Abstract
The European Commission is increasingly supporting collaborative initiatives focused on research into treatments and drugs for rare diseases, but lack of funding continues to be an issue. Gary Humphreys reports.
“Suffering from a rare disease affects your sense of identity.”
Edmund Jessop
“It was like being hit by a tsunami.” French mother of three, Béatrice de Montleau, vividly recalls the day she learned that her four-year-old son had Duchenne muscular dystrophy, a neuromuscular disease characterized by rapidly progressive muscle weakness and wasting. The disease primarily affects males and onset occurs early. People with the disease usually die in young adulthood, succumbing to cardiomyopathy, the effect of the disease on the heart muscle, and respiratory failure. Treatment with steroids offers some relief, but there is no cure. “For a time I was just in shock,” says de Montleau. “It was devastating.”
With an estimated incidence of 1 in 3300, Duchenne muscular dystrophy is considered a rare disease, one of several thousand classified as such. Estimates vary as to exactly how many rare diseases there are, partly because countries define rare diseases differently. “In European Union (EU) countries, any disease affecting fewer than 5 people in 10 000 is considered rare,” explains Antoni Montserrat Moliner, policy officer at the Directorate of Public Health at the European Commission in Luxembourg. Most patients suffer from diseases affecting 1 in 10 000 or less. According to the European Medicines Agency, there are between 5000 and 8000 distinct rare diseases in the EU, affecting between 27 and 36 million people.
Rare diseases range from cystic fibrosis and haemophilia to Angelman Syndrome, with an incidence of about 1 in 15 000, to Opitz trigonocephaly syndrome, which is extremely rare with about one case per million people.
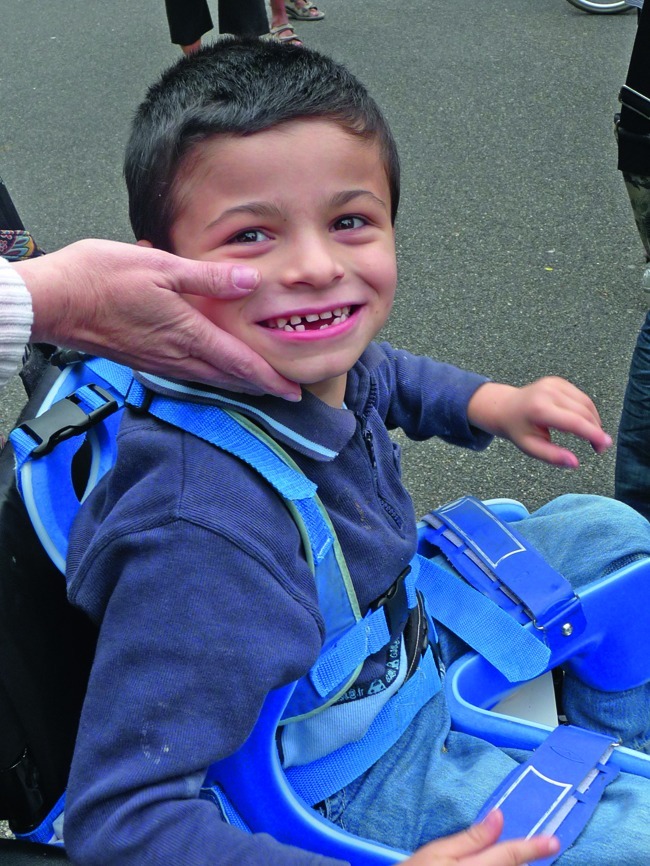
Six-year-old Pierre has Angelman Syndrome, a disease which causes severe developmental delay. Its prevalence is about one in 15 000
Aurelie Trahard
While the parents of children with rare diseases may initially be isolated by grief, they soon realize that talking to people going through similar experiences is invaluable. “They have to reach out,” says Paloma Tejada, communications director at the European Organization for Rare Diseases (EURORDIS), a nongovernmental, patient-driven alliance of patient organizations and individuals active in the field of rare diseases. For her part, Béatrice de Montleau reached out to the French Muscular Dystrophy Assocation, Association Française Contre les Myopathies.
Reaching out goes beyond looking for people who understand what you are going through. It is also a way of finding out about new treatments or research that looks promising. This drive to connect is one of the reasons that patients’ associations such as EURORDIS naturally cross borders and forge links, strategies that have until quite recently been largely unexploited in Europe. “In the area of health, the EU has had very little power when compared to agriculture or the environment,” explains Montserrat, noting that because of this, EU Member States have tended to focus on national health concerns. National identity is something that people living with rare diseases don’t tend to think about, according to Edmund Jessop, a rare disease specialist and the United Kingdom of Great Britain and Northern Ireland (UK) representative on the new EU Committee of Experts on Rare Diseases. “Suffering from a rare disease affects your sense of identity,” he says. “You start to think of yourself not as being French or English or Danish, but as a person with this particular disease. I think that is one of the reasons why the rare disease patient groups tend to be much more international and collaborative in their focus.”
While a domestic focus is not particularly problematic for many health issues, for rare diseases it is disastrous. This is because not one of the 27 EU Member States, however big, can hope to offer treatment for the full spectrum of rare diseases. “Even in the UK [with a population of 62 million] some patients have to be sent overseas for treatment,” says Jessop.
Meanwhile, individual countries struggle to find the resources to conduct research, including the patients needed to conduct clinical trials. “There are just three cases of progeria in France,” says Tejada. “You cannot organize clinical trials on that basis. You have to achieve critical mass and that means international collaboration.”
It is with the aim of promoting international collaboration that the European Commission is increasingly supporting collaborative initiatives, including the patients’ association, but especially Orphanet, created in 1997 by rare disease expert and advocate Ségolène Aymé, to establish a freely accessible database of rare diseases and the drugs needed to treat them, so-called orphan drugs. Orphanet launched a rare disease and orphan drug information portal in 2000 and is also coordinating the Topic Advisory Group for Rare Diseases of the World Health Organization, in charge of the revision of the International classification of diseases.
Orphanet launched a rare disease and orphan drug information online portal in 2000
Orphanet
The Commission is also behind the International Rare Disease Research Consortium also known as IRDiRC, pronounced ‘ear-dirk’, an ambitious international effort to pool resources and harmonize policy to promote research. Launched in April 2011, the Consortium started out as a joint project of the European Commission and the National Institutes of Health in the United States of America; but since then other countries have become involved, including Canada and Japan. It aims to bring together regulatory agencies, researchers, patient group representatives, members of the biopharmaceutical industry and health professionals, and among the objectives that it has publicly set is the generation of 200 new therapies for rare diseases and diagnostic tools for most rare diseases by 2020.
Montserrat believes that collaboration on rare diseases is also going to increase as a result of the European Reference Networks, which came out of the European Commission Directive on Cross Border Health Care and Patient Rights that was adopted at the beginning of 2011. The European Commission will adopt legislation defining the European Reference Networks before October 2013. “This is very positive because the European Reference Networks have been discussed as an objective for a long time, and now it is something that will be a reality in one and half years,” Montserrat says. The European Reference Networks will cover a range of diseases, but, according to Montserrat, will be “largely concerned with rare diseases”, something that EURORDIS has fought for very hard, according to Tejada.
The European Reference Networks will depend on yet-to-be designated centres of expertise in the 27 EU Member States and will facilitate collaboration across a range of activities. “The European Reference Networks will offer the possibility of consultation, but also of sending patients to other Member States where treatment is available,” Montserrat says. Backed by the Directive on Cross Border Health Care, patients will have the right to be treated in another EU country if their own country is unable to provide care.
Like the IRDiRC, the European Reference Networks is a work in progress and Monserrat is hoping that the EU Committee of Experts on Rare Diseases will play an important part in steering it through the complicated development period that lies ahead. The EU Committee includes representatives from Member States, patient organizations and industry.
Another key aspect of EU work on rare diseases is the adoption in 2009 of the European Council Recommendation on Rare Diseases, which supports, among other activities, the development of registers and databases. This is essential if researchers are to be able to draw on a wide population base for epidemiological and clinical research, according to Domenica Taruscio, director of the National Centre for Rare Diseases in Rome. Taruscio hopes that the recommendation will result in a “burst of initiatives” for rare disease registration, while recognizing obstacles need to be overcome, notably EU regulation on personal data protection which allows data collection and exchange for a “legitimate purpose”, the exact meaning of which is open to argument. Another obstacle is the lack of a common language. “Worldwide sharing of information, data and samples is hampered by the absence of an exhaustive rare disease classification, standard terms of reference and common ontologies,” Taruscio says.
While these are clearly encouraging times for rare disease advocates, certain fundamental problems remain, the most obvious being the continued lack of investment in rare disease research and treatment. For Professor Béla Melegh, head of the Institute of Medical Genetics and leader of the National Coordinating Research Group for Rare Diseases in Hungary, increased collaboration is a good thing as far as it goes, but poorer countries still struggle to achieve sustainable funding. “For collaboration I have lots of nice European friends and at any time I can send somebody to study the techniques if we need to learn more. But money is not so easy to find, and it is mainly money that makes the world go round today,” he says.
Raising more money for research into rare disease is also one of Béatrice de Montleau’s central preoccupations in her work for the French Muscular Dystrophy Association and the EURORDIS patient group. A banker by training, after her initial contact with the association, de Montleau went on to advise the charity on investing the money they collect. “Governments give very little or nothing for research on rare diseases,” she says. But she is optimistic about the future and draws strength from the connections she has made, connections that have allowed her to give as well as receive support.