Abstract
Unrepaired DNA double-strand breaks can lead to apoptosis or tumorigenesis. In mammals double-strand breaks are repaired mainly by nonhomologous end-joining mediated by the DNA–PK complex. The core protein of this complex, DNA–PKcs, is a DNA-dependent serine/threonine kinase that phosphorylates protein targets as well as itself. Although the (auto)phosphorylation activity has been shown to be essential for repair of both random double-strand breaks and induced breaks at the immunoglobulin locus, the corresponding phosphatase has been elusive. In fact, to date, none of the putative phosphatases in DNA double-strand break repair has been identified. Here we show that protein phosphatase 5 interacts with DNA–PKcs and dephosphorylates with surprising specificity at least two functional sites. Cells with either hypo- or hyperphosphorylation of DNA–PKcs at these sites show increased radiation sensitivity.
Unrepaired DNA double-strand breaks (DSB) either trigger apoptosis or lead to genomic rearrangements, which in turn can lead to cancer. To fix DSBs, mammalian cells have at least two competing DSB repair pathways (1), which work either via homologous recombination or, most frequently, via nonhomologous end-joining (NHEJ). NHEJ is mediated by the DNA–PK complex, which is composed of the proteins DNA-dependent protein kinase catalytic subunit (DNA–PKcs), the heterodimer Ku70/80, XRCC4, and ligase IV (2). Further functional interaction partners include the Werner protein (3) and the endo/exonuclease Artemis (4). The importance of the serine/threonine kinase activity of DNA–PKcs for NHEJ was demonstrated in severe combined immunodeficient (SCID) mice (5), in which a mutated DNA–PKcs lacks this activity and thus fails to repair DSBs induced by the immunoglobulin and T cell receptor gene rearrangement processes (6, 7). As a consequence, T and B cell development are impaired, and radiation sensitivity is increased (8). The kinase activity of DNA–PKcs is triggered by binding to the open DNA ends of the damaged site, recruited by the heterodimer Ku70/80 (2). Then DNA–PKcs phosphorylates itself on a cluster of six well conserved sites, from the threonine at position 2609 (T2609) to T2647, and then colocalizes at DNA damage foci with other repair proteins such as H2AX and 53BP1 (9). When this step is blocked by mutations of these autophosphorylation sites, DNA end processing is blocked and radiation sensitivity is increased (9, 10).
Although the phosphorylation activity has been studied in quite some detail, little is known about the corresponding dephosphorylation. Obviously, by the end of the repair process, either the phosphorylated protein has to be degraded or the phosphate group at T2609 or other sites has to be removed. If so, it is not clear whether the dephosphorylation marks the end of the repair process or an intermediate step. The lack of information is partly due to the fact that the putative protein phosphatase responsible for the dephosphorylation is unknown. Here we set out to identify this enzyme.
Materials and Methods
Yeast Two-Hybrid Screen. Fragments 7–9 (amino acid residues 1,885–2,780) and 9–11 (amino acid residues 2,458–3,364) from full-length human DNA–PKcs cDNA were cloned into the matchmaker 3 (Clontech) bait plasmid pAS2-1. Then Saccharomyces cerevisiae strain AH109 was cotransfected with one of the three cDNA libraries contained in plasmid pACT2 and yeast cells were selected on Ade– His– Trp– Leu– -plates. The pACT2 plasmid DNA was reisolated from the selected yeast clones, and the encoding cDNA was amplified by PCR and sequenced.
Cell Lines, Cell Culture, and Irradiation Treatments. For our experiments, we used human HeLa cells and the Chinese hamster ovary cell line V3 (DNA–PKcs mutant) transfected with empty expression vector (V3-JM) or vector with full-length human DNA–PKcs (V3-F18), both under neomycin selection (11). V3 cells were grown in 5% CO2 at 37°C in α-DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, and 200 μg/ml neomycin for selection. HeLa cells were grown in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% glutamax 1 (Invitrogen) in 5% CO2 at 37°C. For irradiation experiments, cells were trypsinized, put into cell culture medium, and placed in a γ-irradiator (288 rad/min). Afterward the cells were recovered in the incubator for graded time periods and then lysed.
GST Pull-Down. cDNA encoding protein phosphatase 5 (PP5) TPR domain (amino acid residues 1–150) was cloned into pQE80 vector (Qiagen, Valencia, CA) and cDNAs encoding DNA–PKcs regions (amino acid residues 1,878–2,261 and 2,500–2,700, respectively) were cloned into pGEX-KG vector. Cell lysates containing GST or GST–DNA–PKcs fusion proteins were incubated with Glutathione Sepharose 4B beads (Amersham Biosciences) at 4°C for 1 h and washed three times with GST binding buffer (20 mM Tris·HCl, pH 7.5/150 mM NaCl/0.1% Nonidet P-40). His-PP5 TRP fusion protein (in GST binding buffer plus 1 mg/ml BSA) was added to the glutathione beads and incubated at 4°C for additional 2 h, followed by three washes with GST binding buffer. The pulled-down His-PP5 TRP was subjected to SDS/PAGE and Western blotting using anti-His horseradish peroxidase-conjugated antibody (Qiagen).
Retroviral Constructs and Transduction. Full-length human PP5 was cloned into the retroviral vector CRU5–IRES–GFP (IRES, internal ribosome entry site). Dominant negative PP5.GFP was a fusion protein between human PP5 lacking the last 110 aa and GFP. Amphotropic Phoenix packaging cells were transfected with 2 μg of plasmid DNA by using Fugene 6 transfection reagent (Roche Diagnostics). After 2 days, cultural supernatant containing the retrovirus were used to infect HeLa cells by spin-infection at 300 × g for 1 h with polybrene (5 μg/ml). After 3 days, HeLa cells were sorted for GFP-positive subclones by fluorescence-activated cell sorting (FACS). Subclones were grown up and tested for stable GFP expression by flow cytometry. Expression of PP5 and PP5.GFP fusion protein was confirmed by Western blotting of whole cell and nuclear extracts.
Cell Extracts and Immunoblotting. Nuclear extracts (P10 fraction) were prepared as described (12). Protein concentration was measured by the Bradford assay, and equal protein amounts (20–60 μg) were separated by SDS/8% PAGE. The proteins were transferred by Western blotting onto a nitrocellulose membrane (Bio-Rad) and incubated with antibody 18-2 to DNA–PKcs (Neomarkers, Fremont, CA), antibody to pT2609, antibody to pS2056 site, antibody to DNA polymerase δ (Transduction Laboratories, Lexington, KY), antibody to PP5 (Transduction Laboratories), or antibody to ATM pS1981 (Rockland, Gilbertsville, PA). Secondary antibodies were horseradish peroxidase-conjugated rabbit anti-mouse and goat anti-rabbit antibodies, respectively (Southern Biotechnology Associates).
Small interfering RNA (siRNA) Inhibition. siRNA duplexes were synthesized by Dharmacon. The coding strand for PP5 was AACCCCCGGCTGATGGAGCTC. Transfection of HeLa with siRNA duplexes was carried out by using Oligofectamine (Invitrogen) according to manufacturer's instruction. Forty-eight hours after initial transfection, the HeLa cells were divided into two cultures and subjected to 10-Gy irradiation or mock treatment at 72 h. Thirty minutes after irradiation, the HeLa were harvested for Western blotting analysis.
Flow Cytometry Survival Assay. A total of 100,000 cells each of various subclones were irradiated and immediately seeded in triplicates in six-well plates. Irradiated cells were collected after 72 h and 120 h, and undamaged cells were collected after 96 h. For this the supernatant, presumably containing the less viable or dead cells, the PBS wash fraction and the trypsinized adherent cells were pooled and spun down. Then they were resuspended in PBS containing 1% FBS and analyzed by flow cytometry.
Results and Discussion
PP5 Is a Binding Partner of DNA–PKcs. We performed a yeast two-hybrid screen with two overlapping cDNA fragments of the human DNA–PKcs as baits, of ≈3 kb length each (fragments 7–9 and 9–11, Fig. 1A). The screened cDNA libraries originated from the cell line 18-81, an Abelson virus-transformed mouse line of the B lymphocyte lineage that hypermutates and switches its Ig heavy chain gene constitutively (13); from human activated B cells, which undergo Ig class switching; and from human brain. Among the cDNAs encoding putative interaction partners there were five (four mouse and one human) that encoded different peptides derived from the N terminus of PP5 (Fig. 1B). All these cDNA fragments were fused to the GAL4 activation domain in frame. Sequence analysis showed that all hits contained a minimal region of the amino acid positions 1–89 of PP5, which is remarkable, because the 5′ terminus of transcripts is usually underrepresented in cDNA libraries. This PP5 region contains TPR motifs that have been implicated in protein–protein interactions (14). The PP5 peptides were recovered four times by DNA–PKcs fragment 9–11 and once by fragment 7–9 (Fig. 1 A). The region covering these fragments stretches from amino acid positions 2,458 to 2,780 and contains the major autophosphorylation site cluster that is phosphorylated in response to DNA damage (9, 10). We therefore considered PP5 a candidate for an enzyme, that dephosphorylates DNA–PKcs.
Fig. 1.
Binding of PP5 to DNA–PKcs. (A) Schematic representation of human DNA–PKcs and fragments used in yeast two-hybrid (7–9 and 9–11) and GST pull-down experiments. The numbers correspond to amino acid residues. (B) Schematic representation of human PP5 and fragments isolated by yeast two-hybrid screen from the corresponding libraries. The dashed line indicates that the fragments are in fact longer, but sequencing ended at the amino acid residue indicated. (C) Pull-down of GST-fusion proteins. (Upper) His-tagged TPR domain of PP5 (lane 1; input) was coimmunoprecipitated with the GST-DNA–PKcs fragment 2,500–2,700 with wild-type threonine (lane 4), aspartic acid (lane 5) at position 2,609, and fragment 1,878–2,267 (lane 6), beads only (lane 2), or GST only (lane 3), and visualized with anti His-horseradish peroxidase-conjugated antibody. (Lower) Pull-down input controls of GST-DNA–PKcs peptides were stained with Commassie blue.
PP5 is a ubiquitously expressed serine/threonine protein phosphatase that is present in both cytoplasm and nucleus (15, 16). It dephosphorylates the proapoptotic protein ASK1 (17) and interacts with a variety of proteins including the Hsp90–GR (18) and Hsp90–HRI (19) heterocomplexes, CDC16/27 of the anaphase-promoting complex (20), the A-subunit of PP2A (21), hCRY2 (22), and the Gα12/Gα13 subunits of heterotrimeric G proteins (23). Furthermore PP5 can dephosphorylate the Alzheimer's disease protein tau in vitro (24) and regulates p53 function (25). It is intriguing that PP5 is sensitive to okadaic acid (15), considering that cells incubated with this compound have decreased DNA–PKcs kinase activity (26).
Perhaps because of short interaction time in vivo, we were unable to coprecipitate PP5 and DNA–PKcs. Instead, to confirm and further map the interaction in vitro, we tried to precipitate the PP5-TPR domain with two different peptides of DNA–PKcs fused to GST. These peptides contained either the T2609 or related sites (amino acids 2,500–2,700) or the serine at position 2,056 (S2056) (amino acids 1,878–2,261), which has been recently identified as an exclusive DNA–PKcs autophosphorylation site (B.P.C.C. and D.J.C., unpublished result) (Fig. 1 A). Whereas the T2609 fragment retrieved the PP5-TPR domain very well, the S2056 fragment did not (Fig. 1C). When T2609 was changed to aspartic acid (T2609D) to mimic phosphorylation, the interaction seemed to be somewhat weaker. These experiments suggested a strong interaction between PP5 and DNA–PKcs with the fragment spanning the 2609 site, and a much weaker interaction, if any, with the fragment spanning the S2056 site.
Overexpression of PP5 Decreases Phosphorylation of DNA–PKcs but Not of ATM. To determine the functional relevance of the PP5/DNA–PKcs interaction, we stably overexpressed human wild-type PP5 in HeLa cells by using a retroviral vector. We then compared the phosphorylation level of T2609 in transduced subclones with the one in untransduced HeLa cells, with or without irradiation. When the cells were irradiated, we monitored phosphorylation after 30-, 60-, 120-, 240-, and 410-min recovery time. To this end, we prepared nuclear extracts and analyzed them by Western blotting with a polyclonal rabbit antibody that specifically reacts with pT2609 but not with unphosphorylated T2609 (9) (Fig. 2A). Under these conditions, the T2609 phosphorylation in the PP5 overexpressing subclones PP5.C4 and PP5.C13 was diminished, as compared to nontransduced HeLa cells, whether undamaged or damaged by irradiation. Furthermore, the dephosphorylation at T2609 occurred much faster in clones PP5.C4 and PP5.C13, with the pT2609 signal returning to undamaged levels at 410 min.
Fig. 2.
Phosphorylation status of DNA–PKcs after irradiation in PP5 overexpressing clones. PP5.C4 and PP5.C13 subclones were irradiated with IR (11.5 Gy) and recovered for 30–410 min. The undamaged control (UD) is shown on the left. Nuclear extracts were prepared, and 20–60 μg of protein was applied to a SDS gel. After Western blotting, the membrane was stained with rabbit antibody to DNA–PKcs pT2609 (A), rabbit antibody to DNA–PKcs pS2056 (B), or rabbit antibody to ATM pS1981 (C). For loading controls the same membranes were stripped and incubated with antibody to total DNA–PKcs or to DNA polymerase δ. PP5 overexpresssion was visualized by anti-PP5 antibody. * and ** denote phosphorylated DNA–PKcs degradation products.
Besides the 450-kDa band, which represents DNA–PKcs, we also noticed two other bands stained by the antibody, at 240 kDa and 150 kDa respectively; these bands might result from degradation of DNA–PKcs. The 240-kDa fragment is probably due to the apoptotic cleavage at position 2,708–2,713 (27); we do not know anything about the identity of the 150-kDa band. At any rate, compared to HeLa control cells, the 240-kDa band is less intense in the PP5.C4 and PP5.C13 subclones; and the 150-kDa band appears later after damage.
Apart from T2609, another functional DNA–PKcs phosphorylation site at S2056 has been recently defined (B.P.C.C. and D.J.C., unpublished result). To find out whether this site is also regulated by PP5, we used a polyclonal rabbit antibody generated against this site, on the same nuclear extracts as before. Whereas HeLa cells showed weak phosphorylation under undamaged conditions, it was absent in clones PP5.C4 and PP5.C13 and weaker in these clones at 30 min after irradiation (Fig. 2B). After 410 min, the S2056 was almost completely dephosphorylated in PP5.C13 but not in HeLa and PP5.C4. Despite this difference, and the slight difference in the level of PP5 overexpression between subclones PP5.C4 and PP5.C13 (Fig. 2 A), the observed similarities of the phosphorylation patterns seem to exclude a site-specific effect of the retroviral vector integration. Therefore, we conclude that PP5 is the phosphatase that is responsible for removing the phosphate group from T2609 and S2056 of DNA–PKcs after irradiation damage. However, ATM can also phosphorylate DNA–PKcs at T2609 (B.P.C.C. and D.J.C., unpublished result) (but not at S2056) and may be dephosphorylated by PP5 as well. Because this, in part, may contribute indirectly to the phosphorylation differences, we tested our subclones for ATM phosphorylation at the S1981 site (28). As we observed no obvious differences (Fig. 2C), it seems that PP5 is fairly specific for DNA–PKcs.
Dominant Negative and Decreased Expression of PP5 Increase Phosphorylation of DNA–PKcs. The import of PP5 into the nucleus apparently depends on its terminal 80 aa residues (29). One of the transduced HeLa subclones, PP5.C7, expressed a protein that encodes the first 389 residues of PP5 fused to GFP, thus lacking the sequence required for nuclear import. The PP5.GFP fusion protein had a molecular mass of ≈70 kDa, accumulated in the cytoplasm (Fig. 3A), and could be detected by PP5 antibody (Fig. 3B). We reasoned that this fusion protein may act as a dominant negative form. One possible way would be that PP5.GFP blocks the import mechanism while still binding to (some of) the proteins that are needed for this import; endogenous PP5 import would be competed out in this way. Another possibility came to mind when we considered the surprising finding that the fusion protein was not completely denatured by alcohol, as it still was fluorescent (not shown). Thus, it may be that the fusion protein does not fold correctly for the import, but still takes away the protein(s) required for it. Both before irradiation (undamaged) and after, the subclone PP5.C7 was heavily phosphorylated at position T2609 at all time points (Fig. 3B). Even after 410 min, when the signal began to wane in the untransduced HeLa cells (Fig. 2 A, which was part of the same experimental series and thus serves as a control), phosphorylation remained strong. Apart from confirming that PP5 provides the dephosphorylation activity on DNA–PKcs, the findings in clone PP5.C7 indicate that no other phosphatase can substitute for PP5 at T2609. In line with the phosphorylation patterns of ATM in the PP5 overexpressing subclones, no pronounced differences occurred in the PP5.C7 clone (data not shown). But obviously a possible regulation of ATM by PP5 needs a more detailed investigation.
Fig. 3.
Effect of dominant negative PP5.GFP on phosphorylation of DNA–PKcs. (A) Cellular localization in unfixed cells of either GFP in subclone PP5.C13 (Left) or PP5.GFP fusion protein in subclone PP5.C7 (Right). (B) The PP5.C7 subclone expressing dominant negative PP5.GFP was irradiated with 11.5 Gy and recovered for 30–410 min. Nuclear extracts, Western blotting, and staining with anti-pT2609 antibody were part of the experiment series shown in Fig. 2; the HeLa wild-type cells of Fig. 2 A thus serve as control. * and ** denote phosphorylated DNA–PKcs degradation products. (C) Knock-down of PP5 by RNA interference. HeLa cells were transfected with siRNAs targeted against PP5. As control, HeLa cells were either mock treated or transfected with unspecific siRNAs. As before, nuclear extracts were prepared from undamaged cells and cells irradiated with 10 Gy after 30-min recovery time and stained with anti-pS2056 antibody and anti-PP5 antibody.
Because phosphorylation levels at S2056 showed less pronounced differences between wild-type HeLa and PP5.C7 clone, we further assessed whether or not PP5 is the only phosphatase mediating the dephosphorylation of DNA–PKcs. We used specific siRNAs to knock down PP5 expression in HeLa cells and irradiated or mock treated cells transfected with specific or unspecific siRNAs and let them recover for 30 min. Although the reduction in PP5 expression was mild, nuclear extracts stained with antibody to the phosphorylated S2056 site (Fig. 3C) and also to pT2609 (data not shown) showed somewhat increased phosphorylation at both sites, under undamaged and damaged conditions. This points out that PP5 regulates both S2056 and T2609, but the effects of overexpression of wild-type and dominant negative PP5 were weaker for the S2056 site. These differences can be explained by the different mechanisms of RNA interference and dominant negative effects and may underscore the importance of nuclear import of PP5 for its regulation. Considering the multitude of PP5 substrates, it is possible that more efficient elimination of the enzyme cannot be achieved without arresting or killing the cells. Thus, selection against efficient siRNA treatment may occur in the cultures. At any rate, we conclude that PP5 cannot be replaced completely by another phosphatase at the two phosphorylation sites investigated here.
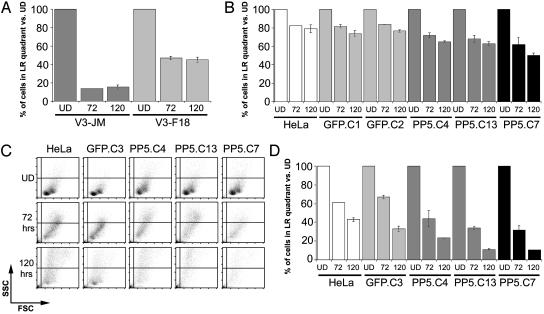
Both Hypo- and Hyperphosphorylation Increase Radiation Sensitivity. From the experiments described above, it seems to be clear that PP5 can dephosphorylate DNA–PKcs. But does this enzymatic activity have a biological consequence? This seemed likely to us, as the expression of DNA–PKcs with mutations in the major autophosphorylation cluster (including T2609) are defective in NHEJ (9, 10). Consequently, all of the transduced clones described above PP5.C4 and PP5.C13, and PP5.C7, ought to show increased radiation sensitivity. To address this question, we used flow cytometry analysis, which allows quantitation of viable and dividing cells, as they appear in defined quadrants of the forward (FSC) and side scatter (SSC) profiles. We determined the number of these cells per 10,000 events in the cytometer after irradiation with either 4 or 11.5 Gy at 72 and 120 h. Although the protocol does not provide absolute numbers of cell survivors, it does give ratios of live and dividing cells over all events that can be compared between cultures. To ensure that such an assay reasonably reflects cell viability, we compared DNA–PKcs deficient Chinese hamster ovary cells containing an empty vector (V3-JM) or reconstituted with a DNA–PKcs-expressing vector (V3-F18) (9) 72 and 120 h after irradiation with 4 Gy. As expected, the DNA–PKcs-positive cells clearly had a higher number of viable and dividing cells per culture than the DNA–PKcs-deficient culture at both time points (Fig. 4A). Next, we irradiated our transduced HeLa subclones and three different GFP only controls (GFP.C1, GFP.C2, and GFP.C3) with either 4 or 11.5 Gy. After irradiation with 4 Gy, there was no difference between the HeLa and the GFP controls (Fig. 4B). In contrast, subclones PP5.C4 and PP5.C13 were more radiosensitive, whereas subclone PP5.C7 was most sensitive. To investigate the effects of a higher dose, we irradiated the cells with 11.5 Gy (Fig. 4 C and D). The differences are easily seen in the FSC/SSC panels (Fig. 4C), which after 5 days showed a distinct population of viable and dividing cells for HeLa and GFP.C1, whereas this was not the case for the cells with aberrant PP5 expression. As another way to measure the viability of the transduced cells, we determined their ability to express GFP, with similar results. When we determined the numbers of GFP-positive cells in the very same experiments, they were in line with the survival rates calculated by counts in the FSC/SSC (data not shown). Because overexpression of PP5 protects cells against apoptosis after oxidative stress mediated by the dephosphorylation of ASK1 (17), it was surprising that both the overexpression of wild-type PP5 and the expression of a dominant negative mutant lead to increased radiation sensitivity. A model for DNA–PKcs autophosphorylation suggests at least two clusters of phosphorylation sites, which are used in specific ways to regulated DNA–PKcs function. Whereas the major cluster around the T2609 site is crucial for end processing, other sites might be essential for kinase disassembly (30). Thus, impaired phosphorylation or premature dephosphorylation caused by PP5 overexpression might block end processing and lead to radiation sensitivity. Classically, autophosphorylation of DNA–PKcs has been linked to kinase dissociation and inactivation (30). Dephosphorylation increases kinase activity in vitro; it has been postulated that this may allow re-use of DNA–PKcs in subsequent repair events in vivo. Thus, in the absence of efficient dephosphorylation, caused by less PP5 enzyme, radiation sensitivity may also be increased. However, we know that the initiation of repair process was intact, because damage foci formation of DNA–PKcs phosphorylated at pT2609 seemed to be normal and stable in all subclones (data not shown). Thus, downstream events must be responsible for decreased survival.
Fig. 4.
Survival of irradiated cell lines, assayed by flow cytometry. (A) DNA–PKcs-deficient Chinese hamster ovary V3 cells transfected with either empty vector (V3-JM) or DNA–PKcs expressing vector (V3-F18) irradiated with 4 Gy. (B) HeLa subclones irradiated with 4 Gy and analyzed after 72 h and 120 h. (C and D) HeLa subclones were irradiated with 11.5 Gy and analyzed after 72 h and 120 h. FSC, forward scatter; SSC, side scatter. For all cell lines, the percentage of viable cells represented in the lower right quadrant of FSC/SSC as shown in C was plotted compared to undamaged (UD) cultures.
Conclusion
Although numerous protein kinases are implicated in DSB repair pathways, so far not a single protein phosphatase could be shown to directly regulate DSB repair proteins (26). Here we demonstrated that PP5 interacts with DNA–PKcs and dephosphorylates it. We showed dephosphorylation mainly for the pT2609 site, and, to some extent, also for S2056; but, of course, other sites recently described in this region of DNA–PKcs (10) may also be regulated by PP5. Nor is it excluded that PP5 affects other protein targets; indeed, it has been shown that PP5 regulates p53 (25). However, in the experiments on p53, depletion of PP5 resulted in G1 growth arrest, whereas our HeLa cells expressing the dominant negative PP5 divide normally. Only in response to irradiation is there a difference in survival, caused either by growth arrest or apoptosis (31). The dominant negative PP5 clone is also more sensitive to phleomycin (data not shown), a radiomimetic chemical related to cancer therapeutics. This may make the nuclear import mechanism of PP5 an attractive drug target for enhancing the efficiency of radiotherapy in cancer.
Acknowledgments
We thank Cliff Wang and Christoph Baumann for discussion. This work was supported by National Institutes of Health Grant AG20684 and the Sandler Family Supporting Foundation (to M.W.) and National Institutes of Environmental Health Sciences Grant 1 R01 ES8061 (to J.E.C). The work from D.J.C.'s laboratory was supported by National Institutes of Health Grant CA50519, CA86936, and PO1-CA92584. T.W. is a recipient of a Boehringer-Ingelheim Fonds fellowship and is very grateful for a stay in Dr. S. P. Jackson's laboratory at The Wellcome Trust/Cancer Research UK Institute of Cancer and Developmental Biology in Cambridge, United Kingdom. This work has been performed as part of requirement for the Ph.D. degree at the Ludwig-Maximilians-University, Munich (supervisor Dr. Elisabeth Weiss).
Abbreviations: PP5, protein phosphatase 5; NHEJ, nonhomologous end-joining; DSB, double-strand breaks; FSC, forward scatter; SSC, side scatter; siRNA, small interfering RNA.
References
- 1.Allen, C., Kurimasa, A., Brenneman, M. A., Chen, D. J. & Nickoloff, J. A. (2002) Proc. Natl. Acad. Sci. USA 99, 3758–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith, G. C. & Jackson, S. P. (1999) Genes Dev. 13, 916–934. [DOI] [PubMed] [Google Scholar]
- 3.Yannone, S. M., Roy, S., Chan, D. W., Murphy, M. B., Huang, S., Campisi, J. & Chen, D. J. (2001) J. Biol. Chem. 276, 38242–38248. [DOI] [PubMed] [Google Scholar]
- 4.Ma, Y., Pannicke, U., Schwarz, K. & Lieber, M. R. (2002) Cell 108, 781–794. [DOI] [PubMed] [Google Scholar]
- 5.Schuler, W., Weiler, I. J., Schuler, A., Phillips, R. A., Rosenberg, N., Mak, T. W., Kearney, J. F., Perry, R. P. & Bosma, M. J. (1986) Cell 46, 963–972. [DOI] [PubMed] [Google Scholar]
- 6.Lieber, M. R., Hesse, J. E., Lewis, S., Bosma, G. C., Rosenberg, N., Mizuuchi, K., Bosma, M. J. & Gellert, M. (1988) Cell 55, 7–16. [DOI] [PubMed] [Google Scholar]
- 7.Blunt, T., Finnie, N. J., Taccioli, G. E., Smith, G. C., Demengeot, J., Gottlieb, T. M., Mizuta, R., Varghese, A. J., Alt, F. W., Jeggo, P. A., et al. (1995) Cell 80, 813–823. [DOI] [PubMed] [Google Scholar]
- 8.Fulop, G. M. & Phillips, R. A. (1990) Nature 347, 479–482. [DOI] [PubMed] [Google Scholar]
- 9.Chan, D. W., Chen, B. P., Prithivirajsingh, S., Kurimasa, A., Story, M. D., Qin, J. & Chen, D. J. (2002) Genes Dev. 16, 2333–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding, Q., Reddy, Y. V., Wang, W., Woods, T., Douglas, P., Ramsden, D. A., Lees-Miller, S. P. & Meek, K. (2003) Mol. Cell. Biol. 23, 5836–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurimasa, A., Kumano, S., Boubnov, N. V., Story, M. D., Tung, C. S., Peterson, S. R. & Chen, D. J. (1999) Mol. Cell. Biol. 19, 3877–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lees-Miller, S. P., Chen, Y. R. & Anderson, C. W. (1990) Mol. Cell. Biol. 10, 6472–6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burrows, P. D., Beck, G. B. & Wabl, M. R. (1981) Proc. Natl. Acad. Sci. USA 78, 564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das, A. K., Cohen, P. W. & Barford, D. (1998) EMBO J. 17, 1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, M. X., McPartlin, A. E., Brown, L., Chen, Y. H., Barker, H. M. & Cohen, P. T. (1994) EMBO J. 13, 4278–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chinkers, M. (2001) Trends Endocrinol. Metab. 12, 28–32. [DOI] [PubMed] [Google Scholar]
- 17.Morita, K., Saitoh, M., Tobiume, K., Matsuura, H., Enomoto, S., Nishitoh, H. & Ichijo, H. (2001) EMBO J. 20, 6028–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen, M. S., Silverstein, A. M., Pratt, W. B. & Chinkers, M. (1996) J. Biol. Chem. 271, 32315–32320. [DOI] [PubMed] [Google Scholar]
- 19.Shao, J., Hartson, S. D. & Matts, R. L. (2002) Biochemistry 41, 6770–6779. [DOI] [PubMed] [Google Scholar]
- 20.Ollendorff, V. & Donoghue, D. J. (1997) J. Biol. Chem. 272, 32011–32018. [DOI] [PubMed] [Google Scholar]
- 21.Lubert, E. J., Hong, Y. & Sarge, K. D. (2001) J. Biol. Chem. 276, 38582–38587. [DOI] [PubMed] [Google Scholar]
- 22.Zhao, S. & Sancar, A. (1997) Photochem. Photobiol. 66, 727–731. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi, Y., Katoh, H., Mori, K. & Negishi, M. (2002) Curr. Biol. 12, 1353–1358. [DOI] [PubMed] [Google Scholar]
- 24.Liu, F., Zaidi, T., Iqbal, K., Grundke-Iqbal, I. & Gong, C. X. (2002) Neuroscience 115, 829–837. [DOI] [PubMed] [Google Scholar]
- 25.Zuo, Z., Dean, N. M. & Honkanen, R. E. (1998) J. Biol. Chem. 273, 12250–12258. [DOI] [PubMed] [Google Scholar]
- 26.Douglas, P., Moorhead, G. B., Ye, R. & Lees-Miller, S. P. (2001) J. Biol. Chem. 276, 18992–18998. [DOI] [PubMed] [Google Scholar]
- 27.Song, Q., Lees-Miller, S. P., Kumar, S., Zhang, Z., Chan, D. W., Smith, G. C., Jackson, S. P., Alnemri, E. S., Litwack, G., Khanna, K. K. & Lavin, M. F. (1996) EMBO J. 15, 3238–3246. [PMC free article] [PubMed] [Google Scholar]
- 28.Bakkenist, C. J. & Kastan, M. B. (2003) Nature 421, 499–506. [DOI] [PubMed] [Google Scholar]
- 29.Borthwick, E. B., Zeke, T., Prescott, A. R. & Cohen, P. T. (2001) FEBS Lett. 491, 279–284. [DOI] [PubMed] [Google Scholar]
- 30.Merkle, D., Douglas, P., Moorhead, G. B., Leonenko, Z., Yu, Y., Cramb, D., Bazett-Jones, D. P. & Lees-Miller, S. P. (2002) Biochemistry 41, 12706–12714. [DOI] [PubMed] [Google Scholar]
- 31.Woo, R. A., Jack, M. T., Xu, Y., Burma, S., Chen, D. J. & Lee, P. W. (2002) EMBO J. 21, 3000–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]