Abstract
Background and methods
Myocarditis and inflammatory cardiomyopathies can be caused by infections, drugs, toxic substances, and autoimmune diseases. We present their clinical features, diagnostic evaluation, treatment, and prognosis on the basis of a selective review of the literature, current expert opinion, and our own clinical experience.
Results
The pathological mechanisms that are accessible to treatment lie at the cellular and molecular levels and generally give rise to nonspecific disease manifestations. Specific treatment is possible only on the basis of a standardized diagnostic evaluation of a biopsy specimen, rather than clinical examination alone. Therapeutic decisions must be based on the results of thorough myocardial biopsy studies while taking account of the individual patient’s clinical course. Moreover, treatment can help only if a treatable cause is present (e.g., a viral infection, an inflammatory process, or cardiodepressive antibodies), and only if the myocardium still has regenerative potential. Once irreversible myocardial injury has occurred—for example, if the diagnosis of post-infectious or post-inflammatory dilated cardiomyopathy has been missed until it is too late—then the development or progression of heart failure in the long term can no longer be prevented.
Conclusion
Recent studies have shown that specific treatment can help patients with viral, inflammatory, or autoimmune cardiomyopathy that has been precisely characterized by means of a myocardial biopsy. More randomized trials with larger patient cohorts are needed for further optimization of treatment.
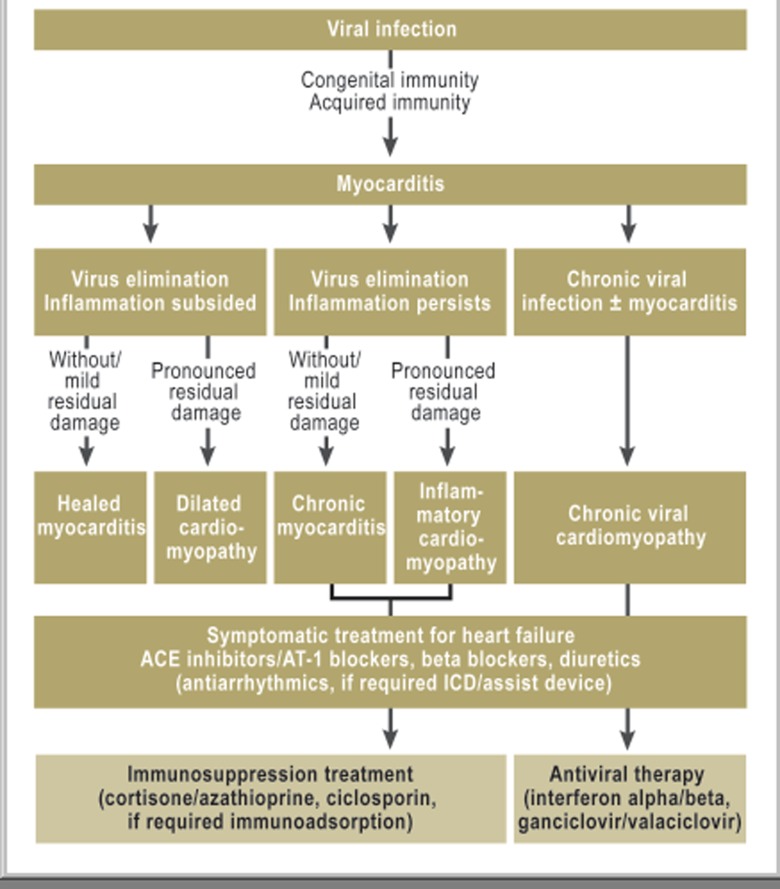
The term myocarditis describes inflammatory disorders of the heart muscle of varied infectious and non-infectious origin (Box). In acute myocarditis, infectious strains usually cause myocardial inflammation with subsequent disturbance of left ventricular or right ventricular function. In Western industrialized countries these pathogens are primarily viruses, whereas in developing countries the cause may be bacterial, protozoal, or fungal infections. Myocardial processes triggered by infectious and non-infectious causes also underlie the chronic-inflammatory myocardial disorders (Figure 1). If the immune system does not eliminate the infectious pathogen early on—owing to insufficient activation, e.g. on the basis of a genetic predisposition—chronic infection develops, which may or may not be accompanied by inflammation (1). If the inflammatory response does not spontaneously resolve after successful elimination of the pathogen, chronic-inflammatory cardiomyopathy is present (Figure 1) (2). In addition to such postinfectious inflammatory processes, accompanying cellular or humoral inflammations in systemic diseases may cause lasting injury to the myocardium.
Box. Etiology of human infectious and non-infectious inflammatory cardiomyopathy.
-
Viral infections
Adenoviruses
Enteroviruses (Coxsackie A/B, Echo)
Cytomegalovirus
Erythroviruses
Herpesviruses
Influenza A/B
HIV
Hepatitisvirus C
Poliovirus
Varicella zoster
Arboviruses
Mixed infections
-
(Auto-)Immune activation
Postinfectious
Influenza vaccination
SLE (systemic Lupus erythematodes)
Sarcoidosis
Sjögren’s syndrome
Churg-Strauss syndrome
Wegener’s granulomatosis
Takayasu arteritis
Inflammatory bowel disorders
Giant cell myocarditis
-
Bacteria
Mycobacteria
Chlamydia
Streptococci
Mycoplasma
Legionella spp
Salmonella spp
Rickettsia spp
Corynebacteria
Borrelia spp
-
Protozoa
Trypanosoma cruzi
Toxoplasma gondii
Trichinosis/trichinellosis
Echinococci
-
Toxins
Anthracyclines
Catecholamines
Cytokines
Cocaine
Alcohol
Chemotherapeutic drugs
-
Allergic/hypersensitive
Penicillin
Tricyclic antidepressants
Clozapine
Antirheumatic drugs
Sulfonamides
Cephalosporins
-
Physical pathogens
Arsenic
Lithium
Irradiation
Hypothermia
Heat stroke
-
Parasites
Schistosomiasis
Larva migrans
-
Fungal infections
Aspergillus
Candida
Cryptococus
Histoplasmodium spp
Figure 1.
Pathogenesis of virus-induced inflammatory cardiomyopathy and therapeutic decisions resulting from the diagnostic evaluation of biopsy specimens
Because of the non-specific symptoms and clinically indistinguishable etiological factors the prevalence of infectious and non-infectious causes of myocarditis is not known. Biopsy of the myocardium is the only way to arrive at a diagnosis that is relevant to treatment (3). The present article will focus on the discussion of pathophysiological insights, indications, and options for myocardial biopsy, considering existing guidelines for biopsy (4) and the therapeutic options in acquired myocardial disorders based thereon.
Clinical symptoms
Myocarditis can manifest like a myocardial infarction with sudden-onset angina pectoris, arrhythmias, and/or heart failure developing within days. Most patients with myocarditis initially have such non-specific symptoms that these are often categorized in the context of the preceding infection and not as being of cardiac origin. Cardiac involvement is often considered as the differential diagnosis only when cardiac symptoms, such as palpitations, angina, and/or exertional dyspnea, persist for a long period after the underlying infection has resolved, or if they develop de novo in the course of the recovery. At this point in time, the electrocardiography results and laboratory chemical findings that are characteristic of acute myocarditis—such as the changes to the ST segment and raised cardiac enzymes that are typical for acute myocardial involvement—are no longer present. Diagnostic evaluation starting at this point can only collect data on the extent of myocardial injury, even when using imaging methods (echocardiography, angiography, magnetic resonance imaging [MRI]); exclude specific cardiac disorders—such as ischemic cardiomyopathy or valve deficiencies; or provide clues for a suspected diagnosis of infectious myocarditis; it can’t, however, diagnose the cause of the existing disorder. While 60% to 70% of patients improve clinically and hemodynamically, the remaining patients will develop chronic heart failure or dilated cardiomyopathy within months or years (2, 5).
An unequivocally confirmed bioptic diagnosis is the crucial prerequisite for differential diagnostic evaluation and the specific treatment strategies derived from this (Figure 1, Table 1). As the pathophysiological changes of infectious and non-infectious myocarditis occur at a cellular and subcellular level, confirmation of a specific pathogen or inflammation requires a direct examination of myocardial tissue, which can be obtained without problems by means of a biopsy. In terms of therapeutic considerations it needs to be taken into account that a positive treatment effect will occur only if treatable causes—such as viral infections, inflammatory processes, or cardiodepressive autoantibodies—are present and the myocardium still has regenerative potential (6). If irreversible myocardial damage has occurred, for which no specific treatment options exist (Figure 1)—such as in postinfectious or postinflammatory dilated cardiomyopathy that is diagnosed too late—the development or progression of heart failure in the long term cannot be prevented.
Table 1. Stage dependent treatment options for virus-induced inflammatory cardiomyopathies.
| Disease stage | Pathomechanism and infectious strain | Therapeutic option |
| Symptomatic treatment for heart failure | ||
| Acute myokarditis (early phase) | Direct cytopathic myocardial injury | Antiviral therapy? |
| Congenital immune response (macrophages, natural killer cells, cytokines) | Antiviral therapy? | |
| Intravenous immunglobulins? | ||
| Postinfectious (auto)immunity | adaptive immune response (T/B cells, antibody production) | Immune modulation
|
| Chronic viral cardiomyopathy | Enterovirus | Interferon-β |
| Adenovirus | Interferon-β | |
| Erythro-/Parvovirus | Intravenous immunglobulins (acute infection)Type I interferons (chronic infection) | |
| Human herpesvirus type 6A/B | Valaclovir/ganciclovir | |
| Cytomegalovirus | Valaclovir/ganciclovir Foscanet\ Cidofovir | |
| Epstein-Barr virus | Vala-/GanciclovirFoscanet Cidofovir | |
| Herpes simplex virus | Aciclovir | |
| Varicella | Aciclovir | |
| Respiratory syncytial virus | Ribavirin | |
| Hepatitis C virus | Pegylated interferon-α + ribavarin | |
| HIV | Antiretroviral medications | |
Prognosis
Acute myocarditis mostly does not sufficiently respond to symptomatic medication for heart failure, and mortality is high in spite of treatment. The long-term disease course depends on the pathogen, the extent and type of inflammation, and the initial injury to the myocardium. Focal borderline myocarditis often undergoes spontaneous clinical healing if no serious heart failure developed initially. The early mortality of fulminant lymphocytic myocarditis requiring intensive care is in excess of 40% in the first 4 weeks (7). Untreated giant cell and eosinophilic myocarditis also have an extremely poor prognosis, with 4 year survival rates of less than 20% (8). Granulomatous necrotizing myocarditis is lethal if overlooked and untreated. Non-fulminant active myocarditis has a mortality rate of 25% to 56% within 3 to 10 years, owing to progressive heart failure and sudden cardiac death, especially if symptomatic heart failure manifests early on (9– 11, e1). In addition to impaired left ventricular (LV) and right ventricular (RV) function, virus persistence, chronic inflammation, and cardiodepressive autoantibodies are independent predictors of a poor prognosis (9, 12, 13).
Diagnostic evaluation
The effects of virus-induced inflammatory processes on myocardial functioning and the disease course can be easily identified clinically be means of the medical history, laboratory tests, ECG/long-term ECG, echocardiography, computed tomography (CT)/magnetic resonance imaging (MRI), or diagnostic catheterization. Systolic or diastolic heart failure, hemodynamically effective arrhythmias, or injury to the cardiac valves are easy to identify qualitatively and quantitatively, and it is uncomplicated to differentiate between dilated, restrictive, or hypertrophic types of cardiomyopathy.
Acute “infarct-like” changes to the ECG, a positive troponin-T/I measurement, raised NT-proBNP, and a finding of edema, or early contrast enhancement, in patients with clinically suspected myocarditis indicates, non-specifically, virus-associated or inflammatory cell–associated injury to the myocardium. However, they do not provide any information on the type of infectious pathogen or the inflammation, nor as to whether the infectious strain has been completely eliminated or the inflammation subsided. Since the toxic, infiltrative, or infectious-inflammatory processes that are responsible for the clinical phenotype “myocarditis” take place at the cellular level, they cannot be identified at all or only unsatisfactorily by means of non-invasive clinical diagnostic modalities, including MRI. To make a specific diagnosis on which to base causative treatment, it is therefore important to take a biopsy specimen and analyze this early on, in accordance with the recommendations from European and US medical specialty societies (4). Giving treatment that is supposedly specific (immunosuppression) without running exact molecular biology diagnostic tests to exclude a viral cause can have fatal consequences for patients (12). Tissue specimens should be analyzed only in institutions that provide the complete range of molecular biological and histological/immunohistological diagnostic techniques. Specimens can also easily be sent off for diagnostic evaluation in special media.
In case of a biventricular biopsy, virus genome (12.6% versus 7.1%) or inflammation (18.7% versus 7.9%) is slightly more commonly found in the specimens from the left ventricle (14). However, the fact that the detection rate in samples from the left ventricle is some 12% higher is also partly owing to the larger number of biopsies. Severe complications after right ventricular biopsy arise in some 0.12% and are notably lower after LV biopsy (e2).
Identifying infectious agents
With the exception of borreliosis, which is accompanied by cardiac symptoms in 8% of cases, non-viral infections are not of major importance in the Western world (6). 10% to 15% of virus associated cases of myocarditis are caused by enteroviruses. Other pathogens include adenoviruses, herpesviruses, erythroviruses, cytomegalovirus, HIV, and hepatitis viruses; the prevalence rates differ by geographical location. Non-infectious autoimmune processes in systemic disease affect some 10% (e3).
Molecular biology diagnostic testing for the causative agent is done by means of polymerase chain reaction (nPCR) and identifies relevant infectious pathogens with a very high degree of sensitivity (Box). Qualitative diagnosis of viral pathogens is complemented by quantitatively determining the viral load (real-time PCR) and sequencing for the purpose of identifying the viral subtypes or quality assurance (15). Acute or latent infections and infections that replicate actively in the myocardium can be differentiated from one another by parallel analyses of blood composition (peripheral cells, plasma, serum) and confirmation of transcriptional activity. Since different viruses and viral subtypes respond differently to antiviral medications and are in some cases not completely eliminated—merely blocked in their continual replication—this information is important for making a tailored decision regarding treatment and the success thereof.
Tissue-based diagnostics and diagnostic evaluation of inflammation
In cases with an acute inflammatory disease course, the histology or immunohistology specimens often contain focal or diffuse cell infiltration by lymphocytes and/or macrophages, more rarely, by eosinophils or giant cells (Figure 2). In contrast to borderline myocarditis, active lymphocytic myocarditis is characterized by inflammatory cell–associated acute myocardial cell necrosis (Figure 2). The density of the inflammatory cell infiltrate determines the acute and long-term disease course; the clinical relevance of the extent of inflammation and the inflammatory cell subtypes is not known (7, 11, 16, e4). Giant cell myocarditis, idiopathic eosinophilic myocarditis, inflammatory processes in granulomatous disorders, and allergic medication-induced types of myocarditis are rare and found in less than 20% of acute cases of myocarditis.
Figure 2.
Diagnostic evaluation of inflammation in a myocardial biopsy specimen (histology and immunohistology)
a) normal myocardium,
b) acute lymphocytic myocarditis with focal cell infiltrates and necrosis of myocytes,
c) advanced postinflammatory dilated cardiomyopathy with hypertrophy of the cardiomyocytes and pronounced fibrosis/scarring
In patients with chronic heart failure and dilated cardiomyopathy (DCM), inflammatory cell infiltrations occur in some 30%; in patients with systemic disease, in 10% to 20%. In these settings the inflammatory cells do not have a focal distribution but a diffuse one, and they are notably lower in number than in acute cases. Since immune cells are difficult to identify in histological specimens, histology staining is therefore supported by more sensitive immunohistological investigations (6, e5, e6). Hypertrophy of the cardiomyocytes, interstitial fibrosis, and scarring in patients with inflammatory and post-inflammatory cardiomyopathies indicates a longstanding process of damage with loss of myocardium (scars) (Figure 2).
Treatment
The cornerstone of any therapeutic approach is to treat the heart failure or arrhythmia, which—independent of the actual cause—is done symptomatically in accordance with general, evidence based guidelines (1, 17). Specific treatment depends on the results of the diagnostic myocardial biopsy, while also taking into account the disease course so far and the individual patient’s current clinical symptoms. Since most therapeutic studies involved no biopsy diagnostics and, in particular, no virus identification, the therapeutic recommendations discussed in this article are based on only two randomized studies of immunosuppression, or rather, one open-label trial and one controlled antiviral study, as well as long years of experience accumulated in a few larger centers (12, 18, 19). Therapeutic guideline recommendations from the medical specialty societies are thus far lacking.
Acute disease course
Virally induced acute myocarditis is initially cardioprotective and aims rapidly to eliminate the viral infection, before irreversible myocardial injury has developed. Whether early inhibition of the inflammation or early antiviral therapy has a beneficial effect on the disease course is not known as data are lacking (10, 20). If anti-inflammatory treatment is given before the virus has been completely eliminated, the result may be virus persistence and an unfavorable disease course over the longer term (12).
Since acute viral-inflammatory cardiomyopathies after spontaneous viral elimination and receding inflammation often improve spontaneously within weeks or months during treatment with ACE inhibitors, beta blockers, and diuretics (60%), watchful waiting is justified in patients who can be stabilized and whose biopsy results are known. Indications for early implantation of a mechanical circulatory support device or implantable cardioverter defibrillator (ICD) should be defined with caution and undertaken only if symptoms are persistent. A wearable LifeVest defibrillator can be used as an interim measure.
If progressive deterioration of cardiac pump function develops in spite of optimal drug treatment or if intractable arrhythmias are present in a specific finding of inflammation, speedy and if possible etiologically targeted treatment is required. Giant cell and eosinophilic myocarditis and acute heart failure in necrotizing myocarditis are among the treatable disorders that require immediate treatment because of their high mortality. If treatment is initiated too late then often the only remaining option is that of mechanical circulatory support or—as the method of last resort—heart transplantation, because of the rapidly developing irreversible myocardial injuries. Owing to the high rates of spontaneous improvement and thus small numbers of patients, no reliable data are available regarding the frequency of transplantation in acute myocarditis.
Specific treatment regimens
For giant cell myocarditis an aggressive treatment regimen with anti-CD3-antibodies, ciclosporin (trough level 100–120 µg/mL), and cortisone is required (Table 2) (21). In the following period, cortisone can be reduced stepwise in two-week intervals by 10 mg each time down to a maintenance dose of 5–10 mg/day. This regimen is maintained with continued ciclosporin for a minimum of 12 months (Table 2).
Table 2. Immunosuppression in acute giant cell myocarditis, chronic myocardis, and inflammatory cardiomyopathy.
| Giant cell myocarditis | |
| Oral steroids 3 (anti-CD3-antibodies)*1 | 5 mg/day i.v. for 7 days10 mg/kg body weight (3 days) |
| Ciclosporin*2 | Targeted trough level: 100–120 µg/mL |
| Methylprednisolone*3 | 1 mg/kg body weight (1 week)Reduction: 10 mg/4 weeks |
| Chronic/autoimmune myocarditis, eosinophilic myocarditis, inflammatory cardiomyopathy | |
| Methylprednisolone*3 | 1 mg/kg body weight (2 weeks), then reduction by 10 mg each week for 4 weeks to a maintenace dose of 10 mg (duration of treatment 6 months) |
| Azathioprine*2, 3, 4 | 50–150 mg/day (6 months) |
| Stomach protection | |
| Pantoprazole | 20 mg/die |
| Calcium substitution | 1×1 g/die |
*1Caution: initial hypotension;
*2Monitor liver function and renal function;
*3Check differential blood count, blood glucose;
*4reduce/stop if leukocytes <1000/nL or liver function more than three times higher than normal
Eosinophilic myocarditis is—like chronic lymphocytic myocarditis and autoimmune myocarditis—treated with cortisone and azathioprine, with the cortisone being stepped down at fortnightly intervals from an initial dose of 1 mg/kg body weight by 10 mg each time until a maintenance dose of about 10 mg has been reached and then gradually tapered off after 6 months (Table 2).
Granulomatous myocarditis with a fulminant course is usually identified post mortem. Other granulomatous disorders with myocardial involvement, such as sarcoidosis or rheumatoid arthritis, respond well to cortisone but often require a lengthy course of treatment (e7). The prognosis of giant cell myocarditis can be improved only by early immunosuppression treatment. Too few data to enable reliable assessment of mortality are available for eosinophilic and granulomatous myocardial diseases, although individual positive case reports exist.
Chronic inflammatory cardiomyopathies
Chronic post-infectious or autoimmune inflammatory processes with a duration of illness in excess of 6 months respond well to 6 months’ immunosuppression treatment with cortisone and azathioprine, as long as virus persistence has been excluded on biopsy (12, 19, 20, 22). Two randomized studies showed that patients treated with immunosuppressants had a significant treatment advantage compared with patients receiving purely symptomatic treatment (Figure 3) (19, 22). Especially the TIMIC study confirmed in the placebo group that persistent inflammation exerts a harmful effect on the myocardium (22).
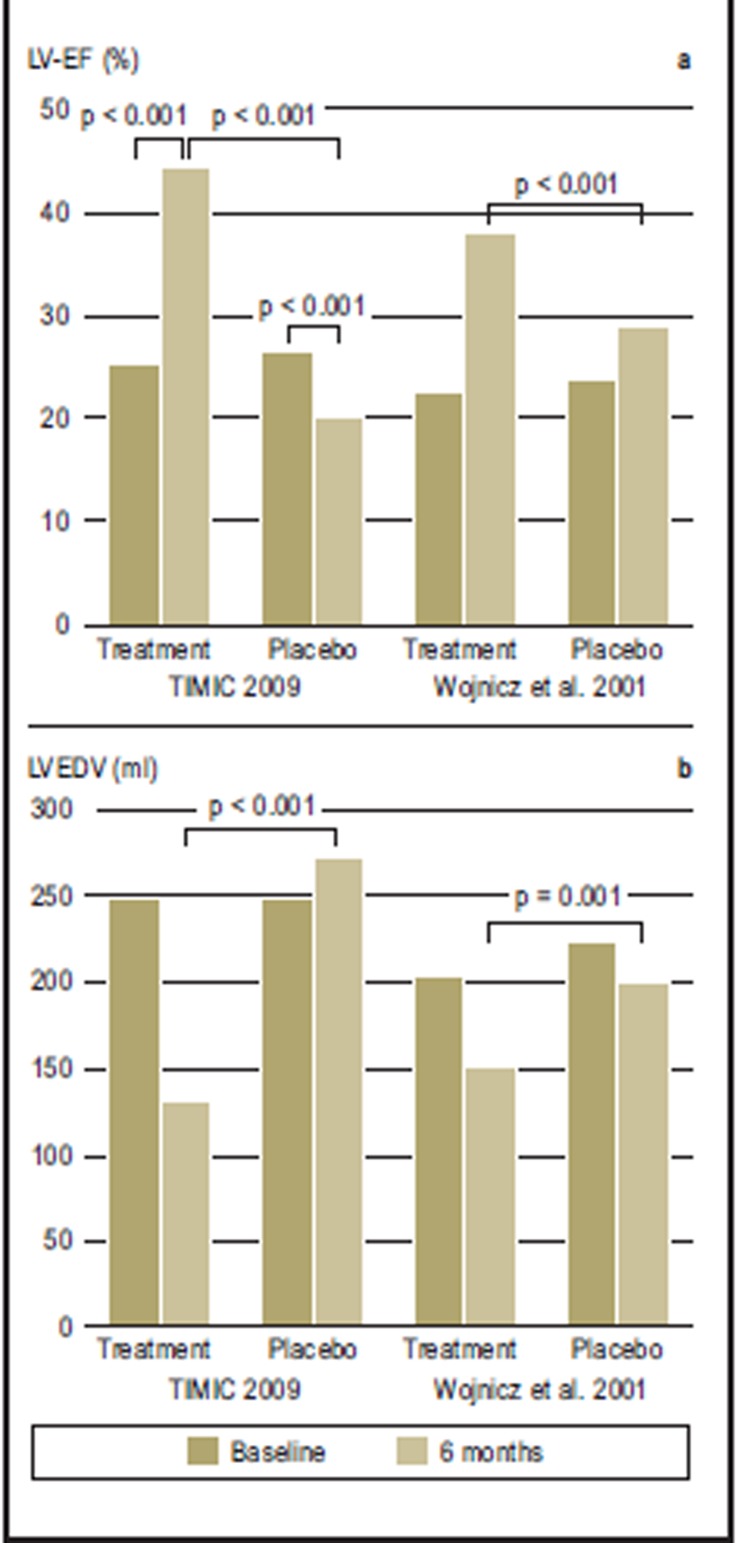
Figure 3.
Immunosuppressive treatment of myocarditis/inflammatory cardiomyopathy (from 24, 25). In the placebo controlled TIMIC study (22), 85 patients were treated for six months. Immunosuppressive treatment was given in addition to optimized treatment for heart failure. The patients in the treatment group (n = 43) showed significant improvement in left ventricular pump function, with a mean increase in left ventricular ejection fraction (LV-EF) of 25%. In the placebo group (n = 42), the LV-EF fell by more than 6%. In parallel, the left ventricle end-diastolic diameter (LVEDV) normalized, whereas in the placebo group it increased in the same time period (p<0.001 for both groups). The data confirmed a second randomized study from 2001 (19), which also showed a treatment advantage for the patients receiving immunosuppressive treatment, which was still present two years after the end of the treatment
The TIMIC study investigated patients with chronic active myocarditis and restricted LV function (LV ejection fraction [EF] less than 45%) who had displayed symptoms of chronic heart failure in spite of having received symptomatic medication for heart failure for more than 6 months. Viral infection was excluded by molecular biology tests before treatment was started. The primary end point of the study was an improvement in LV-EF after 6 months. 89% of treated patients, but none in the placebo group, improved according to the NYHA classification. After the end of the study, five of the 42 placebo patients (12%) had to be admitted to inpatient care because of progressive symptoms of heart failure. Within the following 10 to 72 months, two patients died and two patients received heart transplants. No further events were seen in the treatment group.
In a further placebo controlled study, the positive treatment effect (Figure 3) was sustained over two years (19). No data are available for the long-term survival rate after immunosuppression, although mortality in the US myocarditis trial after four years showed a trend towards a treatment advantage (10). The crucial issue is that, as a previous study reported by the TIMIC authors showed, a favorable course was achieved only in patients who were virus-negative (12). In this uncontrolled treatment study, almost exclusively virus-negative patients had improved after 12 months under immunosuppressant treatment (LV-EF rise 21.4%). The diagnostic evaluation for viruses that was conducted retrospectively showed a myocardial viral infection in 85% of non-responders. Within a year, five of these patients had died and three required a transplant.
Treatment of viral myocardiopathy
The prognosis of viral (inflammatory) cardiomyopathy is negatively affected by virus persistence (9, 12, 13, 23). The course of viral cardiomyopathy is for certain viruses closely associated with the spontaneous course of the viral infection, as spontaneous elimination of the virus is accompanied by clinical improvement whereas this does not apply to patients who develop virus persistence (23).
Antiviral therapy
Thus far only few antiviral treatment studies have been conducted. Enterovirus and adenovirus infections respond well to interferon beta (IFN-ß) (24). The treatment scheme in chronic viral cardiomyopathy closely follows the experiences gained in multiple sclerosis. A dose of initially 2 × 106 IU IFN-ß is administered subcutaneously every other day and increased at weekly intervals, first to 4 × 106 IU and then to 6–8 × 106 IU; this is continued for 24 weeks. The symptomatic treatment for heart failure is maintained. Slowly increasing the dose of IFN-ß, at least initially, or administering non-steroidal anti-rheumatics, notably reduces the flu-like side effects of the medication.
A first open-label treatment study, 6 months of antiviral treatment of patients who were positive for enterovirus or adenovirus showed complete virus elimination and a reduction in the virus associated myocardial inflammatory reaction (24). In parallel, significant clinical and hemodynamic improvements were seen in two thirds of treated patients. The efficacy of antiviral therapy was independent of the duration of illness; especially patients with higher-grade impaired left ventricular pump function (EF<45%) benefited from the treatment. Similar results were obtained in a placebo controlled treatment study (BICC trial), which in addition to enterovirus and adenovirus positive patients predominantly included patients who were positive for parvovirus B19 (1, 25). The finding that, in addition to significant virus elimination or reduction in viral load, significant clinical improvement also occurred in patients in whom the parvovirus was not completely eliminated shows that immunomodulation with interferon affects different pathomechanisms.
Key Messages.
Viral infections are the most common triggers of inflammatory myocardiopathies and can, if persistent, damage the myocardium even without accompanying inflammation.
Since the pathophysiological processes in myocarditis take place at the cellular and subcellular levels, myocardial biopsy is the only method by which the causative strain can be identified and/or inflammation can be confirmed—both of which are important for differential treatment.
Cases of subacute myocarditis that initially is accompanied by non-specific symptoms are frequently identified and cardiologically evaluated only at an advanced stage.
Because the clinical course of myocarditis is unpredictable, all patients with etiologically unexplained heart failure have to undergo myocardial biopsy, before irreversible and thus untreatable damage to the myocardium has developed.
Numerous chronic viral infections and postinfectious or autoimmune inflammations of the myocardium are treatable.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists. The research projects used as the basis for the review article were funded by the German Research Foundation (DFG) in the context of Sonderforschungsbereich-Transregio (SFB-TR19), inflammatory cardiomyopathy.
References
- 1.Schultheiss HP, Kuehl U, Cooper LT. The management of myocarditis. Eur Heart J. 2011;32:2616–2665. doi: 10.1093/eurheartj/ehr165. [DOI] [PubMed] [Google Scholar]
- 2.D’Ambrosio A, Patti G, Manzoli A, et al. The fate of acute myocarditis between spontaneous improvement and evolution to dilated cardiomyopathy: a review. Heart. 2001;85:499–504. doi: 10.1136/heart.85.5.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magnani JW, Dec GW. Myocarditis: current trends in diagnosis and treatment. Circulation. 2006;113:876–890. doi: 10.1161/CIRCULATIONAHA.105.584532. [DOI] [PubMed] [Google Scholar]
- 4.Cooper LT, Baughman KL, Feldman AM, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. Eur Heart J. 2007;28:3076–3093. doi: 10.1093/eurheartj/ehm456. [DOI] [PubMed] [Google Scholar]
- 5.Baboonian C, Treasure T. Meta-analysis of the association of enteroviruses with human heart disease. Heart. 1997;78:539–543. doi: 10.1136/hrt.78.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu PP, Schultheiss HP Baunwald. Heart Disease. 8 ed. Philadelphia: W B Saunders co; 2008. Myocarditis; pp. 1775–1792. [Google Scholar]
- 7.McCarthy RE, Boehmer JP, Hruban RH, et al. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342:690–695. doi: 10.1056/NEJM200003093421003. [DOI] [PubMed] [Google Scholar]
- 8.Cooper LT, Jr., Berry GJ, Shabetai R. Idiopathic giant-cell myocarditis—natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N Engl J Med. 1997;336:1860–1866. doi: 10.1056/NEJM199706263362603. [DOI] [PubMed] [Google Scholar]
- 9.Why HJ, Meany BT, Richardson PJ, et al. Clinical and prognostic significance of detection of enteroviral RNA in the myocardium of patients with myocarditis or dilated cardiomyopathy. Circulation. 1994;89:2582–2589. doi: 10.1161/01.cir.89.6.2582. [DOI] [PubMed] [Google Scholar]
- 10.Mason JW, O’Connell JB, Herskowitz A, et al. A clinical trial of immunosuppressive therapy for myocarditis The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333:269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 11.Magnani JW, Suk Danik HJ, Dec GW, DiSalvo TG. Survival in biopsy-proven myocarditis: A long-term retrospective analysis of the histopathological, clinical, and hemodynamic predictors. Am Heart J. 2006;151:463–470. doi: 10.1016/j.ahj.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 12.Frustaci A, Chimenti C, Calabrese F, et al. Immunosuppressive therapy for active lymphocytic myocarditis: virological and immunologic profile of responders versus nonresponders. Circulation. 2003;107:857–863. doi: 10.1161/01.cir.0000048147.15962.31. [DOI] [PubMed] [Google Scholar]
- 13.Caforio AL, Calabrese F, Angelini A, et al. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J. 2007;28:1326–1333. doi: 10.1093/eurheartj/ehm076. [DOI] [PubMed] [Google Scholar]
- 14.Yilmaz A, Kindermann I, Kindermann M, et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation. 2010;122:900–909. doi: 10.1161/CIRCULATIONAHA.109.924167. [DOI] [PubMed] [Google Scholar]
- 15.Kuhl U, Lassner D, Pauschinger M, et al. Prevalence of erythrovirus genotypes in the myocardium of patients with dilated cardiomyopathy. J Med Virol. 2008;80:1243–1251. doi: 10.1002/jmv.21187. [DOI] [PubMed] [Google Scholar]
- 16.Kindermann I, Kindermann M, Kandolf R, et al. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639–648. doi: 10.1161/CIRCULATIONAHA.108.769489. [DOI] [PubMed] [Google Scholar]
- 17.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 18.Kühl U, Strauer BE, Schultheiss HP. Methylprednisolone in chronic myocarditis. Postgrad Med J. 1994;70:35–42. [PubMed] [Google Scholar]
- 19.Wojnicz R, Nowalany-Kozielska E, Wojciechowska C, et al. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: two-year follow-up results. Circulation. 2001;104:39–45. doi: 10.1161/01.cir.104.1.39. [DOI] [PubMed] [Google Scholar]
- 20.Stanton C, Mookadam F, Cha S, et al. Greater symptom duration predicts response to immunomodulatory therapy in dilated cardiomyopathy. Int J Cardiol. 2008;128:38–41. doi: 10.1016/j.ijcard.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Cooper L, Hare JM, Tazelaar HD, et al. Usefulness of immunosuppression for giant cell myocarditis. Am J Cardiol. 2008;102:1535–1539. doi: 10.1016/j.amjcard.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J. 2009 doi: 10.1093/eurheartj/ehp249. [DOI] [PubMed] [Google Scholar]
- 23.Kuhl U, Pauschinger M, Seeberg B, et al. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112:1965–1970. doi: 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]
- 24.Kuhl U, Pauschinger M, Schwimmbeck PL, et al. Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation. 2003;107:2793–2798. doi: 10.1161/01.CIR.0000072766.67150.51. [DOI] [PubMed] [Google Scholar]
- 25.Schultheiss HP, Piper C, Sowade K, et al. The effect of subcutaneous treatment with interferon-beta-1b over 24 weeks on safety, virus elimination and clinical outcome in patients with chronic viral cardiomyopathy. Circulation. 2008 Abstract 3322. [Google Scholar]
- e1.Aoyama N, Izumi T, Hiramori K, et al. National survey of fulminant myocarditis in Japan: therapeutic guidelines and long-term prognosis of using percutaneous cardiopulmonary support for fulminant myocarditis (special report from a scientific committee) Circ J. 2002;66:133–144. doi: 10.1253/circj.66.133. [DOI] [PubMed] [Google Scholar]
- e2.Mason JW. Techniques for right and left ventrikulär endomyocardial biopsy. Am J Cardiol. 1978;41:887–894. doi: 10.1016/0002-9149(78)90729-4. [DOI] [PubMed] [Google Scholar]
- e3.Brehm M, Rellecke P, Strauer BE. Inflammatory cardiac diseases by primary extracardial diseases. Internist. 2008;49:27–33. doi: 10.1007/s00108-007-1949-z. [DOI] [PubMed] [Google Scholar]
- e4.Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol. 1987;18:619–624. doi: 10.1016/s0046-8177(87)80363-5. [DOI] [PubMed] [Google Scholar]
- e5.Kuhl U, Noutsias M, Seeberg B, Schultheiss HP. Immunohistological evidence for a chronic intramyocardial inflammatory process in dilated cardiomyopathy. Heart. 1996;75:295–300. doi: 10.1136/hrt.75.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e6.Noutsias M, Pauschinger M, Ostermann K, et al. Digital image analysis system for the quantification of infiltrates and cell adhesion molecules in inflammatory cardiomyopathy. Med Sci Monit. 2002;8:MT59–MT71. [PubMed] [Google Scholar]
- e7.Okura Y, Dec GW, Hare JM, et al. A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J Am Coll Cardiol. 2003;41:322–329. doi: 10.1016/s0735-1097(02)02715-8. [DOI] [PubMed] [Google Scholar]