Abstract
Differences between normal adult tissue stem cells and cancer stem/initiating cells remain poorly defined. For example, it is controversial if cancer stem cells can become fully quiescent, require a stem cell niche, are better at repairing DNA damage than the bulk of the cancer cells, and if and how they regulate symmetric sversus asymmetric cell divisions. This minireview will not only provide our personal views to address some of these outstanding questions, but also present evidence that an understanding of telomere dynamics and telomerase activity in normal and cancer stem cells may provide additional insights into how tumors are initiated, and how they should be monitored and treated.
Keywords: Quiescence, Niche, Aging, Senescence, Immortal
1. Introduction
What do we really know about the differences between normal tissue stem cells and cancer stem (initiating) cells? While this is a complex question that covers many areas of previous and ongoing research, this review will focus on comparing and contrasting the role of telomeres and telomerase in normal and putative cancer stem (initiating) cells. Understanding the dynamics of telomeres and telomerase in normal and cancer stem cells may provide some additional insights into defining key differences between these cell types. Since the discovery of rare tumor cells with stem cell-like features, it has been proposed that these stem-like tumor cells are the primary cellular component within a tumor that drives disease progression and metastasis. The alternative to the cancer stem hypothesis is the clonal evolution hypothesis model that suggests tumor progression results from genetic variability within the original population of tumor cells that is permissive for more aggressive subtypes. While the cancer stem cell hypothesis has been difficult to prove, as it makes few predictions, there are some common elements that are generally accepted. In addition to their ability to self-renew and differentiate, cancer stem cells are also enriched in cells postulated to be resistant to conventional radiation and chemotherapy. While normal stem cells are chromosomally stable containing a normal diploid genome, cancer stem cells are almost always aneuploidy and have a significant number of chromosomal rearrangements. In addition, normal stem cells are generally quiescent or very slow growing, reside in a specific niche, and have relatively long telomeres compared to more differentiated somatic cells. In contrast, we find cancer cells expressing stem cell markers are not completely quiescent, and almost universally express cancer levels of telomerase. Importantly, we also find cancer stem/initiating cells have short telomeres (compared to normal stem cells which have relatively longer telomeres). The short telomeres in cancer stem cells may reflect the multistep nature of cancer initiation and progression. The immediate implications of this new tumor growth paradigm not only require a re-evaluation of how tumors are initiated, but also on how tumors should be monitored and treated.
2. Bypass of senescence and crisis to become a cancer initiating cell
Human telomeres consist of repetitive TTAGGG DNA sequences that associate with a series of telomere binding (shelterin) proteins [1] believed to provide genomic stability by protecting the linear chromosome ends from being recognized as DNA breaks needing repair. The inability of the DNA replication machinery to copy the extreme ends of chromosomes, often referred to as the end replication problem [2], is consistent with the observation that cells can lose telomeres without initially affecting cell function. Thus, almost all normal human cells including stem cells of renewal tissues show progressive telomere shortening with ongoing cell division until a subset of telomeres reach a critically shortened length and induce a DNA damage signal that is often referred to as replicative senescence or cell aging [3]. Thus, telomeres not only serve as chromosome ‘caps’ to protect chromosome ends from being recognized as DNA damage, but also serve as a gauge for the mitotic (replication) age of a cell. Telomerase, a RNA-containing enzyme that synthesizes DNA onto the ends of chromosomes, helps to maintain the integrity of the genome in embryonic stem cells and in proliferating progenitor cells derived from quiescent normal stem cells. Telomerase is silent in the vast majority of human tissues and is only expressed in a small number of normal cell types such as dividing male germ-line spermatocytes and a subset of proliferating somatic adult progenitor cells [4].
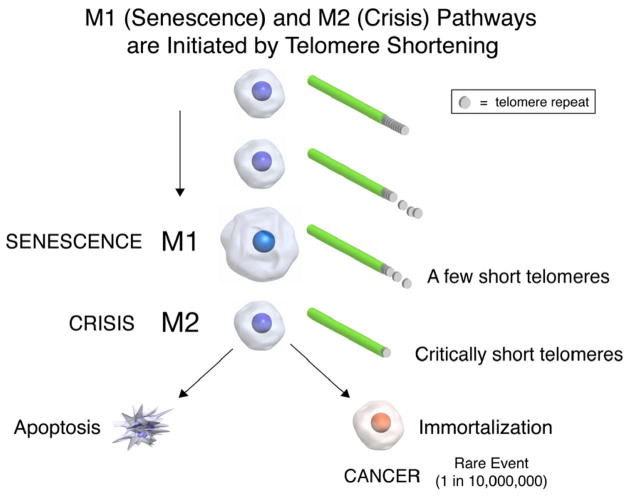
In 1991 we proposed a connection between telomeres, telomerase, aging and cancer [5]. The hypothesis put forth was that most normal human cells lack telomerase activity and their telomeres shorten with each cell division, until they enter replicative senescence (Fig. 1). Cells that lose critical cell cycle checkpoint functions escape this initial growth arrest (replicative senescence) and continue to divide (called extended lifespan by virologists who first identified that one of the important function of DNA tumor viruses is to bypass senescence). Cells that bypass senescence eventually enter a second growth arrest state (crisis) when many shortened chromosome ends fuse, leading to chromosome bridge-breakage-fusion cycles almost universally leading to apoptosis (Fig. 1). In human cells these two mechanisms to restrict cell growth (senescence and crisis) are at least initially potent anticancer protection mechanisms [6]. Most human cells remain in this crisis period with cell growth being balanced by cell death until a rare cell acquires a mechanism, such as telomerase expression, that can maintain or lengthen telomeres [5,6]. This rare cell that can maintain telomeres is then able to grow continuously (i.e. becomes immortal) and this is generally believed to be a critical step in cancer progression [7]. Cells that have escaped crisis generally have two defining hallmarks, telomere stability and reactivation of telomerase [8,9]. This suggests that the cancer stem (initiating) cell was likely to initially have very short telomeres and recent evidence supports this idea [10,11]. In these studies cancer cells with stem-like markers have similar or shorter telomeres compared to the bulk of the tumor [10,11]. There may thus either be an advantage and mechanism to maintain subsets of cancer cells at very short telomere lengths or the length varies with differentiation state of the tumor cells.
Fig. 1.
The M1 and M2 model of senescence and crisis. All normal human somatic cells have progressive shortening of telomeres with each cell division. This is also true in proliferative (transit amplifying) adult stem cells. When a few telomeres in a cell reach a shortened state, a DNA damage signal is initiated. This DNA damage signal indicates that the shortened telomeres is being sensed as uncapped or broken DNA. In cells that have bypassed the M1 senescent state by inactivation of important cell cycle checkpoint genes (e.g. TP53 and/or pRB), cells ignore the ongoing DNA damage signal and continue to divide until many telomeres are critically shortened. During this extended lifespan period, end associations occur eventually leading to breakage-fusion-bridge cycles resulting in M2 or a state of crisis. During crisis apoptotic cell death almost universally occurs. However, in a rare human cell (based on fluctuation analyses calculated to be about one in ten million cells) an immortalization event occurs. This cell has two characteristics, expression of telomerase and stabilization of telomeres.
When telomerase is upregulated or reactivated in cells escaping crisis many outcomes are possible. For example, there can be too little telomerase expressed and these cells may not be able to divide long-term and they are unlikely to become robust cancer cells. If telomerase is made in excess then telomeres would be predicted to grow rapidly leading to long telomeres, but this is only rarely observed (less than 10% of primary cancers). Thus, there may be no selective advantage for cancer cells having more telomerase than is needed to maintain telomeres longer than that which provides protection against DNA-damage signaling/end-fusion. What is observed is that the vast majority of human cancer cells have telomeres generally the same or shorter than adjacent normal tissues.
It is believed that greatly shortened telomeres in initiated but still preneoplastic cells (while initially a potent anti-cancer protection mechanism) may also promote genomic instability and lead to the development of advanced disease. It is widely accepted that genetic instability drives malignant transformation. With only a few cellular alterations, the DNA damage signals from telomere shortening (telomere uncapping) would be predicted to be a very potent tumor suppressor pathway, since the “damage” could not be repaired in the absence of telomerase. Thus, replicative senescence is likely to initially stop cells from proliferating and progressing to cancer. This would certainly have an advantage in large long-lived species such as humans but may be less important in short-lived animals (such as mice). Proof that telomeres shortening and cellular aging are causally and not just correlatively related was provided in 1998 when Bodnar and co-workers [12] showed that introduction of telomerase into normal telomerase silent cells was sufficient to bypass senescence, activate telomerase activity, and lead to cell immortalization. It was further shown that ectopic expression of telomerase (TERT) in pre-senescent cells or in cells between senescence and crisis could be immortalized with ectopic introduction of TERT, demonstrating that telomeres are mechanistically important in both senescence and crisis. In the absence of intact critical check point pathways, genomic instability occurs when telomeres are short, leading to end-to-end fusions, anaphase bridges, the development of aneuploidy, and eventually to telomerase reactivation. One possibility is that the re-expression or upregulation of telomerase in cancer reduces the ongoing chromosomal instability that occurs in cells in crisis to a level compatible with both viability and sufficient instability to generate mutational evolution of the malignancy. In summary telomere shortening may be a common underlying cause of chromosomal rearrangements in cancer.
3. Telomerase in stem cells
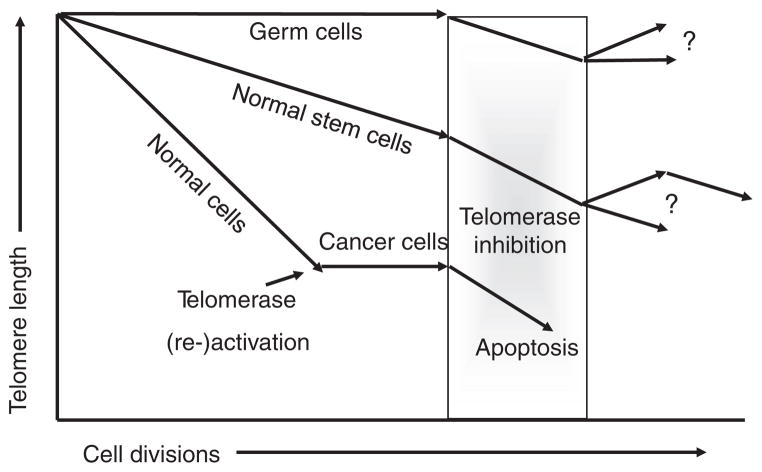
Normal tissue stem cells reside in microenvironmental niches that are tissues/organ specific. Stem cells are negative for differentiation markers, are not actively cycling in vivo, and generally form large self-renewing colonies in vitro. Thus, there are many differences between normal stem cells in vivo and in vitro. The function of stem cells appears to change with increased age and this may be due in part to progressive telomere shortening. Stem cells also show progressive shortening of telomeres with increased age, while embryonic stem cells appear to fully maintain telomeres. This is believed to be due to fully active telomerase in embryonic stem cells that does not occur in stem cells of renewal tissues (Fig. 2). Thus, while proliferative descendents of normal stem cells have detectable telomerase activity, this activity is rarely sufficient to fully maintain telomere length (Fig. 2). Very little is known about the regulation of telomerase in proliferative stem cells. Thus, a major difference between normal tissue stem cells and cancer cells is that in the latter but not the former, stable telomere length are maintained. Normal tissue stem cells show progressive telomere shortening with increased age and telomerase is carefully regulated so that it is not continuously expressed. Thus, normal tissue stem cells are telomerase competent but mostly silent, while cancer cells are almost universally telomerase expressing. There are no apriori reasons to assume that subsets of cancer cells (e.g. cancer stem cells) would maintain their telomeres differently from the bulk of the tumor, even though there are frequent comparisons made between normal stem cells and cancer stem cells as if cancer stem cells are simply derivatives of normal stem cells. This has led to many misconceptions that persist in the general scientific thought collective. Many of these comparisons are based on very marginal data and will be discussed further. Importantly, cancer cells expressing telomerase and forced to differentiate or to become quiescent, either undergo apoptosis or down regulate telomerase [13] suggesting that part of becoming a cancer cell may be the inability to efficiently undergo quiescence as do normal tissue stem cells. Cancer cells may lack the cell cycle checkpoint activities that allow them to completely growth arrest or there may not be a cancer specific niche as occurs in normal stem cells to allow cancer stem cells to become completely quiescent.
Fig. 2.
Changes in telomere length in germline cells, normal stem cells and preneoplastic somatic cells. Telomeres progressively shortening in normal stem and preneoplastic cells but not in proliferating male germline spermatocytes. When telomeres are very short in preneoplastic cells, a rare cell stabilizes its telomeres by upregulating or reactivating telomerase. This cell is likely to initially have very short telomeres and telomerase may be a mechanism to reduce the ongoing genomic instability that occurs when cells are in crisis at the time of immortalization. Thus, the bulk of tumor cells including cancer stem cells have much shorter telomeres compared to germline or normal stem cells. Robust telomerase inhibitors currently in clinical trials are likely to induce apoptosis in cancer cells before adversely affecting normal stem cell functions.
4. Background review of cancer stem cells
While the term “cancer stem cells” is still controversial, the general consensus is that these cells must have potent tumor initiation, self-renewal and differentiation capacity [14]. The evidence for this is that it is difficult to establish tumor cell lines even from metastatic lesions. It generally takes hundreds of thousands (if not millions) of established cancer cells to make a tumor in immunosuppressed mice and tumors that are initially clonal rapidly become heterogeneous. Thus it is believed that the vast majority of tumors do not have the characteristics of a cancer stem cell. One of the main concerns about the cancer stem cell hypothesis is that virtually all the work has involved transplanting human cancer cells into a variety of different types of immunodeficient mice. Thus, the experiments supporting the cancer stem cell hypothesis may not accurately reflect what happens during cancer initiation and progression in humans. In addition, the idea that only a very rare cell can initiate tumor formation has recently been challenged. In one study, approximately 25% of single melanoma cells from unselected melanoma cells isolated directly from patients were able to make tumors in NOD/SCID/interleukin-2 receptor gamma chain null mice [15]. If cancer stem cells are very rare as the hypothesis suggests, it is hard to explain how a large fraction of even single cells can reproducibly make tumors.
Even with these caveats, the tumor initiation aspect of cancer stem cells refers to the capacity of these cells to form tumors in immunocompromised mice using very small numbers of cells. Self-renewal capacity is tested by serial transplantation experiments, where re-isolated cancer stem cells can be transplanted in secondary and tertiary recipients. The differentiation ability of these cells does not refer to multilineage differentiation but rather to the capacity of the resulting tumors to be a phenocopy of the original tumor. An important characteristic of cancer stem cells is their ability to survive various therapies by activating anti-apoptotic pathways, increasing activity of membrane transporters and high DNA repair capacity [16,17]. The currently accepted definition of cancer stem cells does not imply the cell type from which these cells originated and thus the term the term tumor-initiating cells may be more appropriate. There are still many outstanding questions, such as when do cancer stem cells arise, are they ever completely quiescent, is there a cancer stem cell niche, and are cancer stem cells derived from normal stem cells or can transit amplifying cancer progenitor cells also revert to cancer stem cells?
5. Review of telomerase in cancer stem cells
Embryonic stem cells derived early in embryogenesis, are believed to proliferate by equal division where the two daughter cells produced by the division share the same stem cell characteristics. Later during organogenesis, tissue stem cells are believed to divide by unequal division where the two daughter cells differ in their characteristics such than one remains a stem cell and the other becomes a progenitor or transit amplifying cell. There is robust experimental evidence that progenitor or transit amplifying normal cells express high levels of telomerase while the remaining daughter stem cell rapidly becomes quiescent (e.g. not dividing or very slowly dividing) and does not express telomerase. How does this asymmetric cell division occur? What regulates symmetric cell division in embryonic stem cells but asymmetric cell division in normal tissue stem cells? What regulates telomerase activity in quiescent stem cells versus proliferating transit amplifying cells? While technological advances have made it possible to isolate stem and progenitor cells and there is a beginning description of the molecular characteristic of these various cell types, there is still much we do not know. Clearly the progenitor cells can undergo many divisions to eventually differentiate into the functional cells of the specific tissue but these are almost universally “end” cells that are eventually lost from the tissue after completing their physiological functions. In some tissues there are many levels of transit amplifying cells while in others there are not. The mass of a tissue is maintained by the balance between differentiated cell death and production of new transit amplifying cells. Some tissues turn over rapidly such as in the gastrointestinal tract and thus there is a high number of transit amplifying cells. In the brain there is almost no cell turnover and interestingly, the telomeres of neuronal cells do not change with increased age. The terminally differentiated cells of the brain and mature differentiated gastrointestinal cells also do not express telomerase activity.
6. Evidence for short telomeres in cancer stem cells
While there are many physical and cell surface markers that have been used to identify cancer initiating/stem cells none are universal and the biomarkers vary depending on the tissue of origin of the cancer. For example, in brain tumors, there is controversy over whether a single cell marker (such as CD133/Prominin 1) can identify the tumor-initiating population [14,16,17]. However, there is general agreement that brain tumors cells which can be propagated in vitro as non-adherent neurospheres and produce intracranial tumors retain the genotype and phenotype of the patient’s original tumor [18,19]. In a recent study Marian et al. [10] demonstrated that CD133+ primary glioblastoma mutiformi (GBM) cells could form neurospheres, were capable of make orthotopic tumors at low seeding numbers, and could differentiate into three different brain lineages. Neurospheres generated in vitro by primary GBM cells are enriched in stem/progenitor cells. This technique is based on the unique property of stem/progenitor cells to survive and grow in serum-free suspension, while more differentiated cells undergo anoikis and die in these conditions. Thus, these cells, by well accepted criteria, have the characteristics of cancer stem or initiating cells. The studies found that these putative glioma stem cells expressed telomerase. More importantly, not only were the telomere lengths of tumors shorter than normal brain telomeres but that the telomere length of GBM tumor-initiating cells expressing CD133 had even shorter telomeres (~3.5 kb), than the bulk of tumor cells. Thus, the average telomere lengths of GBM putative tumor stem cells were approximately three times shorter compared to normal human brain cells.
In another study examining the dye Hoechst 33342 dye exclusion marker called SP (side population), Ponti et al. [20] demonstrated that primary breast carcinoma-derived cultures were capable of self-renewal, extensive proliferation as clonal non-adherent spherical clusters, and could differentiate along different mammary epithelial lineages (ductal and myoepithelial). As with the GBM study, breast cancer-initiating cells in this study displayed a similar extent of telomerase activity as the bulk of the tumors. In addition, the telomere length was similar to adjacent non-cancerous tissue telomere length.
Using specific surface markers (CD44, integrin α2β1 and CD133), Hoechst 33342 dye exclusion, and holoclone formation, tumor initiating cells were isolated [11] from a panel of prostate cancer cell lines (DU145, C4-2 and LNCaP). Tumor cells with putative stem cell markers [21–25] were isolated from these cell lines and all had significant telomerase activity and the telomeres were of similar average length as the telomeres of the main population of cells. Holoclones (tightly packed round colonies of cells with distinct morphology) were also able to re-initiate tumor growth [23] and these had both telomerase and short telomeres [11]. Finally in a series of studies involving multiple myeloma both CD138+ plasma cells and CD138− precursors were observed to express telomerase activity. These investigators demonstrated that the malignant CD138+ plasma cells in multiple myeloma had limited replicative potential while the clonogenic cancer stem cells resemble normal memory B cells (CD138− CD19+CD27+) [26]. Using a telomerase inhibitor currently in clinical trials (Imetelstat or GRN163L), they found a marked reduction of telomerase activity in both the CD138+ and CD138− cells and more importantly that Imetelstat inhibited the in vitro clonogenic growth of CD138− putative cancer stem cells isolated from the bone marrow aspirates of myeloma patients [W. Matsui et al., ASH, 2006]. These findings provide added support that multiple myeloma stem cells are not quiescent since they express telomerase activity and also that they are likely to have short telomeres since the stem cells lost their clonogenic potential rapidly when telomerase was inhibited. Finally, recent results suggest that the majority of leukemia stem cells are not quiescent and may have features of aberrantly self-renewing committed progenitors or precursors, as opposed to quiescent adult tissue stem cells [27].
Investigating the telomere length of putative cancer stem cells is important, since it is theoretically possible for these cells to have longer telomeres, similar to normal stem cells. However, since these cells had to bypass senescence and crisis, it is more logical to assume that the cancer cell that first became immortal by upregulating telomerase would have had short telomeres. For example, in most cases of preneoplasia it has been shown that cells have very short telomeres [28–30]. In prostate cancer for example, telomere shortening is detected in low grade prostatic intraepithelial neoplasia (PIN) lesions, and is restricted to the luminal compartment [28–30]. This indicates that the tumor initiating cells are likely to originate from a subset of transient amplifying cells which may have critically shortened telomeres perhaps due to chronic inflammation. While speculative, this not only leads to short telomeres but also genomic instability and eventually re-activation of telomerase. That 60% of high grade PIN lesions express telomerase activity is evidence in support of this scenario [28].
7. Self-renewal in cancer stem cells versus bulk tumor cells
The concept of self-renewal has significant meaning when applied to normal stem cells, where the evidence for a unidirectional differentiation (from progenitor to transient amplifying or other cell) is strong and where distinct properties of the cells exist. However, there are a variety of reasons why one needs to be cautious in applying the concept of self-renewal to cancer stem cells. On the one hand, a telomerase-expressing normal fibroblast is immortal and thus clearly capable of self-renewal, so self-renewal cannot be an exclusive property of cancer stem cells. In addition, there are many examples where investigators have studied cancer cell lines in which only a subset of cells expresses markers of cancer stem cells. There are at least three situations in which a cell line could stably maintain this characteristic. Stable populations would exist if the stem cells grew faster than the bulk of the cells but constantly generated more differentiated progeny, in which case stem cell numbers could be maintained. This situation certainly conflicts with a general assumption that cancer stem cells would be quiescent or grow less rapidly than the bulk of tumor cells, but is consistent with self-renewal. The growth rate of the cancer stem cells in culture could be identical to that of the bulk tumor cells, which is possible. Finally, the differentiation characteristics of tumor cells could be fluid, where progeny of more differentiated cells could assume more stem-like characters and vice versa. Clones of cells expressing cancer stem-cell markers can clearly give rise to populations dominated by “bulk population tumor cells”, and clones of cells that seem to lack stem cell markers give rise to populations in which cells expressing cancer stem cell markers can be found. It is unclear what self-renewal would mean if cells can transition back and forth between these states. Given the multiple genetic and epigenetic changes associated with malignancy it would not be surprising that one might need a much more flexible interpretation of stem cells in the context of tumors.
8. Do cancer initiating/stem cells have different DNA strands?
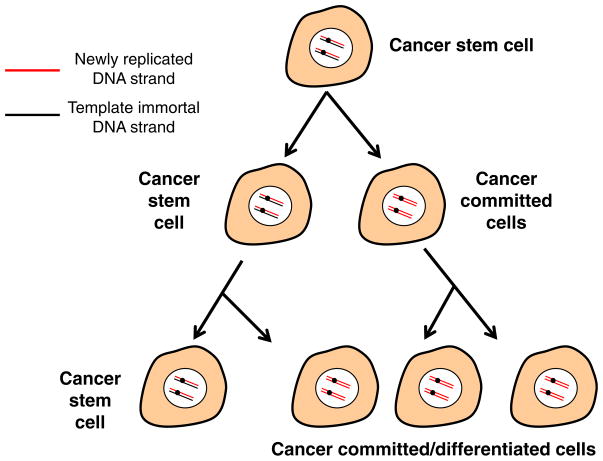
Similar to normal stem cells is it reasonable to speculate that in order for the cancer initiating/stem cell to retain a more stabilized genome compared to the bulk of the tumor that they have to retain a mechanism to minimize ongoing DNA damage? The cancer stem cell model proposes that tumor progression, metastasis and relapse after therapy may be driven by a rare subset of tumor cells that possess the capacity to self-renew while the bulk of the tumor does not (Fig. 3). As already described, there is a robust published literature indicating that preneoplastic cells have very short telomeres [28–30]. Thus progressive telomere shortening results in chromosome end associations, fusions, anaphase bridges and breakages with each cell cycle, and may lead to global genomic instability that is characteristic of most cancer cells. If telomerase upregulation or reactivation is a means to slow down or stabilize the ongoing genomic instability changes, this could help provide a possible explanation for why putative cancer stem cells have short telomeres. It also suggests that robust telomerase inhibition could be an effective anti-cancer therapeutic approach that would target both the bulk of the cancer cells as well as the dividing cancer stem cells.
Fig. 3.
Hypothetical model for retention of template immortal DNA strand in cancer stem cells. Similarly to a proposed mechanism that may exist in certain adult stem cells, it is possible that the cancer initiating/stem cell have engaged a mechanism to retain specific characteristics of stem cells. Perhaps due to distinct epigenetic marks at centromeric DNA as well as at specific genomic sites, specific cancer cells (which are not quiescent, express telomerase, and have shortened telomeres), may preferentially retain the template immortal strand and the parental centriole in the cancer stem cell while the newly replicated DNA strands may segregate with the cancer committed or more differentiated cells. While this would help explain the rare nature of cancer stem cells, currently there is a lack of experimental support.
In normal cells, during anaphase separation of chromosomes, it is believed there is either random segregation of DNA strands or asymmetric segregation of DNA strands to daughter cells. That individual chromosomes can be partitioned non-randomly has been controversial and difficult to prove. While random segregation would not require the engagement of a new regulatory mechanism, asymmetric segregation of DNA strands would require such a mechanism but it would potentially have a selective advantage to minimize the replication errors if the parental “immortal” strand was segregated to the stem cells. In this hypothesis, the stem cell keeps the template DNA strand after a round of DNA synthesis, while the progenitor cells inherit the newly replicated daughter strands. Thus the stem cells that inherit the parental template strand might have fewer errors, while the new replicated strands with possible replication errors would eventually be discarded when the terminally differentiated cells are eliminated from the body. Even though there is limited data to support that there are fewer replication errors in stem cells compared to more differentiated cells, it does pose the question if this same model can be extrapolated to cancer stem cells (Fig. 3)? Approximately 35 years ago a mechanism to avoid replication mediated mutations was proposed by John Cairns [31] and was termed the immortal strand hypothesis. Others had clearly considered non-random segregation of chromatids at mitosis [32] but Cairns was the first to propose this as a mechanism involved in the origin of cancer cells [31,33]. Speculation was that daughter cells inheriting the parental strands may be mostly quiescent or slow dividing and thus much rarer than the transit amplifying or differentiated cells. Thus, the cancer stem cell may retain the template immortal strand while the cancer committed or more differentiated cancer cells may contain the newly replicated DNA strands (Fig. 3). While currently there is no experimental evidence to support this concept in cancer cells, there are recurring reports that such a mechanism may occur in some normal stem cells [34–40].
An alternate hypothesis [41] is that, following DNA replication, sister chromatids in tissue-specific stem or progenitor cells carry distinct epigenetic marks at centromeric DNA as well as at specific genomic sites. This could be regulated by a specific niche or microenvironmental stimulus to protect the cells from replication errors. There is some evidence that epigenetic differences between sister chromatid centromeres may be required to direct non-random segregation of sister chromatids during mitosis, and thus epigenetic differences at certain genes could regulate the expression of those genes following mitosis. According to this hypothesis, selective retention of chromatids with “active” stem cell genes may result in maintenance of self-renewal properties. It also predicts that there will be loss of stem cell properties in the cell that inherits the opposite “silent” sister chromatids. Either the immortal strand or the silent sister chromatids hypothesis for normal stem cells could also be applied to cancer cells. Both of these mechanisms may be dependent on cell polarity and parental centriole retention.
9. Conclusions and perspectives
Clearly long-lived animals protect the steady state of their tissues by continuous replacement of the cells that regularly differentiate and die. An adult human contains approximately 1012 rapidly multiplying cells. During a typical~30 000 day lifespan (~80 years), each person makes and discards an enormous number of cells from the bone marrow, skin, and gastrointestinal track each day [42]. In the absence of a mechanism to deal with spontaneous rates of sporadic mutations, cancer in humans would be a lot more prominent and appear earlier in life than already occurs. Mechanisms to minimize genomic damage are essential for large long-lived species. While much remains to be discovered, organ systems in large long-lived species have evolved mechanisms that dramatically slow the rate of accumulation of replication errors. In this minireview we have presented some evidence that cancer stem/initiating cells have many differences from normal stem cells and presented a variety of hypotheses for how this may occur. Importantly, we have learned that there are differences in telomere lengths and telomerase activity between normal and cancer stem cells. This knowledge may help us identify unique vulnerabilities that can be targeted as specific cancer therapeutic approaches while avoiding targeting our much needed normal stem cells. Howsubsets of tumor cells have co-opted the capacity for self-renewal and differentiation is an active area of research. New knowledge in this field is beginning to build a rationale for targeting pathways of aberrant self-renewal in the treatment of many cancer types.
Acknowledgments
Supported in part by NASA NNX08BA54G and SPORE CA70907 and CA127297.
References
- 1.Moyzis RK, Buckingham JM, Cram LS, Dani M, Deaven LL, Jones MD, Meyne J, Ratliff RL, Wu JR. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;85:6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson J. Origin of concatemeric T7 DNA. Nat New Biol. 1972;239:197–200. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 3.Shay JW, Wright WE. Historical claims and current interpretations of replicative aging. Nat Biotechnol. 2002;20:682–688. doi: 10.1038/nbt0702-682. [DOI] [PubMed] [Google Scholar]
- 4.Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1995;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Shay JW, Wright WE, Werbin H. Defining the molecular mechanism of human cell immortalization. Biochim Biophys Acta. 1991;1072:1–7. doi: 10.1016/0304-419x(91)90003-4. [DOI] [PubMed] [Google Scholar]
- 6.Wright WE, Shay JW. The two-stage mechanism controlling cellular senescence and immortalization. Exp Gerontology. 1992;27:383–389. doi: 10.1016/0531-5565(92)90069-c. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg R. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 8.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 9.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 10.Marian CO, Cho SK, Hatanpaa KJ, Wright WE, Maher E, Madden C, Mickey B, Yang XL, Shay JW, Bachoo R. The telomerase inhibitor GRN163L blocks telomerase activity. Induces telomere attrition and limitsGBM stem cell proliferation and in-vivo growth. Clin Cancer Res. 2010;16:154–163. doi: 10.1158/1078-0432.CCR-09-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marian CO, Wright WE, Shay JW. The effect of telomerase inhibition on prostate tumor-initiating cells. Int J Cancer. 2010;127:321–331. doi: 10.1002/ijc.25043. [DOI] [PubMed] [Google Scholar]
- 12.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 13.Holt SE, Shay JW. Role of telomerase in cellular proliferation and cancer. J Cell Physiol. 1999;180:10–18. doi: 10.1002/(SICI)1097-4652(199907)180:1<10::AID-JCP2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 14.Ward RJ, Dirks PB. Cancer stem cells: at the headwaters of tumor development. Annu Rev Pathol: Mech Dis. 2007;2:175–189. doi: 10.1146/annurev.pathol.2.010506.091847. [DOI] [PubMed] [Google Scholar]
- 15.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single melanoma cells. Nature. 2008;45:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkin C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 17.Wang J, Sakariassen PO, Tsinkalovsky O, Immervoll H, Boe SO, Svendsen A, Prestegarden L, Rosland G, Thorsen F, Stuhr L, Molven A, Bjerkvig R, Enger PO. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122:761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin M, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Tunici P, Bissola L, Lualdi E, Polla B, Cajola L, Broggi G, Sozzi G, Finocchiaro G. Genetic alterations and in vivo tumorigenicity of neurospheres derived from an adult glioblastoma. Mol Cancer. 2004;3:25. doi: 10.1186/1476-4598-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 21.Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+ CD24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008;98:756–765. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins AT, Habib FK, Maitland NJ, Neal DE. Identification and isolation of human prostate epithelial stem cells based on alpha(2)beta(1)- integrin expression. J Cell Sci. 2001;114:3865–3872. doi: 10.1242/jcs.114.21.3865. [DOI] [PubMed] [Google Scholar]
- 23.Locke M, Heywood M, Fawell S, Mackenzie IC. Retention of intrinsic stem cell hierarchies in carcinoma-derived cell lines. Cancer Res. 2005;65:8944–8950. doi: 10.1158/0008-5472.CAN-05-0931. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Chen X, Calhoun-Davis T, Claypool K, Tang DG. PC3 human prostate carcinoma cell holoclones contain self-renewing tumorinitiating cells. Cancer Res. 2008;68:1820–1825. doi: 10.1158/0008-5472.CAN-07-5878. [DOI] [PubMed] [Google Scholar]
- 25.Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6796–6805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh N, Matsui W. Cancer stem cells in multiple myeloma. Cancer Lett. 2009;277:1–7. doi: 10.1016/j.canlet.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cleary ML. Regulating the leukaemia stem cell. Best Practice and Research Clinical Haematology. 2009;22:483–487. doi: 10.1016/j.beha.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koeneman KS, Pan CX, Jin JK, Pyle JM, 3rd, Flanigan RC, Shankey TV, Diaz MO. Telomerase activity, telomere length, and DNA ploidy in prostatic intraepithelial neoplasia (PIN) J Urol. 1998;160:1533–1539. [PubMed] [Google Scholar]
- 29.Meeker AK, Gage WR, Hicks JL, Simon I, Coffman JR, Platz EA, March GE, De Marzo AM. Telomere length assessment in human archival tissues: combined telomere fluorescence in situ hybridization and immunostaining. Am J Pathol. 2002;160:1259–1268. doi: 10.1016/S0002-9440(10)62553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meeker AK, Hicks JL, Platz EA, March GE, Bennett CJ, Delannoy MJ, De Marzo AM. Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res. 2002;62:6405–6409. [PubMed] [Google Scholar]
- 31.Cairns J. Mutation, Selection and cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- 32.Lark KG, Consigli RA, Minocha HC. Segregation of sister chromatids in mammalian cells. Science. 1966;154:1202–1205. doi: 10.1126/science.154.3753.1202. [DOI] [PubMed] [Google Scholar]
- 33.Cairns J. Cancer and the immortal strand hypothesis. Genetics. 2006;174:1069–1072. doi: 10.1534/genetics.104.66886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karpowicz P, Morshead C, Kam A, Jervis E, Ramuns J, Cheng V, van de Kooy D. Support for the immortal strand hypothesis: neural stem cells partition DNA strands asymmetrically in vitro. J Cell Biol. 2005;170:721–732. doi: 10.1083/jcb.200502073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merok JR, Lansita JA, Tunstead JR, Sherley JL. Cosegregation of chromosomes containing immortal DNA strands in cells that cycle with asymmetric stem cell kinetics. Cancer Res. 2002;62:6791–6795. [PubMed] [Google Scholar]
- 36.Potten CS, Hume WJ, Reid P, Cairns J. The segregation of DNA in epithelial stem cells. Cell. 1978;15:899–906. doi: 10.1016/0092-8674(78)90274-x. [DOI] [PubMed] [Google Scholar]
- 37.Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci. 2002;115:2381–2388. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- 38.Conboy MJ, Karasov AO, Rando TA. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol. 2007;5:1120–1126. doi: 10.1371/journal.pbio.0050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rando TA. The immortal strand hypothesis: segregation and reconstruction. Cell. 2007;129:1239–1243. doi: 10.1016/j.cell.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 40.Shinin V, Gayraud-Morel B, Gomes D, Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol. 2006;8:677–682. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- 41.Landsdorp PM. Immortal Strands: Give me a break. Cell. 2007;129:1244–1247. doi: 10.1016/j.cell.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 42.Potten CS, Morris RJ. Epithelial stem cells in vivo. J Cell Sci. 1988;10 (Suppl):45–62. doi: 10.1242/jcs.1988.supplement_10.4. [DOI] [PubMed] [Google Scholar]