by patients and healthcare professionals. Drug Saf. 2010;33:775–88. doi: 10.2165/11536510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Silvers LE, Varricchio FE, Ellenberg SS, Krueger CL, Wise RP, Salive ME. Pediatric deaths reported after vaccination – the utility of information obtained from parents. Am J Prev Med. 2002;22:170–6. doi: 10.1016/s0749-3797(01)00430-5. [DOI] [PubMed] [Google Scholar]
- 16.Department of Health. The Meningitis C Campaign. London: Department of Health; 2009. Available at http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/Aboutus/MinistersandDepartmentLeaders/ChiefMedicalOfficer/ProgressOnPolicy/ProgressBrowsableDocument/DH_5852428 (last accessed 17 May 2011) [Google Scholar]
- 17.Trotter CL, Edmunds WJ. Modelling cost effectiveness of meningococcal serogroup C conjugate vaccination campaign in England and Wales. Br Med J. 2002;324:809. doi: 10.1136/bmj.324.7341.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine. 2001;20(Suppl. 1):S58–67. doi: 10.1016/s0264-410x(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 19.Donaldson L, Mullally S, Smith J. Current vaccine and immunisation issues. Department of Health; 2001. Available at http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4013406.pdf (last accessed 31 October 2011)
- 20.Committee on the Safety of Medicines, part of the MHRA. SSI BCG vaccine and local reactions. Current Problems in Pharmacovigilance. 2004:8. [Google Scholar]
- 21.Donaldson L, Mullally S, Smith J. New vaccinations for the childhood immunisation programme. Department of Health; 2004. Available at http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4087347.pdf (last accessed 17 May 2011)
- 22.Donaldson L, Beasley C, Smith J. Changes to the BCG vaccination programme. Department of Health; 2005. Available at http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4114996.pdf (last accessed 17 May 2011)
- 23.Donaldson L, Beasley C, Ridge K. Important changes to the childhood immunisation programme. Department of Health; 2006. Available at http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4137175.pdf (last accessed 17 May 2011)
- 24.Donaldson L, Beasley C, Ridge K. Introduction of Human Papilloma virus vaccination into the national immunisation programme. Department of Health; 2008. Available at http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_084546.pdf (last accessed 17 May 2011)
- 25.Bates G. Drug treatments for attention-deficit hyperactivity disorder in young people. Adv Psychiatr Treat. 2009;15:162–71. Available at http://apt.rcpsych.org/ (last accessed 31 October 2011) [Google Scholar]
- 26.Strom BL. Pharmacoepidemiology. 2nd. Chichester, UK: J. Wiley; 2005. [Google Scholar]
- 27.Belton KJ. Attitude survey of adverse drug-reaction reporting by health care professionals across the European Union. Eur J Clin Pharmacol. 1997;52:423–7. doi: 10.1007/s002280050314. [DOI] [PubMed] [Google Scholar]
- 28.Crombie I. Inherent limitations of the yellow card system for the detection of unsuspected adverse drug reactions. Hum Exp Toxicol. 1984;3:261–9. doi: 10.1177/096032718400300402. [DOI] [PubMed] [Google Scholar]
- 29.Clarkson A, Choonara I. Surveillance for fatal suspected adverse drug reactions in the UK. Arch Dis Child. 2002;87:462–6. doi: 10.1136/adc.87.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacristán JA, Gómez JC, Badía X, Kind P. Global index of safety (GIS): a new instrument to assess drug safety. J Clin Epidemiol. 2001;54:1120–5. doi: 10.1016/s0895-4356(01)00384-5. [doi: DOI: 10.1016/S0895-4356(01)00384-5] [DOI] [PubMed] [Google Scholar]
- 31.Rawlins MD. Pharmacovigilance – paradise-lost, regained or postponed – the William Withering lecture 1994. J R Coll Physicians Lond. 1995;29:41–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Committee on the Safety of Medicines, part of the MHRA. Current Problems in Pharmacovigilance 1998.
- 33.Committee on the Safety of Medicines, part of the MHRA. Safety of meningococcal group C conjugate vaccines. Current Problems in Pharmacovigilance: Medicines Control Agency/Committee on Safety of Medicines. 2000. p. 14.
- 34.Impicciatore P, Choonara I, Clarkson A, Provasi D, Pandolfini C, Bonati M. Incidence of adverse drug reactions in paediatric in/out-patients: a systematic review and meta-analysis of prospective studies. Br J Clin Pharmacol. 2001;52:77–83. doi: 10.1046/j.0306-5251.2001.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yergeau A, Alain L, Pless R, Robert Y. Adverse events temporally associated with meningococcal vaccines. Can Med Assoc J. 1996;154:503–7. [PMC free article] [PubMed] [Google Scholar]
- 36.Teo SS, Smeulders N, Shingadia DV. BCG vaccine-associated suppurative lymphadenitis. Vaccine. 2005;23:2676–9. doi: 10.1016/j.vaccine.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 37.Purssell E. Uncertainties and anxieties about vaccination, answering parent's concerns. J Pediatr Nurs. 2009;24:433–40. doi: 10.1016/j.pedn.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Deer B. How the case against the MMR vaccine was fixed. BMJ. 2011;342:c5347. doi: 10.1136/bmj.c5347. [DOI] [PubMed] [Google Scholar]
- 39.Prescott LF. Therapeutic misadventure with paracetamol: fact or fiction? Am J Ther. 2000;7:99–114. doi: 10.1097/00045391-200007020-00007. [DOI] [PubMed] [Google Scholar]


