Abstract
AIMS
There is no consensus as to what extent the results of thorough QT interval/corrected QT interval (QT/QTc) studies need to be bridged.
METHODS
The results of two studies using levofloxacin in Japanese and Caucasian subjects were compared in a post hoc analysis to investigate the similarity of dose–effect responses.
RESULTS
Concentration–response analysis based on the change of QT interval corrected using Fridericia's formula (QTcF) from time-matched placebo was planned and performed in the combined data sets. At the geometric maximum mean concentration for the two doses in the Caucasian study, a predicted effect on QTcF comparable to the effects observed was found. For the Japanese study, the predicted effect was lower, but the difference was not statistically significant.
CONCLUSIONS
No statistically significant differences in QTc-prolonging effect between Japanese and Caucasian subjects were observed following levofloxacin dosing. However, a trend suggests that Caucasian subjects may be more sensitive. Age and sex did not have an impact.
Keywords: cardiac safety, clinical trial methodology, electrocardiogram, ethnic comparison, levofloxacin, pharmacology
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Although the ICH guidelines state that it is not expected that the results of a ‘thorough QT interval/corrected QT interval (QT/QTc)’ study would be affected by ethnic factors and would consequently be independent of the race of the study population, there is little documented evidence to support or refute this.
Since 2010, the ICH E14 guidelines have been fully adopted in Japan, and the question has arisen of whether specific ethnic studies will become necessary to assess cardiac safety in Japanese individuals.
WHAT THIS STUDY ADDS
The findings of this study suggest that there is no difference in QTc-prolonging effect between Japanese and Caucasian subjects following levofloxacin dosing. However, a trend (not statistically significant) suggests that Caucasian subjects are more sensitive. Age and sex did not have an impact on the comparison of QT interval corrected using Fridericia's formula (QTcF) effects in this study.
Introduction
Since 2010, the ICH E14 guidelines have been fully adopted in Japan, and the question has arisen as to whether specific ethnic studies will become necessary to assess cardiac safety in Japanese individuals. Although the ICH guidelines state that it is not expected that the results of a ‘thorough QT interval/corrected QT interval (QT/QTc)’ study would be affected by ethnic factors and would consequently be independent of the race of the study population, there is little documented evidence to support or refute this [1].
Inherited genotypes in combination with environmental factors may result in ethnic variations producing certain basic and drug-induced changes in the electrocardiogram (ECG). One of the earliest studies in this field, by Macfarlane et al., compared Caucasian and Chinese populations (1555 adult Caucasians and 503 adult Chinese participants), demonstrating small racial differences in some measurements, including slightly longer QRS and QTc intervals in Chinese participants [2]. Whilst there are available data on the ethnic variations within basic ECGs, there are few to demonstrate whether there are any ethnic susceptibilities to drug-induced ECG changes, in particular comparing Caucasians and Japanese. A study by Shin et al. in 24 Korean and 13 Caucasian subjects demonstrated that Korean subjects were less sensitive to the QT-prolonging effects of quinidine than their Caucasian counterparts [3].
The fluoroquinolone moxifloxacin has become well established in its use as a positive control in confirming assay sensitivity by causing modest QT prolongation above the threshold of regulatory concern. This effect of moxifloxacin, together with further pharmacokinetics and pharmacokinetics/ECG analysis, has shown that there is no racial difference [4]. Levofloxacin, another fluoroquinolone, has also been shown to cause changes in the QTc interval, which are close to the threshold of regulatory concern (approximately 6 ms [5]), even at supratherapeutic doses of three to six times the therapeutic doses of 250 and 500 mg.
The effects of levofloxacin have been demonstrated in two studies, one carried out in Japan with Japanese volunteers (Sugiyama et al. [6]) and another Caucasian study carried out in the UK (Taubel et al. [5]). Sugiyama et al. studied the effect of a single intravenous dose of 500 mg levofloxacin on the QT/QTc interval in healthy Japanese male and female volunteers [6]. Taubel et al. studied the effect of 1000 and 1500 mg oral doses of levofloxacin on the QT/QTc interval in Caucasians and its potential to be used as a positive control in thorough QT/QTc studies [5]. In order to assess whether there is any ethnic variation between Caucasian and Japanese volunteers, a retrospective comparison of the pharmacokinetics and ECG parameters of the two studies was performed to analyse the concentration–effect response to levofloxacin between the two races and investigate whether there is any significant difference.
Methods
Two studies (a QT/QTc study and a study with similar quality to a QT/QTc study) evaluating the effect of levofloxacin on the QT interval in Caucasian and Japanese subjects were compared. Both the Caucasian and the Japanese studies received relevant ethics and regulatory approvals from their respective authorities [5, 6]. All subjects provided written informed consent prior to any study-specific procedures being undertaken. Sample size considerations were addressed in the relevant study protocols.
Data analysis
In the Caucasian study, the effect on QT/QTc interval was analysed using the largest time-matched mean difference between moxifloxacin/levofloxacin and placebo (baseline adjusted) [5, 7]. For the purpose of this analysis, QT interval corrected using Fridericia's correction (QTcF = QT/RR0.33) was used when analysing the data. In the Japanese study, the measurement of QT interval and subsequent over-reading was performed in a similar manner to that in the Caucasian study.
Comparison of data between the two studies (statistical methods)
The initial data collected from both studies were at different dose levels; therefore, to make the two data sets comparable, a concentration–response model was fitted. However, only the Caucasian study established a new baseline for each period of the crossover, whereas the Japanese study used a common baseline for both periods; therefore, the baseline was not used in this analysis.
In the concentration–response model, the time-matched difference to placebo in QTcF was used as a response variable; the plasma concentration of levofloxacin and its interaction with race were used as covariates. In the primary model, an intercept was only allowed as a random effect per subject, together with random slopes for concentration. Other models, one with additional fixed intercepts per race and one without any intercepts, were used to assess linearity of the relationship [4]. The slopes of the mean regression lines for both races and their differences are given with 95% confidence intervals. As an alternative, the predicted effect on QTcF at the geometric maximum mean concentration (Cmax) of the two oral doses used was calculated based on the primary model for each race together with a two-sided 95% confidence interval. The same was done for the difference of these effects between races.
Results
Effect on QTc
Both studies demonstrated a modest QTcF prolongation below the regulatory threshold of concern as defined in the ICH E14 guidelines. The Japanese subjects dosed with 500 mg levofloxacin demonstrated a mean ΔΔQTcF of 3.4 ms (upper bound of one-sided 95% confidence interval was 5.2 ms). The Caucasian subjects who received two doses (1000 and 1500 mg) of levofloxacin demonstrated a mean ΔΔQTcF of 4.7 and 7.1 ms, respectively (upper bound of one-sided 95% confidence interval was 7.0 and 9.1 ms, respectively).
Pharmacokinetics of levofloxacin
The mean Cmax for the intravenous 500 mg dose was found to be 10.0 ± 1.3 µg ml−1 in the Japanese study, and the geometric mean Cmax 9.3 ± 1.2 and 12.5 ± 1.2 µg ml−1 for the oral 1000 and 1500 mg doses, respectively, in the Caucasian study (Table 1). Levofloxacin parameters observed in the Caucasian study were in the same range as those presented in the literature [8–10]. The area under the curve (AUC) values showed an approximately linear dose relationship, considering that the 500 mg dose was administered intravenously and allowing for an absolute bioavailability of approximately above 90% of the oral form [11, 12].
Table 1.
Pharmacokinetic parameters of levofloxacin in Japanese and Caucasian subjects
| Dose | Cmax (µg/mL; arithmetic mean) | Cmax (µg/mL; geometric mean) | AUC24 (µg h/mL) |
|---|---|---|---|
| 500 mg | 10.2 ± 2.3 | 10.0 ± 1.3 | 66.3 ± 14.8 |
| 1000 mg | 9.6 ± 2.1 | 9.3 ± 1.2 | 99 ± 17 |
| 1500 mg | 13 ± 2.1 | 12.5 ± 1.2 | 150 ± 26 |
Values are means ± SD. AUC, area under the curve; Cmax, maximum mean concentration.
Relationship between QTcF change from baseline and levofloxacin concentration
Plasma concentrations of levofloxacin had a significant effect on ΔQTcF for both races, with that of Caucasians being larger. However, the difference between slopes for the two groups was not statistically significant, i.e. there was insufficient statistical evidence for a difference between races (Figure 1 and Table 2). The other fitted models showed similar results, but the Akaike Information Criterion indicated that the model chosen had a slightly better fit. At the geometric mean Cmax of the two oral doses in the Caucasian study, a predicted effect on QTcF comparable to the effects observed was found (Table 3). Additional analyses were performed to investigate the effect of age and sex, which had no effect on the outcome of the analysis.
Figure 1.
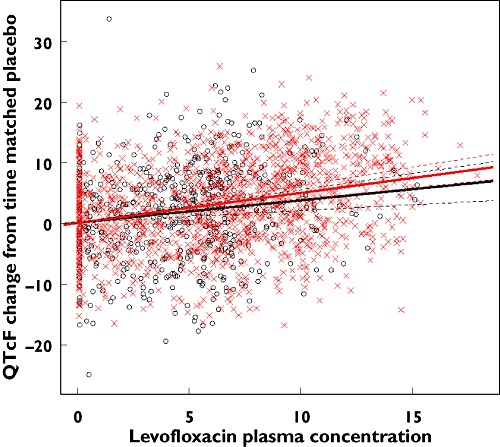
Levofloxacin effects in Caucasian and Japanese subjects. Japanese ( ); Caucasian (
); Caucasian ( )
)
Table 2.
Effect of plasma concentrations of levofloxacin on change of QT interval corrected using Fridericia's formula from time-matched placebo
| 95% Confidence interval | |||
|---|---|---|---|
| Effect | Estimate | Lower | Upper |
| Slope, Japanese | 0.364 | 0.189 | 0.539 |
| Slope, Caucasians | 0.492 | 0.380 | 0.604 |
| Slope, difference | −0.128 | −0.336 | 0.080 |
Analysis based on primary concentration-response model.
Table 3.
Predicted effect of levofloxacin at geometric maximum mean concentration (Cmax) of oral doses
| Prediction for | Estimate (ms) | 95% Confidence interval | ||
|---|---|---|---|---|
| at (µg/mL) | Lower | Upper | ||
| Japanese | 9.3* | 3.4 | 1.8 | 5.0 |
| Caucasians | – | 4.6 | 3.6 | 5.6 |
| Japanese | 12.5† | 4.5 | 2.4 | 6.7 |
| Caucasians | – | 6.1 | 4.7 | 7.5 |
| Difference | 9.3 | 1.2 | −0.7 | 3.1 |
| 12.5 | 1.6 | −1.0 | 4.2 | |
Geometric mean Cmax at 1000 mg oral dose.
Geometric mean Cmax at 1500 mg oral dose.
Discussion
The ICH E14 guidelines state that there is no expectation that ethnic factors would have an impact on the results of QT/QTc studies. Although these guidelines have been fully adopted in Japan since 2010, there is still the question of whether specific ethnic studies are required to assess cardiac safety in Japanese subjects.
The present analysis is an extension of the previous study [5] and, combined with the levofloxacin data from a Japanese study, explored the potential for ethnicity to have an impact on QTc. The outcome would confirm whether there is a need for specific ethnic studies or whether the findings of Caucasian studies are sufficient and can generally be applied to an ethnic population in which the investigated product may be used. This comparison between data sets demonstrated no statistically significant differences between Caucasians and Japanese following doses of levofloxacin. However, the lack of statistical significance does not mean that there is no effect; rather, we cannot tell if there is one, based on the data at hand (absence of evidence is not evidence of absence). In addition, regardless of significance, as the data for Japanese were obtained in different conditions from those on Caucasians, we cannot tell whether any difference observed is due to race or to the conditions of measurement. It is noted that this analysis has its limitations, including the fact that the doses and method of administration, age and time points are different between the races, and any difference observed could be attributable to study design, clinical conduct or racial difference. Further comparison of differences between Japanese and Caucasian subjects is being investigated in an ongoing study using moxifloxacin (EudraCT no. 20011-002423-17).
A study by Sohaib et al., which reviewed the QTc intervals of 4252 British men aged between 60 and 79 years found that amongst other factors, such as medications and co-existing cardiovascular disease, with increasing age there was a propensity for increasing QTc interval [13]. This was confirmed in the study by Sugiyama et al. [6], which used two separate age groups (between 20 and 45 and between 65 and 79 years old). However, as there is little literature on this topic, further investigation would be needed. There is a good case for the further conduct of bridging studies where all external factors are standardized and each group will be in direct comparison with each other, thereby providing data suitable to explore or exclude ethnic differences; alternatively, pharmacokinetics/ECG analyses between races and regions could be performed as in the present analysis.
Conclusion
The data show a clear concentration–response relationship that, in the concentration range investigated, can be well described with a linear model. The effect could be shown for both races, but although the slope for Japanese subjects is approximately 74% of that observed in Caucasians, the difference between the two is not statistically significant in the clinically relevant plasma concentration range investigated in this analysis. However, a trend suggests that Caucasian subjects are more sensitive, in agreement with Shin et al. [3]. Age and sex did not have an impact on the comparison of QTcF effects in this study.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1. ICH E14 The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhytmic Potential for Non-antiarrhythmic Drugs. International Conference of Harmonisation Step 4 Guideline, EMEA, CHMP/ICH/2/04. 25-5-2005.
- 2.Macfarlane PW, McLaughlin SC, Devine B, Yang TF. Effects of age, sex, and race on ECG interval measurements. J Electrocardiol. 1994;27:14–9. doi: 10.1016/s0022-0736(94)80039-1. [DOI] [PubMed] [Google Scholar]
- 3.Shin JG, Kang WK, Shon JH, Arefayene M, Yoon YR, Kim KA, Kim DI, Kim DS, Cho KH, Woosley RL, Flockhart DA. Possible interethnic differences in quinidine-induced QT prolongation between healthy Caucasian and Korean subjects. Br J Clin Pharmacol. 2007;63:206–15. doi: 10.1111/j.1365-2125.2006.02793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florian JA, Tornøe CW, Brundage R, Parekh A, Garnett CE. Population pharmacokinetic and concentration-QTc models for moxifloxacin: pooled analysis of 20 thorough QT studies. J Clin Pharmacol. 2011;51:1152–62. doi: 10.1177/0091270010381498. [DOI] [PubMed] [Google Scholar]
- 5.Taubel J, Naseem A, Harada T, Wang D, Arezina R, Lorch U, Camm AJ. Levofloxacin can be used effectively as a positive control in thorough QT/QTc studies in healthy volunteers. Br J Clin Pharmacol. 2010;69:391–400. doi: 10.1111/j.1365-2125.2009.03595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugiyama A, Fukuda R, Mori K, Fujita T, Kumagai Y. Effect of single 500 mg iv. levofloxacin dose on QT interval in healthy subjects. Jpn J Chemother. 2009;57:106–14. [Google Scholar]
- 7.Strnadova C. The assessment of QT/QTc interval prolongation in clinical trials: a regulatory perspective. Drug Inf J. 2005;39:407–33. [Google Scholar]
- 8.Noel GJ, Goodman DB, Chien S, Solanki B, Padmanabhan M, Natarajan J. Measuring the effects of supratherapeutic doses of levofloxacin on healthy volunteers using four methods of QT correction and periodic and continuous ECG recordings. J Clin Pharmacol. 2004;44:464–73. doi: 10.1177/0091270004264643. [DOI] [PubMed] [Google Scholar]
- 9.Chien SC, Chow AT, Natarajan J, Williams RR, Wong FA, Rogge MC, Nayak RK. Absence of age and gender effects on the pharmacokinetics of a single 500-milligram oral dose of levofloxacin in healthy subjects. Antimicrob Agents Chemother. 1997;41:1562–65. doi: 10.1128/aac.41.7.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubasch A, Keller I, Borner K, Koeppe P, Lode H. Comparative pharmacokinetics of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, trovafloxacin, and moxifloxacin after single oral administration in healthy volunteers. Antimicrob Agents Chemother. 2000;44:2600–03. doi: 10.1128/aac.44.10.2600-2603.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chien SC, Rogge MC, Gisclon LG, Curtin C, Wong F, Natarajan J, Williams RR, Fowler CL, Cheung WK, Chow AT. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob Agents Chemother. 1997;41:2256–60. doi: 10.1128/aac.41.10.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galan-Herrera JF, Poo JL, Rosales-Sanchez O, Fuentes-Fuentes E, Cariño L, Burke-Fraga V, Namur S, Parra MG. Bioavailability of two oral formulations of a single dose of levofloxacin 500 mg: an open-label, randomized, two-period crossover comparison in healthy Mexican volunteers. Clin Ther. 2009;31:1796–803. doi: 10.1016/j.clinthera.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Sohaib SM, Papacosta O, Morris RW, Macfarlane PW, Whincup PH. Length of the QT interval: determinants and prognostic implications in a population-based prospective study of older men. J Electrocardiol. 2008;41:704–10. doi: 10.1016/j.jelectrocard.2008.01.010. [DOI] [PubMed] [Google Scholar]


