Abstract
Plasma hydrophilization and subsequent hydrophobic recovery are studied for ten different polymers of microfabrication interest: polydimethylsiloxane (PDMS), polymethylmethacrylate, polycarbonate, polyethylene, polypropylene, polystyrene, epoxy polymer SU-8, hybrid polymer ORMOCOMP, polycaprolactone, and polycaprolactone/D,L-lactide (P(CL/DLLA)). All polymers are treated identically with oxygen and nitrogen plasmas, in order to make comparisons between polymers as easy as possible. The primary measured parameter is the contact angle, which was measured on all polymers for more than 100 days in order to determine the kinetics of the hydrophobic recovery for both dry stored and rewashed samples. Clear differences and trends are observed both between different polymers and between different plasma parameters.
INTRODUCTION
Plasma treatments can be used to modify the surface properties of polymers and improve their performance in various applications. In particular, hydrophilization of polymers by oxygen or nitrogen plasmas has found wide use. In biomedical applications, plasma hydrophilization can improve the biocompatibility of polymers1, 2, 3 as well as affect the attachment density of cells4, 5, 6, 7 and the adsorption of proteins.8, 9 Capillary filling of polymer microfluidic channels10, 11, 12, 13 and structured substrates9, 14, 15 is also enhanced by hydrophilization, since most polymers in their native form are either hydrophobic or only slightly hydrophilic. Furthermore, plasma hydrophilization can be used for improved adhesion and bonding of polymers.16, 17, 18
Typically, polymer surfaces exposed to oxygen or nitrogen plasmas become more hydrophilic due to formation of high energy surface groups in reactions between the native surface groups of the polymer and the reactive plasma species.19, 20 In addition to surface chemistry, plasma treatment also often affects the surface topography,20, 21, 22, 23 which further enhances the effect of the surface chemistry on the contact angle. However, the hydrophilization is typically not stable, and either a partial or complete hydrophobic recovery is usually observed. While the hydrophobic recovery can sometimes be exploited to produce multiple different contact angles for experiments,12, 14, 24 typically the hydrophobic recovery is a significant drawback for most applications. On dry storage, the principal reasons for the recovery have been identified as being the reorientation of the surface layer, or the migration of the polymer chains from the bulk of the polymer to the surface or from the surface to the bulk.19, 25, 26, 27, 28, 29, 30 If the samples are stored or washed in a solvent, polymer fragments can also leave the surface by dissolving into the solvent.19, 25, 31
The plasma hydrophilization and hydrophobic recovery have been studied for many polymers, in both mechanistic and application oriented studies. However, while these studies have greatly illuminated the fundamental physics and chemistry of plasma treatments, as well as provided valuable applications for plasma treatments, comparisons between different polymers are not always easy, since most studies focus on only one or a few polymers, and the plasma reactor types, processing parameters and the surface characterization methods vary greatly between studies. Because of that, we report here the hydrophilization and hydrophobic recovery, as characterized by the contact angle, for ten different polymers of microfabrication interest, all treated in the same reactor and under the same experimental conditions. The purpose of the study is to help other researchers in choosing polymers for their applications, as well as help future studies of the hydrophilization and recovery mechanisms of various polymers.
Both oxygen and nitrogen plasmas, with two different treatment times, were tested for all samples, and the recovery is followed on samples exposed to air ambient as well as for samples that are rewashed after each measurement. The polymers chosen for the study were: (1) polydimethylsiloxane (PDMS), (2) polymethylmethacrylate (PMMA), (3) polycarbonate (PC), (4) polyethylene (PE), (5) polypropylene (PP), (6) polystyrene (PS), (7) epoxy polymer SU-8, (8) hybrid polymer ORMOCOMP, (9) polycaprolactone (PCL), and (10) a branched poly(ɛ-caprolactone/D,L-lactide)copolymer with 70/30 monomer ratio (P(CL/DLLA)).
EXPERIMENTAL
Polymers
PDMS (Sylgard 184, Dow Corning) was prepared by mixing a 10:1 ratio of prepolymer to curing agent, casting the mixture on a Petri dish, and curing the PDMS for 3 h in an oven in 50 °C. PMMA (Foiltek Oy, Vantaa, Finland) and PC (Arla Plast AB, Borensberg, Sweden) were purchased as 1 mm and 2 mm thick sheets, respectively. PE (PE 300) and PP were purchased as 1 mm and 2 mm thick sheets, respectively (VINK Finland Oy, Kerava, Finland). The PS surfaces were the bottoms of plastic Petri dishes (Greiner Bio-One GmbH, Kremsmünster, Germany). SU-8 (SU-8 50 from Microresist Technology, Berlin, Germany) films were prepared on top of a silicon wafer by spin coating for 30 s at 9000 rpm, soft baked on a hot plate for 5 min at 65 °C and 8 min at 95 °C, exposed for 10 s (MA 6 mask aligner, Süss Microtech, Germany) and post exposure baked for 8 min at 95 °C. ORMOCOMP (ORMOCOMP® from Microresist Technology, Berlin, Germany) was prepared on top of silicon wafer by spin coating for 30 s at 4000 rpm, soft baking for 2 min at 95 °C, exposed for 5 s and baked for 5 min at 95 °C. Both the SU-8 and the ORMOCOMP layers were also developed (mr-Dev 600 and Ormodev, respectively, from Microresist) after preparation in order to render the initial surface properties similar to what would be found in a lithographically fabricated microfluidic device. PCL was synthesized in house by ring opening polymerization of ɛ-caprolactone (Solvay), with 1,4-butanediol (Acros Organics) as a co-initiator (0.3 mol. %) and stannous octoate (Sigma) as an initiator (0.01 mol. %). The polymerization was carried out for 6 h at 160 °C. The weight average molecular weight of the PCL was 60 000 g/mol. Discoid specimens (thickness 2 mm, diameter 6 mm) of PCL were then compression moulded (Fontijne TP400) at 80 °C for plasma treatments. P(CL/DLLA) was prepared as reported previously32 and cast 2 mm thick on a plate and cured in a Triad 2000 light curing system (350–550 nm, DeguDent) for 30 min on both sides.
Plasma treatments
All plasma treatments were carried out by Plasma System 400 batch reactor (PVA Tepla AG, Kirchheim, Germany), which uses microwave (2.45 GHz) generated plasma to activate the surface. Both oxygen and nitrogen plasmas were used, with the gas flows being 800 ml/min for both O2 and N2 precursors. The microwave power was held constant at 500 W. Two treatment times, 1 min and 10 min were tested for both plasmas.
Contact angle measurements
Static contact angles of the samples were monitored by sessile droplet contact angle goniometry (Cam-101, KSV Instruments Ltd, Helsinki, Finland). A water droplet of 1-2 μl was gently brought into contact with the surface using a precision pipette mounted on a movable stage, and the contact angle was measured immediately after the droplet stopped spontaneously advancing on the surface. The reported contact angles are the averages of three repeat measurements. Contact angles near the limit of complete wetting could not be reliably measured, and an arbitrarily chosen value of 5° was used as the contact angle in these cases. Pooled standard deviations of the contact angle measurements were calculated for all polymers and they were less than 4° for all polymers.
The contact angles were measured immediately before and after the plasma treatments to measure the effect of the treatment, as well as multiple times after the treatment to measure the hydrophobic recovery. The hydrophobic recovery was monitored under two different conditions, similar to Walther et al.20 The first set was measured from previously unused surface to simulate long term storage in a shelf, while the second set was rewashed after each measurement in order to simulate constant use and reuse in a laboratory. The washing was done by immersing the sample in isopropanol for 10 s, then immersing the sample in deionized water for 10 s, and blowing the sample dry by a nitrogen pistol.
Samples
Two plasmas, two treatment times, and two storage methods produced a total of eight samples for each of the ten polymers. Table TABLE I. presents the nomenclature used for naming the samples.
TABLE I.
Sample parameters used in the study.
| Sample | Plasma | Time (min) | Storage |
|---|---|---|---|
| O2-long-dry | O2 | 10 | Dry stored |
| O2-long-wash | O2 | 10 | Rewashed |
| O2-short-dry | O2 | 1 | Dry stored |
| O2-short-wash | O2 | 1 | Rewashed |
| N2-long-dry | N2 | 10 | Dry stored |
| N2-long-wash | N2 | 10 | Rewashed |
| N2-short-dry | N2 | 1 | Dry stored |
| N2-short-wash | N2 | 1 | Rewashed |
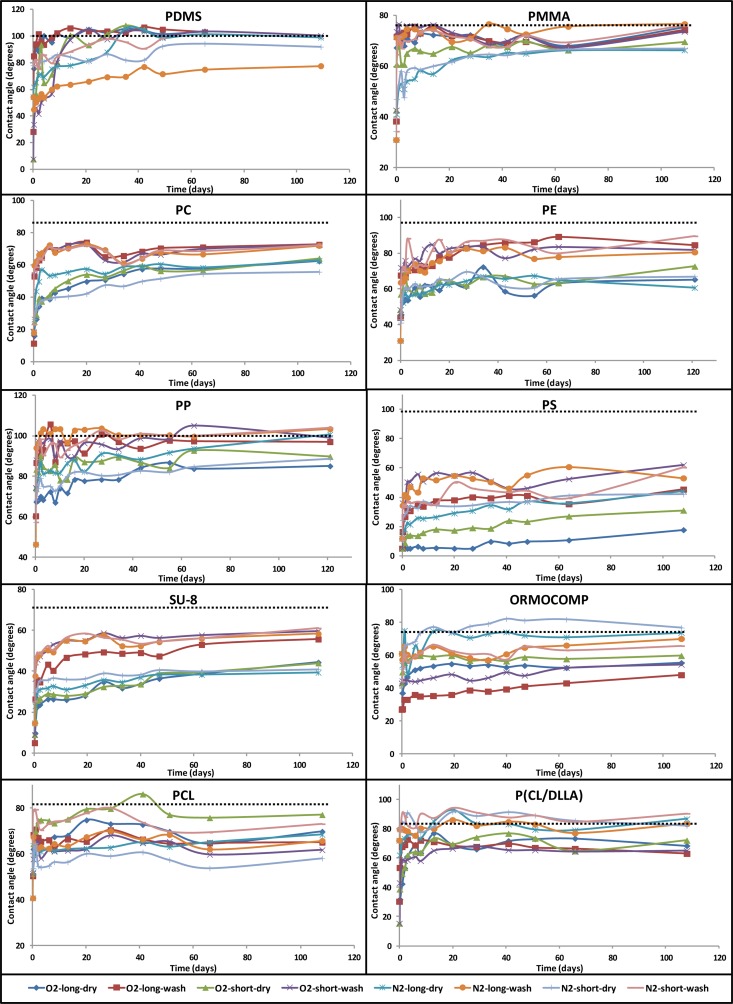
POLYMER CONTACT ANGLES
PDMS
The hydrophilization of PDMS, which has a native contact angle in the 100°–110° range, by oxygen plasma has been widely studied.3, 19, 27, 28, 30, 33, 34, 35 A near universal finding is that the oxygen plasma treatment initially brings the contact angle to very hydrophilic values, after which a rapid and nearly complete hydrophobic recovery takes place for dry stored samples. The time scale for the recovery varies greatly between studies, from hours35 to days19, 27 and weeks.30, 34 Storing the PDMS surfaces permanently under water19 or solvent extraction of non cross linked low molecular weight species prior to plasma treatment13 can help to preserve the hydrophilicity for longer times. Hydrophilization of PDMS by nitrogen plasma is less studied. Williams et al.2 report contact angles of 60° after a nitrogen plasma treatment, with a recovery back to 100° in a month. Owen and Smith28 report that completely wetting PDMS could be obtained by nitrogen plasma treatments, but do not elaborate further.
The native contact angle of our PDMS surfaces was 100° and the results of contact angle measurements of plasma treated samples are shown in Figure 1. Initially, the shorter oxygen plasma treatment produced the most hydrophilic PDMS (θ ≈ 10°), followed by the longer oxygen plasma treatment (θ ≈ 30°). Nitrogen plasma treated PDMS was not as hydrophilic, but the recovery was slower than that of oxygen plasma treated PDMS. The shorter treatment time was also preferable in the case of nitrogen, which supports the conclusion of Owen and Smith28 that longer treatment times cause cracking of PDMS surface, which is a detriment to the hydrophilization effect. Ultimately, by around day 50, most of the samples had recovered completely to their native contact angle, which is a well known characteristic of plasma based PDMS hydrophilization. There was no consistent difference between the dry stored and rewashed samples in the recovery behavior. Curiously, two of the nitrogen plasma treated samples did not recover quite to the same extent as the other samples, but even in those cases, the hydrophobicity recovered significantly. Overall, PDMS was a polymer that had some of the lowest contact angle values immediately after the treatment, but also one of the polymers that had the most complete hydrophobic recoveries.
Figure 1.
Contact angle evolution of the polymers. The dashed line shows the native contact angle. The error bars have been omitted for clarity.
PMMA
The surface modification of PMMA by oxygen plasma treatment has been used in various applications to improve the surface wettability and adhesion properties.6, 7, 8, 9, 21, 23, 36 Several studies9, 21, 23 have demonstrated that longer treatment times induce more hydrophilic surfaces. Similar results have been observed for higher plasma powers as compared with lower ones,6 implicating that the higher the plasma dose the lower the measured contact angle. Some groups have also investigated the aging behaviour of oxygen plasma treated PMMA surfaces for different periods of times. Both Schmalenberg et al.8 and Tsougeni et al.9, 23 reported that the hydrophobic recovery begun almost immediately after relatively short exposure (30 s-5 min). On the other hand, longer treatments (20 min-60 min) were reported to make the surface superhydrophilic for several days. However, after 120 days the samples with longer treatment had recovered back to 70°, while the samples with shorter treatment stabilized to just under 60°.
The native contact angle of our PMMA surfaces was 77° and the results of contact angle measurements of plasma treated samples are shown in Figure 1. Initially, the PMMA samples treated with nitrogen plasma were more hydrophilic (θ ≈ 30°) than the samples treated with oxygen (θ ≈ 40°). Our experimental results do not replicate the result that higher doses lead to better hydrophilization, as the dose dependency in our case was rather weak and actually favoured shorter treatments. The hydrophobic recovery of the rewashed samples was greater than the recovery of the dry stored samples and the contact angles of all rewashed samples recovered to near the original level (θ ≈ 75°) in approximately 10 days. Slightly less recovery was observed among dry stored samples and the dry stored nitrogen and oxygen plasma treated surfaces stabilized around 65° and 70°, respectively. Overall, we find that PMMA was one of the polymers with least stable hydrophilization, which also agrees well with previous studies.
PC
Larsson and Dérand37 studied the hydrophilization of PC with oxygen plasma using different plasma intensities and observed the stability of the surface wettability for 200 days. Although the contact angles (θ ≈ 5°) measured immediately after the exposure did not differ remarkably between different plasma conditions, the aging behaviour varied significantly. The least recovery was observed for the highest plasma intensities (around 15° after 200 days) and the most recovery was observed for lowest plasma intensities (around 60° after 200 days). Washing effect was also investigated by immersing the plasma treated samples in diluted ethanol solution. In this case, the intensity of the plasma had significant effect and the contact angle varied from 5° to 60° after the washing step, but the recovery over time was not reported. PC has also been treated with nitrogen plasma17 and the hydrophobic recovery was observed up to 12 months, and the contact angle was reported to stabilize around 40°.
The native contact angle of our PC surfaces was 85° and the results of contact angle measurements of plasma treated samples are shown in Figure 1. The longer oxygen plasma treatment made the surface most hydrophilic (θ ≈ 15°) immediately after the exposure, whereas the shorter nitrogen plasma was the least effective but still produced very hydrophilic PC (θ ≈ 25°). These results are in qualitative agreement with previous studies, which also report very hydrophilic PC surfaces immediately after the plasma treatment. Very rapid recovery was observed for all rewashed samples and the final level (θ ≈ 75°) was reached in 20 days. Dry stored samples underwent much slower hydrophobic recovery and the contact angles seemed to still be increasing after 110 days except for the shorter nitrogen plasma treated sample, which seemed to have stabilized to 55°.
PE
Hydrophobic recovery of PE surface after oxygen plasma treatment has been investigated by Behnisch et al.38 The PE surface was treated with the plasma and the hydrophobic recovery at room temperature was followed for several days. The native contact angle of the surface was measured to be 106° and the contact angle immediately after the treatment was around 40°. In 18 days, the contact angles had recovered to around 60°.
The native contact angle of our PE surfaces was 95° and the results of contact angle measurements of plasma treated samples are shown in Figure 1. For PE the longer nitrogen plasma treatment produced the most hydrophilic surface (θ ≈ 30°) while 10°-20° higher contact angles were observed for samples treated with other plasmas. Longer treatment time was beneficial also in the case of oxygen plasma. There was a clear difference in the intensity of the hydrophobic recovery between the rewashed and dry stored samples, so that the rewashed samples recovered almost completely to 80°–90°, while the dry stored samples recovered only partially to 60°–70°. Overall, our results agree reasonably well with those of Behnisch et al.38
PP
Hydrophilization of PP surface has been studied by Morra et al.26 The polymer samples were treated with oxygen plasma and the contact angles were measured as a function of aging time and temperature. At room temperature, the contact angles decreased from the native 95° to 24° immediately after the treatment. However, the recovery was relatively fast and after a day the measured contact angle was already 60°, and a complete recovery was observed after 16 days. Another study38 reported somewhat different results: the native contact angle of PP was determined to be 116° and the contact angle immediately after an oxygen plasma treatment was around 80°. Interestingly, the surface did not seem to suffer from the hydrophobic recovery at all when stored at room temperature for 18 days.
The native contact angle of our PP surfaces was 101° and the results of contact angle measurements of plasma treated samples are shown in Figure 1. Initially, the lowest contact angles are measured from samples treated with the longer nitrogen plasma (θ ≈ 45°). However, it is interesting to note that the very same longer nitrogen plasmas samples (both rewashed and dry stored) undergo the most significant hydrophobic recovery and finally they reach the level of native PP surface. The other samples show initial contact angles of only 60°–75°, but the recovery is also slower. All rewashed samples eventually reach levels similar to the native contact angle, but the dry stored oxygen plasma and lower duration nitrogen plasma samples retained some hydrophilization even after 100 days, but even there the contact angle recovered to around 85°. Both the initial and final contact angle results of our oxygen plasma treated sample lie in-between the results reported in the aforementioned studies.
PS
Wetting properties of oxygen plasma treated PS surfaces have been studied by several groups.19, 25, 37, 39 Murakami et al.19 report that PS contact angles decreased from native 92° to 7° during oxygen plasma treatment, but these recovered to 64° after a methanol washing step after the treatment, while the hydrophobic recovery of samples stored in a nitrogen gas atmosphere was more moderate. Larsson and Derand37 reported completely wetting PS surfaces after oxygen plasma treatment. With higher plasma intensities, the hydrophobic recovery was moderate and after 200 days the contact angles were still below 20°, while contact angles of the samples with less intensive plasma treatment recovered to above 45° during the same time period.
The native contact angle of our PS surfaces was 98° and the results of contact angle measurements of plasma treated samples are shown in Figure 1. For PS, the oxygen plasma treatment was clearly the most efficient way to make the surface hydrophilic and complete wetting was observed on those surfaces for both treatment times. The level of recovery of the dry stored oxygen plasma treated samples was the least of all the samples in this study and at the end of the experiment the contact angles of the longer and shorter oxygen plasma treated samples had recovered to around 18° and around 30°, respectively. These results agree well with previous reports of highly hydrophilic oxygen plasma treated PS surfaces. The initial contact angles of nitrogen plasma treated surfaces were around 10° and after 100 days the contact angles of dry stored and rewashed samples had recovered to around 42° and 60°, respectively. Overall, PS was the material which kept its hydrophilicity for the longest time, although most of the samples seem to still be ongoing slow hydrophobic recovery after 100 days.
SU-8
Walther et al.20 studied the hydrophilization of SU-8 by oxygen plasma and the hydrophobic recovery of both dry stored surfaces and rewashed surfaces. They report a native contact angle of 74° and the samples became extremely hydrophilic, showing contact angles of less than 5° immediately after the treatment. For dry stored samples, relatively mild recovery was observed, with the contact angles recovering to around 10° in a week and to around 20° in ten weeks. On the other hand, samples rewashed after each measurement rapidly recovered to around 35° in a week, after which the recovery proceeded to around 45° in 61 days.
The native contact angle of our SU-8 surfaces was 72° and the results of contact angle measurements of plasma treated samples are shown in Figure 1. The initial contact angles ranged from 5° to 25°, with oxygen precursors and higher plasma doses leading to more hydrophilic surfaces. The contact angles of dry stored surfaces recovered first to 25°–35° in a week, after which the contact angles further recovered to final values of around 40° in 100 days. The extent of recovery on the rewashed samples was greater, and the contact angles recovered to around 50° in a week and to 60° in 100 days. Overall, our results are in good agreement with those of Walther et al. and show that SU-8 can be made almost permanently hydrophilic by oxygen and nitrogen plasmas.
ORMOCOMP
ORMOCOMP belongs to a class of inorganic-organic hybrid polymers called ORMOCERs, which consists of an inorganic silica network backbone, with organic cross linking units and functional moieties.40 Applications of ORMOCERs include abrasion resistant transparent coatings,40 barrier layers for food packaging,40 elements for optical applications,40 microchip capillary electrophoresis,15, 41 and surface assisted laser desorption ionization mass spectrometry.42 We have recently studied the hydrophilization and pore formation process of ORMOCOMP by an oxygen plasma process using a different oxygen plasma process (reactive ion etching).43 In that study, we found that the contact angles of all but the most clearly porous samples recovered to around 45 in three weeks, while the strongly porous samples retained complete wetting during that period.
The native contact angle of our ORMOCOMP surfaces was 72° and the results of contact angle measurements of plasma treated samples are shown in Figure 1. Oxygen plasma treated ORMOCOMP surfaces had initially contact angles of 25°–40°, while the nitrogen plasma surfaces had contact angles around 40°-60°. A longer plasma treatment produced a more hydrophilic surface for both plasmas. A salient feature of ORMOCOMP is the slow pace of the hydrophobic recovery. Most of the samples were still in the process of slowly recovering after 100 days from the measurements. Interestingly, in the case of ORMOCOMP, the effect of rewashing the surfaces seemed to stabilize the hydrophilicity instead of hastening the recovery like was observed for most polymers. The oxygen plasma treated samples had contact angles of around 50° after 100 days, consistent with the less porous samples of our previous study.43 The nitrogen plasma treated samples had recovered completely to around 70° during 100 days. Compared with our previous study,43 we do not find as stable hydrophilization due to the fact that the samples of this study are nonporous.
PCL
PCL is a biodegradable polymer that is used in biomedical applications such as implants and tissue engineering scaffolds. Hirotsu et al.44 studied oxygen plasma treatment and hydrophobic recovery of PCL. They report native contact angles of 70°, which was reduced to 45°–50° after a minute long plasma treatment. The contact angles recovered to 60°–65° in a few days.
The native contact angle of our PCL surfaces was 80° and the results of contact angle measurements of plasma treated samples are shown in Figure 1. Initially, the higher plasma dose nitrogen treated sample had contact angles around 40°, both oxygen plasma treated samples had contact angles around 50°, and the lower plasma dose nitrogen treated sample had contact angles around 60°. From there, the contact angles of most samples rapidly increased by 20° in a few days. Our results thus agree quite closely with those of Hirotsu et al.44 Overall, the hydrophobic recovery on all PCL samples was only partial, but did not follow any easily identifiable pattern along the lines of the experimental parameters studied.
P(CL/DLLA)
PCL can be crosslinked with PDLLA in order to tune the mechanical properties of the polymer,32 and the resulting copolymers have been used as a tissue engineering scaffolds.45, 46 Plasma surface modifications of the copolymers have not been studied before.
The native contact angle of our P(CL/DLLA) surfaces was 82° and the results of contact angle measurements of plasma treated samples are shown in Figure 1. The oxygen plasma treated samples initially became very hydrophilic, having contact angles in the 15°–30° range. The oxygen plasma treated samples partially recovered to final values of around 65° in 10 days, without any major differences between plasma doses or storage method. On the other hand, P(CL/DLLA) had the strongest plasma composition dependency of all the polymers and was almost totally unaffected by nitrogen plasma, and even the initial values of nitrogen plasma treated samples were close to the native contact angle.
CONCLUSIONS AND SUMMARY
Clear differences in the contact angles were observed both between different experimental parameters and between different polymers. Compared with the existing literature, our results are overall in a good qualitative agreement with the majority of studies, while the exact contact angle values can vary significantly. In our study, all of the polymers were treated identically, making both quantitative and qualitative comparisons between various polymers easier. The results of our experiments are summarized in Table TABLE II. and below.
TABLE II.
Summary of the contact angle measuremens. The contact angle range shows the lowest and highest contact angle for that polymer among all the experimental parameters.
| Contact angle range after plasma process | |||||
|---|---|---|---|---|---|
| Polymer | Native contact angle | Immediately | 2 Days | 100 Days | Notes (preferred parameters for hydrophilicity and noteworthy trends) |
| PDMS | 100° | 8°–78° | 42°–102° | 78°–104° | Shorter treatments; strong recovery. |
| PMMA | 77° | 31°–43° | 53°–72° | 66°–77° | N2 plasma; strong recovery. |
| PC | 85° | 11°–23° | 34°–68° | 56°–73° | No clear trend between plasmas; rewashed samples recover more; moderate recovery. |
| PE | 95° | 31°–48° | 54°–76° | 61°–90° | Long treatments; rewashed samples recover more; dry stored moderate recovery. |
| PP | 101° | 46°–74° | 70°–101° | 85°–104° | No clear trend between plasmas; rewashed samples recover more. |
| PS | 98° | 5°–12° | 5°–50° | 18°–62° | O2 and long treatments; very hydrophilic after treatment; least recovery. |
| SU-8 | 72° | 5°–25° | 24°–48° | 39°–61° | Long treatments; rewashed samples recover more; dry stored only moderate recovery. |
| ORMOCOMP | 72° | 27°–61° | 33°–66° | 48°–77° | O2 and long treatments, rewashed samples recover less; slow pace of recovery. |
| PCL | 80° | 41°–65° | 55°–74° | 58°–77° | No clear trend between plasmas. |
| P(CL/DLLA) | 82° | 15°–80° | 53°–88° | 63°–91° | Very hydrophilic after O2 plasma; almost no effect for N2 plasma. |
Oxygen/nitrogen plasma of dry stored samples
The polymers were split between whether oxygen or nitrogen plasma produced a better hydrophilization (lower initial and final values). Comparing only the dry stored samples, oxygen plasma was superior to nitrogen plasma for PS, ORMOCOMP, and P(CL/DLLA), while the opposite was true for PMMA. For PDMS and SU-8, the initial contact angles of oxygen plasma treated samples were superior to the nitrogen plasma treated samples, but during the recovery, this reversed and the final values of the nitrogen samples were lower. No major differences between the plasmas were noticed for PC, PE, PP, and PCL.
Long/short treatment
Whether the 10 min or the 1 min treatment produced better hydrophilization was highly polymer specific. For PE, PS, SU-8, and ORMOCOMP, the longer treatments were preferable for both plasmas, while for PDMS the shorter treatments were superior. In the case of PC, PP, and PCL, the longer treatment was better for oxygen and the shorter treatment for nitrogen plasmas. For PMMA, the shorter treatment time was better for oxygen plasma and there was no big difference for nitrogen. For P(CL/DLLA) both treatment times produced similar results for both plasmas.
The extent of hydrophobic recovery of dry stored samples
There were clear differences in the extent of the hydrophobic recovery between the polymers. Comparingonly the dry stored samples, the polymers that exhibited the least hydrophobic recovery were in order PS, SU-8, PE, and PC and the final contact angles for these polymers ranged from 80° (for PS) to 20° (for PC) lower than the native contact angles. PCL and PP for both plasmas and ORMOCOMP and P(CL/DLLA) treated with oxygen plasma, were intermediary cases that had 10°-20° of the hydrophilization effect remaining after 100 days. Almost a complete recovery (less than 10° of the original hydrophilization remaining) after 100 days was observed for PDMS and PMMA as well as nitrogen plasma treated ORMOCOMP and P(CL/DLLA)samples.
Dry stored/rewashed samples
For most polymers (PMMA, PC, PE, PP, PS, SU-8), the hydrophobic recovery of the rewashed samples was more rapid and more complete than the recovery of the dry stored samples. In the case of PDMS, PCL, and P(CL/DLLA), there was not a big effect either way, and for ORMOCOMP, the rewashed samples actually kept their hydrophilicity better than the dry stored samples.
Most hydrophilic polymers initially and after 100 days
The most hydrophilic polymers immediately after the treatment (contact angles below 20° for some combination of parameters) were: PDMS, PS, PC, SU-8, and P(CL/DLLA). The most hydrophilic polymers after the 100 days recovery period (contact angles below 50°) were: PS, SU-8, and ORMOCOMP.
ACKNOWLEDGMENTS
The authors thank Minna Malin for preparation of PCL and P(CL/DLLA) samples. P.S. received financial support from the Graduate School of Chemical Sensors and Microanalytical Systems (CHEMSEM). The authors acknowledge funding from The Academy of Finland Project No. 123708.
References
- Chu P. K., Chen J. Y., Wang L. P., and Huang N., Mater. Sci. Eng. R. 36, 143 (2002). 10.1016/S0927-796X(02)00004-9 [DOI] [Google Scholar]
- Williams R. L., Wilson D. J., and Rhodes N. P., Biomaterials 25, 4659 (2004). 10.1016/j.biomaterials.2003.12.010 [DOI] [PubMed] [Google Scholar]
- Rhodes N. P., Wilson D. J., and Williams R. L., Biomaterials 28, 4561 (2007). 10.1016/j.biomaterials.2007.07.008 [DOI] [PubMed] [Google Scholar]
- Dewez J., Lhoest J., Detrait E., Berger V., Dupont-Gillain C., Vincent L., Schneider Y., Bertrand P., and Rouxhet P., Biomaterials 19, 1441 (1998). 10.1016/S0142-9612(98)00055-6 [DOI] [PubMed] [Google Scholar]
- Khorasani M.T. and Mirzadeh H., Radiat. Phys. Chem. 76, 1011 (2007). 10.1016/j.radphyschem.2006.10.002 [DOI] [Google Scholar]
- Ozcan C., Zorlutuna P., Hasirci V., and Hasirci N., Macromol. Symp. 269, 128 (2008). 10.1002/masy.200850916 [DOI] [Google Scholar]
- Khorasani M. T., Mirzadeh H., and Irani S., Radiat. Phys. Chem. 77, 280 (2008). 10.1016/j.radphyschem.2007.05.013 [DOI] [Google Scholar]
- Schmalenberg K. E., Buettner H. M., and Uhrich K. E., Biomaterials 25, 1851 (2004). 10.1016/j.biomaterials.2003.08.048 [DOI] [PubMed] [Google Scholar]
- Tsougeni K., Petrou P. S., Tserepi A., Kakabakos S. E., and Gogolides E., Microelectron. Eng. 86, 1424 (2009). 10.1016/j.mee.2008.11.082 [DOI] [Google Scholar]
- Delamarche E., Bernard A., Schmid H., Michel B., and Biebuyck H., Science 276, 779 (1997). 10.1126/science.276.5313.779 [DOI] [PubMed] [Google Scholar]
- Delamarche E., Bernard A., Schmid H., Bietsch A., Michel B., and Biebuyck H., J. Am. Chem. Soc. 120, 500 (1998). 10.1021/ja973071f [DOI] [Google Scholar]
- Jokinen V. and Franssila S., Microfluid. Nanofluid. 5, 443 (2008). 10.1007/s10404-008-0263-y [DOI] [Google Scholar]
- Vickers J. A., Caulum M. M., and Henry C. S., Anal. Chem. 78, 7446 (2006). 10.1021/ac0609632 [DOI] [PubMed] [Google Scholar]
- Jokinen V., Leinikka M., and Franssila S., Adv. Mater. 21, 4835 (2009). 10.1002/adma.200901171 [DOI] [PubMed] [Google Scholar]
- Aura S., Jokinen V., Sainiemi L., Baumann M., and Franssila S., J. Nanosci. Nanotechnol. 9, 6710 (2009). 10.1166/jnn.2009.1356 [DOI] [PubMed] [Google Scholar]
- Duffy D. C., McDonald J. C., Schueller O. J. A., and Whitesides G. M., Anal. Chem. 70, 4974 (1998). 10.1021/ac980656z [DOI] [PubMed] [Google Scholar]
- Hegemann D., Brunner H., and Oehr C., Nucl. Instrum. Methods Phys. Res. B 208, 281 (2003). 10.1016/S0168-583X(03)00644-X [DOI] [Google Scholar]
- Pandiyaraj K. N., Selvarajan V., Deshmukh R. R., and Gao C., Appl. Surf. Science 255, 3965 (2009). 10.1016/j.apsusc.2008.10.090 [DOI] [Google Scholar]
- Murakami T., Kuroda S., and Osawa Z., J. Colloid Interface Sci. 202, 37 (1998). 10.1006/jcis.1997.5386 [DOI] [Google Scholar]
- Walther F., Davydovskaya P., Zürcher S., Kaiser M., Herberg H., Gigler A. M., and Stark R. W., J. Micromech. Microeng. 17, 524 (2007). 10.1088/0960-1317/17/3/015 [DOI] [Google Scholar]
- Chai J., Lu F., Li B., and Kwok D. Y., Langmuir 20, 10919 (2004). 10.1021/la048947s [DOI] [PubMed] [Google Scholar]
- Collaud Coen M., Lehmann R., Groening P., and Schlapbach L., Appl. Surf. Sci. 207, 276 (2003). 10.1016/S0169-4332(02)01503-9 [DOI] [Google Scholar]
- Tsougeni K., Vourdas N., Tserepi A., Gogolides E., and Cardinaud C., Langmuir 25, 11748 (2009). 10.1021/la901072z [DOI] [PubMed] [Google Scholar]
- Extrand C. W., Moon S. I., Hall P., and Schmidt D., Langmuir 23, 8882 (2007). 10.1021/la700816n [DOI] [PubMed] [Google Scholar]
- Dupont-Gillain C. C., Adriaensen Y., Derclaye S., and Rouxhet P. G., Langmuir 16, 8194 (2000). 10.1021/la000326l [DOI] [Google Scholar]
- Morra M., Occhiello E., and Garbassi F., J. Colloid Interface Sci. 132, 504 (1989). 10.1016/0021-9797(89)90264-6 [DOI] [Google Scholar]
- Morra M., Occhiello E., Marola R., Garbassi F., Humphrey P., and Johnson D., J. Colloid Interface Sci. 137, 11 (1990). 10.1016/0021-9797(90)90038-P [DOI] [Google Scholar]
- Owen M. J. and Smith P. J., J. Adhes. Sci. Technol. 8, 1063 (1994). 10.1163/156856194X00942 [DOI] [Google Scholar]
- Oláh A., Hillborg H., and Vancso G. J., Appl. Surf. Sci. 239, 410 (2005). 10.1016/j.apsusc.2004.06.005 [DOI] [Google Scholar]
- Hillborg H., Ankner J. F., Gedde U. W., Smith G. D., Yasuda H. K., and Wikström K., Polymer 41, 6851 (2000). 10.1016/S0032-3861(00)00039-2 [DOI] [Google Scholar]
- Strobel M., Dunatov C., Strobel J. M., Lyons C. S., Perron S. J., and Morgen M. C., J. Adhes. Sci. Technol. 3, 321 (1989). 10.1163/156856189X00245 [DOI] [Google Scholar]
- Helminen A. O., Korhonen H., and Seppälä J. V., Macromol. Chem. Phys. 203, 2630 (2002). 10.1002/macp.200290039 [DOI] [Google Scholar]
- Zhu Y. and Petkovic-Duran K., Microfluid. Nanofluid 8, 275 (2009). 10.1007/s10404-009-0516-4 [DOI] [Google Scholar]
- de Menezes Atayde C.and Doi I., Phys. Status Solidi (c) 7, 189 (2010). 10.1002/pssc.200982419 [DOI] [Google Scholar]
- Ginn B.T. and Steinbock O., Langmuir 19, 8117 (2003). 10.1021/la034138h [DOI] [Google Scholar]
- Lim H., Lee Y., Han S., Cho J., and Kim K., J. Vac. Sci. Technol. A 19, 1490 (2001). 10.1116/1.1382650 [DOI] [Google Scholar]
- Larsson A. and Derand H., J. Colloid Interface Sci. 246, 214 (2002). 10.1006/jcis.2001.8032 [DOI] [PubMed] [Google Scholar]
- Behnisch J., Holländer A., and Zimmermann H., Surf. Coat. Technol. 59, 356 (1993). 10.1016/0257-8972(93)90112-2 [DOI] [Google Scholar]
- Morra M., Occhiello E., and Garbassi F., Die Angew. Makromol. Chem. 189, 125 (1991). 10.1002/apmc.1991.051890112 [DOI] [Google Scholar]
- Haas K. H., Adv. Eng. Mater. 2, 571 (2000). [DOI] [Google Scholar]
- Sikanen T., Aura S., Heikkila L., Kotiaho T., Franssila S., and Kostiainen R., Anal. Chem. 82, 3874 (2010). 10.1021/ac1004053 [DOI] [PubMed] [Google Scholar]
- Jokinen V., Aura S., Luosujärvi L., Sainiemi L., Kotiaho T., Franssila S., and Baumann M., J. Am. Soc. Mass Spectrom. 20, 1723 (2009). 10.1016/j.jasms.2009.05.013 [DOI] [PubMed] [Google Scholar]
- Aura S., Jokinen V., Laitinen M., Sajavaara T., and Franssila S., J. Micromech. Microeng. 21, 125003 (2011). 10.1088/0960-1317/21/12/125003 [DOI] [Google Scholar]
- Hirotsu T., Ketelaars A. A. J., and Nakayama K., Polym. Eng. Sci. 40, 2324 (2000). 10.1002/pen.v40:11 [DOI] [Google Scholar]
- Meretoja V. V., Malin M., Seppälä J. V., and Närhi T. O., J. Biomed. Mater. Res. 89A, 317 (2009). 10.1002/jbm.a.31980 [DOI] [PubMed] [Google Scholar]
- Malin M., Korventausta J., Meretoja V., and Seppälä J., Key Eng. Mater. 361–363, 395 (2008). 10.4028/www.scientific.net/KEM.361-363.395 [DOI] [Google Scholar]