Abstract
Rationale
Recent evidence suggests a role for the dynorphin/ kappa-opioid receptor (KOR) system in the expression of stress-induced behaviors. Wistar Kyoto (WKY) rats exhibit increased depression-like and anxiety-like responses in behavioral tests compared to other strains and may be a model of comorbid depression and anxiety characterized by increased activity within the dynorphin/KOR system. Though KOR antagonists produce antidepressant-like effects in WKY rats, their effects in tests of anxiety-like behavior have not been examined in the WKY strain.
Objective
The aim of the current study was to investigate the effects of the KOR antagonist 2-(3,4-dichlorophenyl)-N-methyl-N-[(1S)-1-(3-isothiocyanatophenyl)-2-(1-pyrrolidinyl) ethyl]acetamide hydrochloride (DIPPA) on the behavior of WKY rats and Sprague Dawley (SD) rats in tests of anxiety-like behavior.
Methods
The novelty-induced hypophagia and defensive burying tests were used to measure anxiety-like behavior in WKY and SD rats and determine the effects of DIPPA on anxiety-like behavior in both strains.
Results
WKY rats displayed greater amounts of anxiety-like behavior compared to SD rats. DIPPA produced anxiolytic-like effects in both tests in both strains.
Conclusions
WKY rats display more anxiety-like behavior at baseline compared to SD rats, and DIPPA produced anxiolytic-like effects in both WKY and SD rats. These findings support previous research suggesting that KOR antagonists possess anxiolytic-like properties and may potentially represent a novel class of treatments for mood disorders.
Keywords: Wistar Kyoto rat, Anxiety, Kappa-opioid receptor, Defensive burying, Novelty-induced hypophagia
Introduction
Major depressive disorder (MDD) diagnosed with comorbid anxiety is likely to be more severe (Joffe et al. 1993), accompanied by increased suicidal behavior (Pfeiffer et al. 2009; Sareen et al. 2005), and more resistant to treatment with established antidepressants (Fava et al. 2008) than MDD without anxiety. In fact, treatment with antidepressants can often induce or exacerbate anxiety before therapeutic effects have had an opportunity to emerge (Grillon et al. 2007; Marshall et al. 1995; Zinner 1994). This suggests a pressing medical need for novel treatments that could more effectively address the symptoms of both MDD and anxiety disorders. Given the interplay between environmental stressors and genetic vulnerability in the development of mood disorders (Sullivan et al. 2000), animal models that exhibit increased trait anxiety and stress sensitivity could help identify potential therapeutic agents for the treatment of comorbid depression and anxiety.
The Wistar Kyoto (WKY) rat strain is a putative model of comorbid depression and anxiety. WKY rats exhibit increased depression-like (Armario et al. 1995; Lopez-Rubalcava and Lucki 2000; Pare 1989) and anxiety-like (Pare 1992; 1994; Ramos et al. 1997) behavior compared to other rat strains. The behavioral phenotype of the WKY rat is expressed at baseline without exposure to prior stress regimens, suggesting it is the result of a genetic predisposition toward exaggerated responses to the effects of stress. Indeed, WKY rats exhibit increased and prolonged activation of the hypothalamic–pituitary–adrenal axis in response to swim stress (Rittenhouse et al. 2002) and acute immobilization following chronic cold stress (Pardon et al. 2003) compared to Sprague Dawley (SD) rats. A number of studies have identified potential neurobiological and genetic correlates associated with the behavioral phenotype of the WKY rat (Ahmadiyeh et al. 2003; De La Garza and Mahoney 2004; Jiao et al. 2003; Solberg et al. 2004), but the factors responsible for the increased stress sensitivity of the strain are still unknown.
A large body of evidence suggests that the dynorphin/ kappa-opioid receptor (KOR) system is an important biological substrate underlying aversion related to depression and anxiety (Bruchas et al. 2009; Knoll and Carlezon 2009). Increased activity of the dynorphin/KOR system may contribute to the enhanced stress sensitivity of the WKY strain. A recent microarray study found that WKY rats express the KOR at higher levels in the locus coeruleus (Pearson et al. 2006), a brain region critically involved in the behavioral response to stress (Van Bockstaele et al. 2009), compared to SD rats. A subsequent study found that WKY rats exhibit increased KOR and dynorphin A protein in the nucleus accumbens and piriform cortex compared to SD rats (Carr et al. 2010), suggesting that increased activity of the dynorphin/KOR system in multiple brain regions may contribute to the behavioral differences between the strains. Previous research has implicated the nucleus accumbens and piriform cortex in the antidepressant-like response to swim stress (Sibille et al. 1997; Chartoff et al. 2009). Furthermore, systemic administration of KOR antagonists produced antidepressant-like behavioral effects in the WKY strain in the forced swim test and increased activation of c-fos in the nucleus accumbens and piriform cortex (Carr et al. 2010), consistent with the potential greater sensitivity of WKY rats to modulation of the dynorphin/KOR system.
The purpose of these studies was to extend the comparison of the behavioral response to KOR antagonists between WKY and SD rats to two tests of anxiety behavior, the novelty-induced hypophagia (NIH) test and defensive burying (DB) test. The effects of KOR antagonists on anxiety-related behaviors have recently been studied in SD rats (Knoll et al. 2007) but have not been examined in WKY rats. These tests were chosen due to the complementary nature of the respective measures of anxiety-like behavior. The NIH test is a modified form of the novelty-induced suppression of feeding test (Bodnoff et al. 1988; Britton and Britton 1981; Shephard and Broadhurst 1982), conducted without food deprivation and using palatable food to drive consumption, that measures the inhibition of feeding behavior produced by exposure to a novel environment. Anxiolytic compounds reduce the latency to approach food and increase feeding in the NIH test (Bechtholt et al. 2008; Dulawa et al. 2004; Merali et al. 2003). The DB test measures the tendency of rodents to cover localized sources of threat or potential harm with bedding (Terlecki et al. 1979). In the DB test, the main measure of anxiety/fear is the increased time spent burying produced by exposure to an electrified probe or other aversive objects. Anxiolytic compounds decrease burying behavior in the DB test (Treit 1990; Treit et al. 1981), and anxiogenic drugs increase the duration of burying (De Boer and Koolhaas 2003). These two tests could provide complementary information concerning the potential anxiolytic-like effects of a KOR antagonist.
The current study characterized the effects of the KOR antagonist 2-(3,4-dichlorophenyl)-N-methyl-N-[(1S)-1-(3-isothiocyanatophenyl)-2-(1-pyrrolidinyl)ethyl]acetamide hydrochloride (DIPPA; Chang et al. 1994) on anxiety-like behaviors in SD and WKY rats using the NIH and DB tests. The results of this study showed that WKY rats exhibited increased anxiety-like behavior compared to SD rats. Additionally, DIPPA produced anxiolytic-like effects in both WKY and SD rats.
Materials and methods
Animals
Adult male Sprague Dawley (Charles River, Wilmington, MA, USA) and Wistar Kyoto (Taconic, Germantown, NY) rats, weighing 250–300 g upon arrival, were housed two per cage in a temperature-controlled (22°C) colony room. The room was on a 12-h light/dark cycle with lights on at 0700 h. All rats were handled daily for a week before testing. The care and use of animals was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs
The KOR antagonist DIPPA (Tocris Bioscience, Ellisville, MO) was dissolved in a mixture of 20% dimethyl sulfoxide (DMSO) and 80% distilled water. In both experiments, DIPPA was injected subcutaneously in a volume of 2 ml/kg. Rats in the control groups were injected with 0.9% saline in an equivalent volume. Studies in our lab have shown that DMSO at this concentration does not alter behavior when compared to saline-treated rats in both the SD and WKY strains in either the NIH or DB test.
Behavioral testing
Novelty-induced hypophagia
The NIH procedure utilized in this study was similar to one previously published (Bechtholt et al. 2008). On each of the first 8 days (Training Phase), the rats were separated within their home cages by an opaque, plastic divider for 90 min. Immediately following this 90-min period, a glass bowl containing Graham cracker crumbs (Nabisco, Northfield, IL) was placed on each side of the cage, and the rats were given access to 9–10 g of Graham cracker crumbs for 15 min. This training allowed the development of stable latency and consumption levels (Bechtholt et al., 2008). Immediately following the removal of the Graham cracker crumbs on Day8, the rats were injected with either DIPPA (2.5 or 5 mg/kg) or saline and returned to their home cage. Twenty-four hours later, the rats were again given 15 min of access to the Graham cracker crumbs for the home cage portion of the test. The 24-h pretreatment interval was chosen because DIPPA produces KOR agonist-like activity within the first 4 h after administration, but the drug then shows significant antagonist activity without agonist-like effects for up to 48 h (Chang et al. 1994). The home cage test was identical to the training sessions except that it was videotaped for later scoring. The latency to begin feeding and the amount consumed were recorded. Immediately following the home cage test, rats were given a second treatment injection and returned to their home cage. Twenty-four hours later, rats were given access to Graham crackers for 15 min in a novel arena. The novel arena consisted of a polycarbonate cage of the same dimensions as the home cage (48 cm L× 26 cm W×20 cm H). The novel arena was brightly lit (1,400 lx) and had a wire mesh floor rather than a plastic floor lined with bedding. Graham cracker crumbs were placed in a glass bowl, identical to those used in the home cage sessions, and the bowl was attached to the grid floor at one end of the cage. This session was videotaped, and the latency to begin feeding and amount consumed were recorded.
Defensive burying test
The protocol used for the DB test was adapted from the procedure of Treit et al. (1981) and similar to one previously used in our lab (Howard et al. 2008). The test was conducted under dim light conditions (160 lx). The testing arena was a clear polycarbonate cage of the same dimensions as the home cage (48 cm L× 26 cm W×20 cm H) lined with bedding to a depth of 5 cm. There was a shock probe, consisting of a 1-cm-diameter glass rod, wrapped with two copper wires, attached to one end of the cage and extending 6 cm into the cage. The wires were connected to a shock generator (SGS-004, BRS-LVE, Laurel, MD) set to deliver 4.0 mA of current when the animal made contact with the probe. Rats were placed into the cage at the end opposite the shock probe facing away from the probe. The 15-min testing period began after the rat made its first contact with the probe. The probe remained electrified for the duration of the test. The cage was replaced with an identical clean test cage with fresh bedding prior to testing each rat. In this experiment, DIPPA (2.5 or 5 mg/kg) was administered 24 h before testing. Test sessions were videotaped for subsequent analysis. The behavioral measures recorded were latency to begin burying from first probe contact, time spent burying, and time spent immobile. Immobility time was defined as the amount of time the rat spent motionless facing the probe. The latency to begin burying and time spent burying are considered measures of anxiety. Immobility time is considered a measure of passive avoidance of the shock probe (De Boer and Koolhaas 2003). Latency to contact probe and shock reactivity (four-point scale; Treit and Pesold 1990) were also measured as general markers of activity and pain threshold, respectively.
Statistical analyses
All statistical analyses were conducted using PASW 17.0 (SPSS, Inc., Chicago, IL) software. The data in both experiments were analyzed using two-way ANOVAs (strain × treatment). Follow-up within-strain comparisons were conducted using Dunnett's post hoc test. Planned comparisons were conducted between the saline-treated groups in order to compare the baseline responses between strains. Significance was established at p<0.05.
Results
Effects of DIPPA in the NIH test
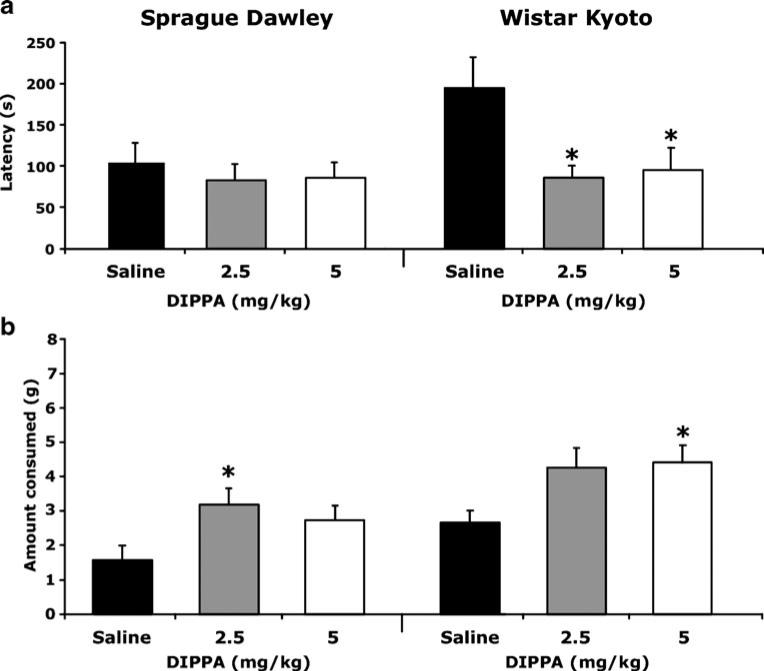
There was a significant effect of drug treatment on novel cage latency (F(2, 36)=4.00, p=0.027). Both doses of DIPPA (2.5 and 5 mg/kg) decreased the latency to feed in WKY rats, but treatment did not alter approach latencies in SD rats (Fig. 1a). There were significant effects of both strain (F(1, 36)=11.57, p=0.002) and treatment (F(2, 36)= 9.31, p=0.001) on food consumption in the novel cage (Fig. 1b). Mean consumption between saline-treated SD and WKY rats was not significantly different. In SD rats, the two doses of DIPPA tested increased novel cage consumption to similar levels, but only the increase caused by the 2.5 mg/kg dose reached statistical significance. A similar pattern was present in the WKY strain as both doses appeared to increase novel cage consumption to similar levels, but only the 5 mg/kg group was significantly different from the saline-treated group.
Fig. 1.
Effects of DIPPA in the NIH novel cage test. a Latency to approach food. b Amount of food consumed. Asterisks represent significant difference from within-strain saline group, *p<0.05. Data are expressed as mean + 1 SEM. n=7/group
There were no significant main or interaction effects on latency to feed in the home cage (Table 1). There was a significant strain × treatment interaction (F(2, 36)=5.11, p=0.011) on home cage consumption (Table 1). Saline-treated SD and WKY rats did not differ in the amount of food consumed. The highest dose of DIPPA (5 mg/kg) decreased consumption in SD rats compared to the 5 mg/ kg group of WKY rats. There were no significant differences between any of the WKY treatment groups.
Table 1.
Effects of DIPPA on home cage consumption and latency
| Strain | Dose (mg/kg) | Latency (s) | Consumption (g) |
|---|---|---|---|
| SD | Saline | 13.71 ± 4.38 | 5.14 ± 0.85 |
| 2.5 | 4.14 ± 0.96 | 5.13 ± 0.31 | |
| 5 | 5.86 ± 2.26 | 3.34 ± 0.53* | |
| WKY | Saline | 8.86 ± 3.05 | 5.46 ± 0.39 |
| 2.5 | 7.43 ± 3.56 | 6.21 ± 0.30 | |
| 5 | 5.57 ± 1.94 | 6.66 ± 0.30 |
Values represent mean ± SEM
p<0.01 compared to the 5 mg/kg WKY group (Bonferroni's post hoc test)
Effects of DIPPA on defensive burying
The latency to begin burying (Fig. 2a) differed overall between strains (F(1, 79)=10.13, p=0.002), with saline-treated WKY rats burying earlier than saline-treated SD rats. DIPPA treatment did not alter burying latency in either strain. Overall, WKY rats also spent more time burying than SD rats (F(1, 79)=19.62, p<0.001), and DIPPA significantly decreased burying time in both strains (F(2, 79)= 7.26, p=0.001). Saline-treated WKY rats spent more time burying than saline-treated SD rats. DIPPA (5 mg/kg) decreased burying in both strains compared to the within-strain control groups (Fig. 2b). Immobility time (Fig. 2c) also showed significant effects of strain (F(1, 79)=13.45, p<0.001) and drug treatment (F(2, 79)=4.06, p=0.021). Saline-treated WKY rats spent more time immobile than saline-treated SD rats. DIPPA (5 mg/kg) increased immobility in SD rats compared to the saline-treated strain control group but not in WKY rats (Fig. 2c). There were no significant effects of strain or treatment on the latency to contact the probe or shock reactivity (Table 2).
Fig. 2.
Effects of DIPPA in the DB test. a Latency to begin burying. b Total time spent burying the shock probe. c Total time spent immobile. Asterisks represent significant difference from within-strain saline group, *p<0.05 and **p<0.01. Number symbol represents significant difference from SD saline-treated group, #p<0.05 and ##p<0.01. Data are expressed as mean + 1 SEM. n=12–17/group
Table 2.
Effects of DIPPA on probe-directed behaviors
| Strain | Dose (mg/kg) | Latency to contact probe (s) | Shock reactivity |
|---|---|---|---|
| SD | Saline | 11.86 ± 3.12 | 1.76 ± 0.13 |
| 1 | 10.77 ± 3.50 | 1.82 ± 0.09 | |
| 5 | 5.58 ± 0.63 | 1.56 ± 0.10 | |
| WKY | Saline | 18.18 ± 4.95 | 1.61 ± 0.11 |
| 1 | 11.85 ± 4.81 | 2.01 ± 0.11 | |
| 5 | 14.25 ± 4.40 | 1.77 ± 0.13 |
Values represent mean ± SEM
Discussion
The major result of this study is that the KOR antagonist DIPPA produced anxiolytic-like effects in the NIH test and the DB test. The effects of DIPPA demonstrated in this study complement previous work documenting the anxiolytic-like effects of the KOR antagonists nor-binaltorphimine and JDTic in two other tests of anxiety-like behavior, the elevated plus maze and fear-potentiated startle measured in SD rats (Knoll et al. 2007).
Novelty suppression of feeding tests are some of the few tests of anxiety-like behavior that are responsive to the anxiolytic effects of antidepressants (Bechtholt et al. 2008; Bodnoff et al. 1988; Borsini et al. 2002; Dulawa et al. 2004). While DIPPA decreased approach latencies in WKY rats, it had no effect on this measure in SD rats. However, food consumption in the novel cage was increased by DIPPA in both strains, an effect that could also be interpreted as anxiolytic-like. Interestingly, the anxiolytic-like effects of DIPPA were evident after acute treatment, whereas chronic treatment with antidepressants is required for the emergence of anxiolytic effects (Bechtholt et al. 2008; Bodnoff et al. 1988). There are some differences in the literature concerning the relative roles for latency and consumption measures in tests of hyponeophagia. Some studies have reported reduced approach latencies and increased food consumption after acute treatment with benzodiazepines or chronic antidepressants (Bechtholt et al. 2008; Dulawa et al. 2004; Shephard and Broadhurst 1982). But other studies have focused exclusively on approach latencies (Bodnoff et al. 1988) or food consumption (Britton and Britton 1981) measures alone. These measures could be disconnected in SD rats due to conflicting effects of the dynorphin/KOR system on anxiety and feeding in this test. Specifically, KOR antagonists may inhibit food consumption while also producing anxiolytic-like effects. KOR agonists increase consumption of both standard chow and palatable foods (Cooper et al. 1985; Silva et al. 2002) while KOR antagonists can reduce consumption of palatable foods (Sipols et al. 2002), suggesting that activity of the dynorphin/KOR system directly regulates feeding behavior. Indeed, DIPPA tended to decrease consumption in SD rats in the home cage but significantly increased feeding in the novel cage where potential anxiolytic-like effects of DIPPA may oppose the hypophagic effects of the compound.
By measuring increases in burying behavior produced after a rat contacts the electrified probe, the DB test examines anxiety by exploiting the predisposition of rats to bury sources of aversive stimulation or threat (Terlecki et al. 1979). The DB test differs from the NIH test because the measure of anxiety is the production of a response actively evoked by a threatening stimulus, instead of the inhibition of a response caused by exposure to a novel environment. The DB test typically measures the effects of anxiolytic drugs with a reduction in burying behavior (Treit et al. 1981). In the present study, the DB test measured a higher level of anxiety-like behavior in WKY rats compared to SD rats at baseline; WKY rats demonstrated a longer duration of burying behavior, a shorter latency to begin burying, and longer immobility times following contact with the probe. These results differ from previous studies with the DB test that showed less burying behavior and increased immobility in WKY rats compared to SD or Wistar rats (Gutierrez-Mariscal et al. 2008; Pare 1992, 1994). Some key methodological differences may account for this discrepancy, principally the use of a lower level of illumination or differences in the vendor stock (Pare and Kluczynski 1997).
In the current study, DIPPA produced clear anxiolytic-like effects in the DB test in WKY rats because it reduced burying but did not alter latency to contact the probe, shock reactivity, or immobility. DIPPA also reduced burying behavior in SD rats, consistent with an anxiolytic-like effect in this strain. However, the increased immobility produced by DIPPA in SD rats, although consistent with the effects produced by some anxiolytic compounds (De Boer and Koolhaas 2003), makes the reduced burying more difficult to interpret because it could represent a sedative effect or a switch to a passive coping strategy without any change in the underlying anxiety/fear state. A higher dose of DIPPA (10 mg/kg) has been shown to decrease locomotor activity in SD rats (Carr et al. 2010).
Although WKY rats are traditionally characterized as exhibiting a greater predisposition toward passive responses to stress, the results of this study suggest that WKY rats can demonstrate exaggerated active and passive responses to stressors depending on the circumstances. In the present study, WKY rats spent more time engaged in both active (increased burying time) and passive (increased immobility) probe-directed behaviors in the DB test compared to SD rats, indicating greater preoccupation with the shock probe. In support of this new characterization, a recent study also found that WKY rats made significantly more inter-trial responses and were more resistant to extinction in a lever-press escape/avoidance task compared to SD rats (Servatius et al. 2008). Thus, one possible explanation for the behavior displayed by WKY rats in both studies is that WKY rats are hypersensitive to the aversive properties of stress.
The convergent anxiolytic-like effects of DIPPA in the NIH and DB tests agree with previous evidence on the role of the dynorphin/KOR system in the behavioral response to stress. Studies utilizing prodynorphin and KOR knockout mice have shown that activation of the KOR through the endogenous release of dynorphin is necessary for the expression of a number of stress-induced behaviors (McLaughlin et al. 2006; McLaughlin et al. 2003; Wittmann et al. 2009). Additionally, KOR activation is required for the expression of aversion produced by multiple stressors in mice (Land et al. 2008). WKY rats have higher KOR tone than SD rats (Pearson et al. 2006; Carr et al. 2010), and this may underlie their increased sensitivity to stress.
In the present study, DIPPA produced anxiolytic-like effects in both the NIH test and the DB test in WKY and SD rats. Convergent effects of DIPPA pretreatment in these two tests suggest that signaling through the dynorphin/KOR system is involved in the expression of both passive and active stress-induced behaviors. These results are consistent with the anxiolytic activity of KOR antagonists in other tests of anxiety-like behavior, such as the elevated plus maze and fear-potentiated startle (Knoll et al. 2007). Other KOR antagonists will need to be tested in order to determine if the effects seen in this study are specific to DIPPA or generalize across the entire class of compounds. Overall, the findings of the current study support the further development of KOR antagonists as a novel class of antidepressant-like compounds with the potential to acutely treat anxiety disorders that present alone or often accompany MDD.
Acknowledgments
The authors are grateful for the valuable technical assistance of Matthew Young. This research was supported by funds from AstraZeneca and the National Institute of Mental Health (T32 MH14652).
Contributor Information
Gregory V. Carr, Department of Psychiatry, University of Pennsylvania, 125 South 31st Street, Room 2204, Philadelphia, PA, USA
Irwin Lucki, Department of Psychiatry, University of Pennsylvania, 125 South 31st Street, Room 2204, Philadelphia, PA, USA; Department of Pharmacology, University of Pennsylvania, Philadelphia, PA, USA.
References
- Ahmadiyeh N, Churchill GA, Shimomura K, Solberg LC, Takahashi JS, Redei EE. X-linked and lineage-dependent inheritance of coping responses to stress. Mamm Genome. 2003;14:748–757. doi: 10.1007/s00335-003-2292-x. [DOI] [PubMed] [Google Scholar]
- Armario A, Gavalda A, Marti J. Comparison of the behavioural and endocrine response to forced swimming stress in five inbred strains of rats. Psychoneuroendocrinology. 1995;20:879–890. doi: 10.1016/0306-4530(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Bechtholt AJ, Valentino RJ, Lucki I. Overlapping and distinct brain regions associated with the anxiolytic effects of chlordiazepoxide and chronic fluoxetine. Neuropsychopharmacology. 2008;33:2117–2130. doi: 10.1038/sj.npp.1301616. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Borsini F, Podhorna J, Marazziti D. Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology (Berl) 2002;163:121–141. doi: 10.1007/s00213-002-1155-6. [DOI] [PubMed] [Google Scholar]
- Britton DR, Britton KT. A sensitive open field measure of anxiolytic drug activity. Pharmacol Biochem Behav. 1981;15:577–582. doi: 10.1016/0091-3057(81)90212-4. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2009;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. Antidepressant-like effects of kappa opioid receptor antagonists in Wistar-Kyoto rats. Neuropsychopharmacology. 2010;35:752–763. doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AC, Takemori AE, Portoghese PS. 2-(3, 4-Dichlorophenyl)-N-methyl-N-[(1S)-1-(3-isothiocyanatophenyl)-2-(1-py rrolidinyl)ethyl]acetamide: an opioid receptor affinity label that produces selective and long-lasting kappa antagonism in mice. J Med Chem. 1994;37:1547–1549. doi: 10.1021/jm00037a001. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Papadopoulou M, MacDonald ML, Parsegian A, Potter D, Konradi C, Carlezon WA., Jr Desipramine reduces stress-activated dynorphin expression and CREB phosphorylation in NAc tissue. Mol Pharmacol. 2009;75:704–712. doi: 10.1124/mol.108.051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SJ, Jackson A, Kirkham TC. Endorphins and food intake: kappa opioid receptor agonists and hyperphagia. Pharmacol Biochem Behav. 1985;23:889–901. doi: 10.1016/0091-3057(85)90088-7. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Mahoney JJ., 3rd A distinct neurochemical profile in WKY rats at baseline and in response to acute stress: implications for animal models of anxiety and depression. Brain Res. 2004;1021:209–218. doi: 10.1016/j.brainres.2004.06.052. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, Biggs MM, Zisook S, Leuchter A, Howland R, Warden D, Trivedi MH. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165:342–351. doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- Grillon C, Levenson J, Pine DS. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: a fear-potentiated startle study. Neuropsychopharmacology. 2007;32:225–231. doi: 10.1038/sj.npp.1301204. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Mariscal M, de Gortari P, Lopez-Rubalcava C, Martinez A, Joseph-Bravo P. Analysis of the anxiolytic-like effect of TRH and the response of amygdalar TRHergic neurons in anxiety. Psychoneuroendocrinology. 2008;33:198–213. doi: 10.1016/j.psyneuen.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Howard O, Carr GV, Hill TE, Valentino RJ, Lucki I. Differential blockade of CRF-evoked behaviors by depletion of norepinephrine and serotonin in rats. Psychopharmacology (Berl) 2008;199:569–582. doi: 10.1007/s00213-008-1179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao X, Pare WP, Tejani-Butt S. Strain differences in the distribution of dopamine transporter sites in rat brain. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:913–919. doi: 10.1016/S0278-5846(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Joffe RT, Bagby RM, Levitt A. Anxious and nonanxious depression. Am J Psychiatry. 1993;150:1257–1258. doi: 10.1176/ajp.150.8.1257. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2009;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA. Anxiolytic-like effects of {kappa}-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Marshall RD, Printz D, Cardenas D, Abbate L, Liebowitz MR. Adverse events in PTSD patients taking fluoxetine. Am J Psychiatry. 1995;152:1238–1239. doi: 10.1176/ajp.152.8.1238b. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Levac C, Anisman H. Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biol Psychiatry. 2003;54:552–565. doi: 10.1016/s0006-3223(02)01827-9. [DOI] [PubMed] [Google Scholar]
- Pardon MC, Ma S, Morilak DA. Chronic cold stress sensitizes brain noradrenergic reactivity and noradrenergic facilitation of the HPA stress response in Wistar Kyoto rats. Brain Res. 2003;971:55–65. doi: 10.1016/s0006-8993(03)02355-2. [DOI] [PubMed] [Google Scholar]
- Pare WP. Stress ulcer susceptibility and depression in Wistar Kyoto (WKY) rats. Physiol Behav. 1989;46:993–998. doi: 10.1016/0031-9384(89)90203-5. [DOI] [PubMed] [Google Scholar]
- Pare WP. The performance of WKY rats on three tests of emotional behavior. Physiol Behav. 1992;51:1051–1056. doi: 10.1016/0031-9384(92)90091-f. [DOI] [PubMed] [Google Scholar]
- Pare WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol Behav. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Pare WP, Kluczynski J. Differences in the stress response of Wistar-Kyoto (WKY) rats from different vendors. Physiol Behav. 1997;62:643–648. doi: 10.1016/s0031-9384(97)00191-1. [DOI] [PubMed] [Google Scholar]
- Pearson KA, Stephen A, Beck SG, Valentino RJ. Identifying genes in monoamine nuclei that may determine stress vulnerability and depressive behavior in Wistar-Kyoto rats. Neuropsychopharmacology. 2006;31:2449–2461. doi: 10.1038/sj.npp.1301100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer PN, Ganoczy D, Ilgen M, Zivin K, Valenstein M. Comorbid anxiety as a suicide risk factor among depressed veterans. Depress Anxiety. 2009;26:752–757. doi: 10.1002/da.20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A, Berton O, Mormede P, Chaouloff F. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behav Brain Res. 1997;85:57–69. doi: 10.1016/s0166-4328(96)00164-7. [DOI] [PubMed] [Google Scholar]
- Rittenhouse PA, Lopez-Rubalcava C, Stanwood GD, Lucki I. Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology. 2002;27:303–318. doi: 10.1016/s0306-4530(01)00052-x. [DOI] [PubMed] [Google Scholar]
- Sareen J, Cox BJ, Afifi TO, de Graaf R, Asmundson GJ, ten Have M, Stein MB. Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch Gen Psychiatry. 2005;62:1249–1257. doi: 10.1001/archpsyc.62.11.1249. [DOI] [PubMed] [Google Scholar]
- Servatius RJ, Jiao X, Beck KD, Pang KC, Minor TR. Rapid avoidance acquisition in Wistar-Kyoto rats. Behav Brain Res. 2008;192:191–197. doi: 10.1016/j.bbr.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Shephard RA, Broadhurst PL. Effects of diazepam and picrotoxin on hyponeophagia in rats. Neuropharmacology. 1982;21:771–773. doi: 10.1016/0028-3908(82)90063-6. [DOI] [PubMed] [Google Scholar]
- Sibille E, Sarnyai Z, Benjamin D, Gal J, Baker H, Toth M. Antisense inhibition of 5-hydroxytryptamine2a receptor induces an antidepressant-like effect in mice. Mol Pharmacol. 1997;52:1056–1063. doi: 10.1124/mol.52.6.1056. [DOI] [PubMed] [Google Scholar]
- Silva RM, Grossman HC, Hadjimarkou MM, Rossi GC, Pasternak GW, Bodnar RJ. Dynorphin A(1-17)-induced feeding: pharmacological characterization using selective opioid antagonists and antisense probes in rats. J Pharmacol Exp Ther. 2002;301:513–518. doi: 10.1124/jpet.301.2.513. [DOI] [PubMed] [Google Scholar]
- Sipols AJ, Bayer J, Bennett R, Figlewicz DP. Intraventricular insulin decreases kappa opioid-mediated sucrose intake in rats. Peptides. 2002;23:2181–2187. doi: 10.1016/s0196-9781(02)00246-2. [DOI] [PubMed] [Google Scholar]
- Solberg LC, Baum AE, Ahmadiyeh N, Shimomura K, Li R, Turek FW, Churchill GA, Takahashi JS, Redei EE. Sex- and lineage-specific inheritance of depression-like behavior in the rat. Mamm Genome. 2004;15:648–662. doi: 10.1007/s00335-004-2326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Terlecki LJ, Pinel JPJ, Treit D. Conditioned and unconditioned defensive burying in the rat. Learn Motiv. 1979;10:337–350. [Google Scholar]
- Treit D. A comparison of anxiolytic and nonanxiolytic agents in the shock-probe/burying test for anxiolytics. Pharmacol Biochem Behav. 1990;36:203–205. doi: 10.1016/0091-3057(90)90151-7. [DOI] [PubMed] [Google Scholar]
- Treit D, Pesold C. Septal lesions inhibit fear reactions in two animal models of anxiolytic drug action. Physiol Behav. 1990;47:365–371. doi: 10.1016/0031-9384(90)90155-w. [DOI] [PubMed] [Google Scholar]
- Treit D, Pinel JP, Fibiger HC. Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacol Biochem Behav. 1981;15:619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Reyes BA, Valentino RJ. The locus coeruleus: a key nucleus where stress and opioids intersect to mediate vulnerability to opiate abuse. Brain Res. 2009;1314:162–174. doi: 10.1016/j.brainres.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H, Schwarzer C. Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology. 2009;34:775–785. doi: 10.1038/npp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinner SH. Panic attacks precipitated by sertraline. Am J Psychiatry. 1994;151:147–148. doi: 10.1176/ajp.151.1.147b. [DOI] [PubMed] [Google Scholar]