Abstract
There is mounting evidence for the existence of an important relationship between telomeres and telomerase and cellular aging and cancer. Normal human cells progressively lose telomeres with each cell division until a few short telomeres become uncapped leading to a growth arrest known as replicative aging. In the absence of genomic alterations these cells do not die but remain quiescent producing a different constellation of proteins compared to young quiescent cells. Upon specific genetic and epigenetic alterations, normal human cells bypass replicative senescence and continue to proliferate until many telomere ends become uncapped leading to a phenomenon known as crisis. In crisis cells have critically shortened telomeres but continue to attempt to divide leading to significant cell death (apoptosis) and progressive genomic instability. Rarely, a human cell escapes crisis and these cells almost universally express the ribonucleoprotein, telomerase, and maintain stable but short telomeres. The activation of telomerase may be thought of as a mechanism to slow down the rate genomic instability due to dysfunctional telomeres. While telomerase does not drive the oncogenic process, it is permissive and required for the sustain growth of most advanced cancers. Since telomerase is not expressed in most normal human cells, this has led to the development of targeted telomerase cancer therapeutic approaches that are presently in advanced clinical trials.
Keywords: Aging, Replicative senescence, Immortalization, DNA damage, Stem cells, Evolutionary considerations
1. The Hayflick limit
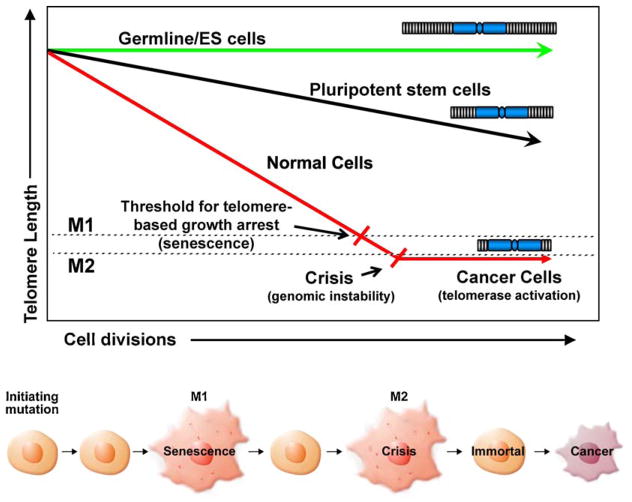
Almost exactly 50 years ago, Leonard Hayflick and his colleague Paul Moorhead discovered that cultured normal human cells have a limited capacity to divide, after which they stop growing, become enlarged, engaging a new pathway in what has been termed replicative senescence [1]. In 1961 this was totally unexpected since the research community firmly believed that cells explanted into cell culture were immortal. To support the idea that normal human cells had a limited number of divisions, Hayflick and Moorhead cultured separate populations of male and female human fibroblasts simultaneously. One was derived from a male that had been passaged in culture longer than the one derived from a female. The older male cells and younger female cells were plated at equal densities and subculture as required. When the “older” unmixed male-derived cell population stopped dividing, they investigated the mixed population and discovered that only female cells were present [1]. Besides providing additional evidence that cells have a limited replicative lifespan, this experiment demonstrated that the older cells had a molecular counting mechanism regardless of being surrounded by younger cells. Ultimately, these experiments demonstrated that a counting mechanism was somehow programmed into each cell and once this biological clock (as opposed to a chronological clock) had expired, the cell would stop dividing [2]. One additional observation that Hayflick reported is that cryogenically preserved cells remembered the number of times that they had divided at the time they were frozen [3,4]. Today, this withdrawal from the cell cycle after a certain number of cellular divisions (replicative senescence) is known to be triggered as a result of shortened telomeres [5]. Studies on replicative senescence have begun to provide valuable information towards our understanding of certain aspects of tissue and organismal aging and, additionally, have created new opportunities in the area of regenerative medicine for aging tissues and telomeropathies (genetic diseases due to premature telomere shortening). Equally important, cancer cells have evolved the ability to overcome senescence [6,7] by using mechanisms capable of maintaining telomere lengths (such as expressing telomerase), which enables cancer cells to divide indefinitely [7], a biomarker of almost all advanced human cancers (Fig. 1).
Fig. 1.
Certain male reproductive cells and embryonic stem cells retain full or almost full telomere length due to expression of telomerase activity. Pluripotent stem cells have regulated telomerase activity and thus they lose telomeres throughout life but at a reduced rate. Most somatic cell do not express telomerase activity and thus lose telomere length with each division at a faster rate until the cells uncap a few of their telomeres and undergo a growth arrest called replicative senescence. In the absence of cell cycle checkpoints (e.g. p53/pRB pathway), cells bypass senescence until they reach crisis. In crisis telomeres are so short that chromosome end fusions occur and there is increased genomic instability (probably due to chromosomal, breakage, fusion, bridge cycles). A rare cell that escapes crisis almost universally does so by reactivating telomerase and this cell can now become a cancer cell with limitless potential to divide. Almost all cancer cells have short telomeres and thus inhibitors of telomerase should drive such cancer cells into apoptotic cell death.
2. Telomeres and telomerase
Progressive telomere shortening from cell division (replicative aging) provides a barrier for tumor progression. However, one of the hallmarks of advanced malignancies is continuous cell growth and this almost universally correlates with the reactivation of telomerase [7]. Telomerase is a cellular reverse transcriptase (molecular motor) that adds new DNA onto the telomeres that are located at the ends of chromosomes [8]. Telomeres consist of many kilobases of TTAGGG nucleotide repeats [9] and an associated protein complex, termed shelterin [10]. The shelterin complex protects chromosome ends from end-to-end fusions and degradation forming special t-loop like structures [11] and thus masking the linear ends of chromosome from being recognized as single and/or double-strand DNA breaks [12].
The telomeric TTAGGG repeats shorten with each cell division due to the end replication problem, oxidative damage and other end processing events [12–16]. When a few telomeres become critically shortened there is a growth arrest state, at which time DNA damage signaling and cellular senescence is triggered [12]. In the absence of other changes, cells can remain in a quiescent/senescent state for years and this can be thought of as a potent anticancer protection mechanism for long-lived species such as humans. However, human tumor cells derived from carcinomas almost universally bypass cellular senescence and DNA damage signaling pathways. In human cell culture models, senescence bypass can be accomplished by abrogating important cell cycle checkpoint genes (such as TP53, p16INK4a and pRb), leading to extended growth of the pre-malignant cells eventually leading to crisis [17]. Crisis (Fig. 1) is a period where cell growth and death are in balance. Due to chromosome end fusions, there are chromosome breakage-fusion-bridge events, leading to genomic instability, rearrangements of chromosomes, and eventually engagement of telomerase. Telomerase, is detected in approximately 90% of all malignant tumors [7], may predict poor or favorable outcome [18], thus making telomerase both a highly attractive biomarker and target for the development of mechanism-based cancer diagnostics, prognostics, and therapeutics.
3. Telomerase therapeutics
Therapy for patients with advanced cancer generally includes surgical tumor resection, intensive multimodal chemotherapy, radiation therapy, or a combination of these regimens. The ideal cancer treatment would specifically target cancerous cells and have little or no effect on normal cells. Because of the compelling correlation that most normal human cells are telomerase silent while telomerase activity is detected in nearly all cancers, telomerase has emerged as an almost universal target for cancer therapeutics. Since telomerase activity is absent from most human somatic cells, telomerase-based therapies should possess greater specificity, lower toxicity, and reduced side effects compared to conventional chemotherapeutic approaches. Thus, targeting telomerase is considered a novel approach to cancer therapeutics and in some instances therapy directed at telomerase has advanced to clinical trials to validate safety, to obtain maximum tolerable doses, and in some cases to determine efficacy as determined by progression free survival or overall survival.
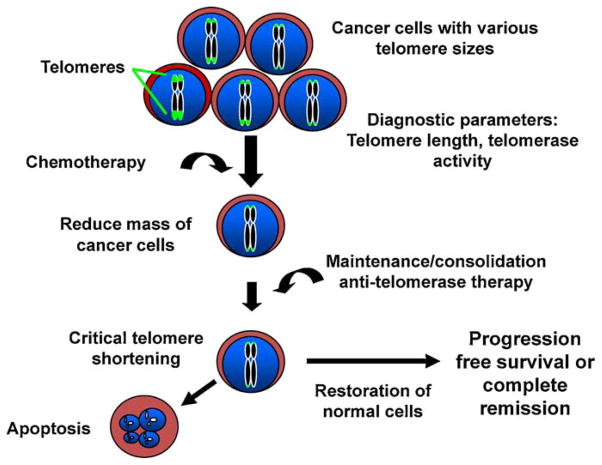
There are three general classes of agents that have been developed to target telomerase biology; gene therapy; immunotherapy; and small molecule inhibitors. The first approach uses either the proximal hTERT (telomerase catalytic protein component) promoter to make a general cancer-specific oncolytic virus or the hTR promoter (telomerase functional or template RNA component) to target a suicide gene therapy vector. The more advanced clinical trials include a telomerase-specific vaccine or immunotherapy currently in phase III trials for advanced pancreatic cancer; and the use of a small molecule oligonucleotide therapy that acts as a telomerase template antagonist (Imetelstat), currently in phase II clinical trials for breast cancer and non-small cell lung cancer. In the case of non-small cell lung cancer telomerase inhibition (Imetelstat) is being used in a maintenance/consolidation manner to prolong remissions after standard doublet chemotherapy. Under these conditions, the lung cancer patients that do well with standard therapy are randomized such that some will receive Imetelstat alone, some will receive an angiogenic inhibitor (Bevacizumab), and some patients may receive both Imetelstat and Bevaizumab (Fig. 2). Imetelstat is also being tested as a biomarker for cancer stem cell depletion in multiple myeloma [19–21 for recent reviews].
Fig. 2.
While most tumor specimens have short telomeres there is heterogeneity such that some cells may have slightly longer telomeres than others. After standard radiotherapy or chemotherapy a subset of residual cancer cells often regrow and become resistant to the initial therapy. In this setting, adding a telomerase inhibitor in the maintenance or consolidation phase of treatment may prolong progression free survival. Clinical trials are in progress to test this idea in humans with non small cell lung cancer following standard doublet chemotherapy.
4. Telomeres and telomerase in normal and cancer stem cells
Over twenty years ago, we speculated that most normal human cells lack telomerase activity and if cells lost critical cell-cycle checkpoint functions, the initial growth arrest (replicative senescence) could be bypassed permitting cells to continue to divide [6]. This was originally called extended lifespan by virologists who first identified that one of the important functions of DNA tumor viruses was to bypass senescence. We further predicted in 1991 that cells that bypass senescence eventually entered a second growth arrest state (crisis) when many shortened chromosome ends fuse, leading to chromosome bridge-breakage-fusion cycles almost universally leading to apoptosis. We proposed that in human cells these two mechanisms to restrict cell growth (senescence or M1 and crisis or M2) were at least initially potent anticancer protection mechanisms [17]. The final part of our hypothesis was that most human cells remain in this crisis period with cell growth being balanced by cell death until a rare cell acquires a mechanism, such as telomerase expression, that would maintain or lengthen telomeres thus permitting cell to continue to proliferate [6,17]. This rare cell that could maintain telomeres was then able to grow continuously (i.e. becomes immortal) and this is believed to be a critical step in cancer progression [22]. Cells that have escaped crisis generally have two defining hallmarks, telomere stability (generally at very short lengths) and reactivation of telomerase [15,23]. This suggests that the cancer stem (initiating) cell was likely to have very short telomeres when telomerase is reactivated, and recent evidence supports this idea [24,25]. In these studies cancer cells with stem-like markers have similar or shorter telomeres compared to the bulk of the tumor [24,25]. Thus, there may either be an advantage and mechanism to maintain subsets of cancer cells at very short telomere lengths or the length varies with differentiation state of tumor cells.
When telomerase is upregulated or reactivated in cells escaping crisis many outcomes could be predicted. For example, cells may not express enough telomerase and these cells would not be able to divide long-term to become robust malignant cancer cells. Another possibility is that telomerase would be expressed in excess and telomeres would be predicted to grow rapidly leading to long telomeres, but this is only rarely observed (less than 10% of primary cancers). Thus, there may be no selective advantage for cancer cells having more telomerase than is needed to maintain telomeres longer than that which provides protection against DNA-damage signaling/end-fusion. What is observed is that the vast majority of human cancer cells (~90%) have telomeres generally the same or shorter than adjacent normal tissues, providing evidence that telomerase is not in excess [26].
It is also generally believed that greatly shortened telomeres in initiated but still preneoplastic cells (while initially a potent anti-cancer protection mechanism) may eventually promote genomic instability and lead to the development of advanced disease. It is widely accepted that genetic instability drives malignant transformation and that most human cancer cells have grossly altered chromosome rearrangements. With only a few cellular alterations, the DNA damage signals from telomere shortening (telomere uncapping) would be predicted to be a very potent tumor suppressor pathway, since the “damage” could not be repaired in the absence of telomerase. Thus, replicative senescence is likely to initially stop cells from proliferating and progressing to cancer. This would certainly have an advantage in large long-lived species such as humans but may be less important in smaller and shorter lived animals (such as mice, see Section 6).
Proof that telomeres shortening and cellular aging are causally and not just correlatively related was provided in 1998 when Bodnar and coworkers [5] showed that introduction of the catalytic protein hTERT, activated telomerase activity in normal telomerase silent cells and was sufficient to bypass senescence leading to cell immortalization. It was further shown that ectopic expression of telomerase (hTERT) in pre-senescent human cells or in cells between senescence and crisis could be immortalized with only the ectopic introduction of hTERT, demonstrating that telomeres are mechanistically important in both senescence and crisis [5]. Finally, it was demonstrated that hTERT immortalized normal human cells were karyotypically normal and not transformed [27].
In the absence of intact critical checkpoint pathways, genomic instability occurs when telomeres are short, leading to end-to-end fusions, anaphase bridges, the development of aneuploidy, and eventually to telomerase reactivation. One possibility is that the re-expression or up-regulation of telomerase in cancer reduces the ongoing chromosomal instability that occurs in cells in crisis to a level compatible with viability. However, most benign or emerging cancer cells have several mutations including the reactivation of telomerase and there remains sufficient genomic instability to generate mutational evolution of the malignancy. In summary telomere shortening may be a common underlying cause of chromosomal rearrangements in cancer [28].
5. Telomerase in stem cells
Normal tissue stem cells are generally slowly cycling in vivo, and form large self-renewing colonies in vitro. Thus, there are many differences between normal stem cells in vivo and in vitro. The function of stem cells appears to change with increased age and this may be due in part to progressive telomere shortening. Stem cells also show progressive shortening of telomeres with increased age, while embryonic stem cells appear to fully maintain telomeres. This is believed to be due to fully active telomerase in embryonic stem cells that does not occur in stem cells of renewal tissues (Fig. 1). Thus, while proliferative descendants of some but not all normal stem cells have detectable telomerase activity, this activity is rarely sufficient to fully maintain telomere length. Very little is known about the regulation of telomerase in proliferative stem cells. Thus, a major difference between normal tissue stem cells and cancer cells is that normal tissue stem cells do not maintain stable telomere lengths while cancer cells do maintain stable telomere lengths. Normal tissue stem cells show progressive telomere shortening with increased age and telomerase is carefully regulated so that it is not continuously expressed. Thus, normal tissue stem cells are telomerase competent but mostly silent, while cancer stem cells almost universally constituitively expressing telomerase.
6. Comparative biology of telomeres
There is a general belief that something as fundamental to human tumor biology as telomere shortening and the reactivation of telomerase should be highly conserved and generally shared among mammals. Long-lived animals must protect the steady state (homeostasis) of their tissues by continuous replacement of the cells that regularly differentiate and die. An adult human contains approximately 1012 rapidly multiplying cells. During an approximate 80 year lifespan (~30,000 days), each person makes and discards an enormous number of cells from the bone marrow, skin, and gastrointestinal tract each day [29]. In the absence of a mechanism to deal with spontaneous rates of sporadic mutations, cancer in humans would be a lot more prominent and appear significantly earlier in life than already occurs. Mechanisms to minimize genomic damage are essential for large long-lived species. While much remains to be discovered, organ systems in large long-lived species must have evolved mechanisms that dramatically slow the rate of accumulation of replication errors (such as increased DNA repair). Alternatively, there could be DNA damage sensing mechanisms that increase cell turnover (e.g. apoptosis) rather to propagate an increased mutational load.
Based on a recent survey of telomere biology covering >60 mammalian species we now have a better conceptual framework for understanding the different uses of telomeres in different species [30,31]. Our results provide evidence that the ancestral mammalian phenotype had short telomeres and repressed telomerase, consistent with the hypothesis that the initial adaptation to homeothermy involved the adoption of replicative aging to compensate for the increased mutational load of elevated body temperatures. In addition, we observed that telomere length inversely correlates with lifespan while telomerase expression correlates with mass. The role of replicative aging as a tumor suppression mechanism is well accepted, however its contribution to lifespan remains controversial. The demonstration that telomere length inversely correlates with lifespan provides support for the interpretation that replicative aging is one of many factors contributing to lifespan in a large number of species. Also, the evidence that oxidative protection mechanisms are lower in species with long telomeres [31] suggests one evolutionary advantage of abandoning replicative aging in favor of long telomeres and not repressing telomerase in smaller mammals. These evolutionary studies now allow the role of telomeres in human cancer and aging to be put in the larger context of mammalian telomere biology.
The telomere shortening based tumor suppressor program is apparently not conserved in laboratory mice [32], which have long telomeres and constitutive telomerase in many tissues. While mice are much smaller and live only a few years compared to humans, they still get cancer at about the same incidence as humans. Thus, mice must have less well regulated telomerase and poorer oxidative damage protective mechanisms to explain these observations. However, more primary data are needed so a conceptual framework for understanding why most short lived animals have exceptionally long telomeres is obtained. For example, could exceptionally long telomeres provide some other advantage such as being G-rich in sequence to deal with increased oxidative damage?
To begin to address this central problem, we used phylogeny based statistical analyses to reconstruct mammalian ancestral states. The ancestral mammalian phenotype of short telomeres and repressed telomerase suggests the initial adaptation to the increased mutational load of homeothermy was the repression of telomerase and the consequent adoption of replicative aging. These studies demonstrated that telomere length inversely correlated with lifespan, while telomerase expression co-evolved with body size [31]. Multiple independent times smaller, shorter lived species changed to having long telomeres and expressing telomerase. Trade-offs involving reducing the energetic/cellular costs of specific oxidative protection mechanisms (needed to protect short telomeres in the absence of telomerase) is one explanation for abandonment of replicative aging. These observations now provide a new framework for understanding different uses of telomeres in mammals, support a role for short telomeres in allowing longer lifespans to evolve, demonstrate the need to include telomere length in the analysis of comparative studies of oxidative protection in the biology of aging, and identify which mammals might be valuable to use as appropriate model organisms for the study of the role of telomeres in human cancer and aging.
7. Conclusions
It is now widely accepted that replicative aging serves as a brake against malignancy. Cancer cells require multiple mutations to become malignant. Each mutation probably uses 20–40 divisions before achieving a population size sufficient for another spontaneous mutation to occur, so premalignant cells usually come up against the barrier of replicative senescence before accumulating enough mutated pathways to become frank malignancies. One critical step in oncogenesis involves the up-regulation or reactivation of telomerase in order to overcome this limit, and approximately 85–90% of all tumor biopsies are telomerase positive [5,23]. An outstanding problem for the future is to obtain an understanding of the regulation of human telomerase in normal and cancer cells. With a better understanding of telomerase, we may not only be able to target telomerase effectively in cancer treatment, but also utilize mechanisms to transiently upregulate telomerase in regenerative medicine to treat patients with diseases of premature stem cell depletion. To accomplish these goals, we need to know more about the mechanisms regulating self-renewal and differentiation in normal stem cells and in cancer stem cells and how these relate to telomeres and telomerase.
References
- 1.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–662. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Hayflick L. How and why we age. Exp Gerontol. 1998;33:639–65. doi: 10.1016/s0531-5565(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 3.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–36. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 4.Hayflick L. The coming of age of WI-38. Adv Cell Cult. 1984;3:303–16. [Google Scholar]
- 5.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–52. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 6.Shay JW, Wright WE, Werbin H. Defining the molecular mechanism of human cell immortalization. Biochim Biophys Acta. 1991;1072:1–7. doi: 10.1016/0304-419x(91)90003-4. [DOI] [PubMed] [Google Scholar]
- 7.Kim NW, Pietyszek MA, Prowse KR, Harley CB, West MD, Ho PLC, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–5. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 8.Collins K, Mitchell JR. Telomerase in the human organism. Oncogene. 2002;21:564–79. doi: 10.1038/sj.onc.1205083. [DOI] [PubMed] [Google Scholar]
- 9.Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;85:6622–6. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–10. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 11.de Lange T. T-loop and the origin of telomeres. Nat Rev Mol Cell Biol. 2004;5(4):323–9. doi: 10.1038/nrm1359. [DOI] [PubMed] [Google Scholar]
- 12.Shay JW. Telomerase therapeutics: telomeres recognized as a DNA damage signal. Clin Cancer Res. 2003;9:3521–5. [PubMed] [Google Scholar]
- 13.Wright WE, Shay JW. The two-stage mechanism controlling cellular senescence and immortalization. Exp Gerontol. 1992;27:383–9. doi: 10.1016/0531-5565(92)90069-c. [DOI] [PubMed] [Google Scholar]
- 14.Wright WE, Shay JW. Time, telomeres and tumors: is cellular senescence more than an anticancer mechanism? Trends Cell Biol. 1995;5(8):293–7. doi: 10.1016/s0962-8924(00)89044-3. [DOI] [PubMed] [Google Scholar]
- 15.Shay JW. Aging and cancer: are telomeres and telomerase the connection? Mol Med Today. 1995;1(8):378–84. doi: 10.1016/s1357-4310(95)93872-9. [DOI] [PubMed] [Google Scholar]
- 16.Harley CB. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991;256:271–82. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- 17.Wright WE, Pereira-Smith OM, Shay JW. Reversible cellular senescence: a two-stage model for the immortalization of normal human diploid fibroblasts. Mol Cell Biol. 1989;9:3088–92. doi: 10.1128/mcb.9.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langford LA, Piatyszek MA, Xu RS, Schold SC, Wright WE, Shay JW. Telomerase activity in ordinary meningiomas predicts poor outcome. Human Pathol. 1997;28(4):416–20. doi: 10.1016/s0046-8177(97)90029-0. [DOI] [PubMed] [Google Scholar]
- 19.Roig AI, Wright WE, Shay JW. Is telomerase a novel target for metastatic colon cancer? Curr Colorectal Cancer Rep. 2009;5:203–8. [Google Scholar]
- 20.Shay JW, Keith WN. Cellular immortality and cancer: from telomerase to cancer stem cells. BBA Mol Basis Dis. 2009;1792:227–8. [Google Scholar]
- 21.Buseman CM, Wright WE, Shay JW. Is telomerase a viable target in cancer? Mut Res. doi: 10.1016/j.mrfmmm.2011.07.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg R. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 23.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–91. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 24.Marian CO, Cho SK, Hatanpaa KJ, Wright WE, Maher E, Madden C, et al. The telomerase inhibitor GRN163L blocks telomerase activity, induces telomere attrition and limits GBM stem cell proliferation and in-vivo growth. Clin Cancer Res. 2010;16:154–63. doi: 10.1158/1078-0432.CCR-09-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marian CO, Wright WE, Shay JW. The effect of telomerase inhibition on prostate tumor-initiating cells. Int J Cancer. 2010;127:321–31. doi: 10.1002/ijc.25043. [DOI] [PubMed] [Google Scholar]
- 26.Hiyama K, Hiyama E, Shay JW. Telomeres and telomerase in humans, Chapter 1. In: Hiyama K, editor. Telomeres and Telomerase in Cancer. Cancer Drug Discovery and Development. Springer Press; 2009. pp. 3–21. [Google Scholar]
- 27.Morales CP, Holt SE, Ouellette M, Kaur KJ, Wilson KS, White MA, et al. Lack of cancer-associated changes in human fibroblasts after immortalization with telomerase. Nat Genet. 1999;21:115–8. doi: 10.1038/5063. [DOI] [PubMed] [Google Scholar]
- 28.Davoli T, Denchi EL, de Lange T. Persistent telomere damage induces bypass of mitosis and tetraploidy. Cell. 2010;141:81–93. doi: 10.1016/j.cell.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potten CS, Morris RJ. Epithelial stem cells in vivo. J Cell Sci. 1988;10(Suppl):45–62. doi: 10.1242/jcs.1988.supplement_10.4. [DOI] [PubMed] [Google Scholar]
- 30.Gomes NM, Shay JW, Wright WE. Telomere biology in Metazoa. FEBS Lett. 2010;584:3741–51. doi: 10.1016/j.febslet.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomes NMV, Ryder OA, Houck ML, Charter SJ, Walker W, Forsyth NR, et al. Comparative biology of mammalian telomeres: hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell. 2011;10(5):761–8. doi: 10.1111/j.1474–9726.2011.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright WE, Shay JW. Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nat Med. 2000;6:849–51. doi: 10.1038/78592. [DOI] [PubMed] [Google Scholar]