Abstract
Background
Matrix metalloproteinases (MMPs) are key enzymes responsible for extracellular matrix degradation contributing to the progressive histological changes seen in lower airway disease, including asthma. MMP-9 and TIMP-1 have also shown some role in the pathogenesis of chronic rhinosinusitis (CRS) and nasal polyposis (NP).
Objective
We aim to determine variability in expression of MMP-9 and its inhibitor, tissue inhibitor of metalloproteinase-1 (TIMP-1), in sinus tissue from distinct patient populations presenting with nasal polyposis.
Methods
The expression of MMP-9 and TIMP-1 was investigated in nasal polyp tissue from 6 aspirin-sensitive (AS) and 6 aspirin-tolerant (AT) patients undergoing endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis (CRSwNP). Sinus mucosa from 6 patients with chronic rhinosinusitis without nasal polyposis (CRSsNP) was used as control. The MMP-9 and TIMP-1 expression was measured using immunofluorescence technique and graded using manual and computerized methods.
Results
Expression of TIMP-1 was significantly reduced in the AS group when compared with both the AT and CRSsNP (control) groups (P < .001). The MMP-9/TIMP-1 ratio was significantly increased in the AS group when compared with other patient groups (P < .001). The MMP- 9 expression was similar between study and control groups.
Conclusion
These results support the importance of MMP-9 and TIMP-1 expression in nasal polyp formation. The decreased expression of TIMP-1 in AS patients may promote the effects of MMP-9 expression and thus contribute to tissue remodeling and inflammatory changes. This finding may lead to further understanding of disease severity and resistance to treatment in this group of patients, as well as the pathogenesis of nasal polyps.
INTRODUCTION
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases that have proteolytic activity and play a role in tissue remodeling. MMPs are key enzymes responsible for extracellular matrix degradation contributing to the progressive histological changes seen in lower airway disease, including asthma.1,2 The MMPs are regulated by a variety of biological functions, including endogenous inhibitors, mainly tissue inhibitors of matrix metalloproteinases (TIMPs).3 So far, 26 MMPs have been characterized, but MMP-9 is the most studied in asthma, whereas four TIMPs have been detected. TIMP-1 is the primary inhibitor for MMP-9.4 The balance of MMP and TIMP has been shown to be important in tissue homeostasis within the extracellular matrix (ECM).1,2,5 The ECM not only acts as a structural support to the cell body but also regulates cell growth, differentiation, migration, proliferation, tissue repair, and apoptosis.3 Normal MMP activity results in tissue remodeling through ECM degradation. Excessive MMP activity results in overaggressive and perhaps unregulated breakdown of ECM components, leading to pathological tissue remodeling.1,4,6
The balance between these enzymes has been studied not only in asthma but in a variety of inflammatory conditions such as arthritis, myocardial infarction, periodontal disease, and chronic rhinosinusitis. The role of MMP-9 and TIMP-1 in the pathogenesis of chronic rhinosinusitis (CRS) and nasal polyposis (NP) is a current question of interest.7 Nasal polyposis in the setting of aspirin sensitivity and asthma, also referred to as Samter’s triad, aspirin-exacerbated respiratory disease, aspirin-induced asthma, or aspirin-sensitive asthma, is a subset of CRS with NP (CRSwNP) that appears to be particularly challenging to understand and manage. Patients with aspirin sensitivity tend to have more severe disease, worse asthma, and are prone to treatment failure.8–10 The aims of this study were to determine variations in expression of MMP-9 and TIMP-1 that might be associated with the development of nasal polyps in aspirin-sensitive (AS) vs aspirin-tolerant (AT) patients with CRS, to add to our understanding of this disease.
METHODS
Tissue Samples
Sinus tissue specimens were obtained from 18 adult patients who met criteria for selected study groups who had sinonasal tissue banked after undergoing endoscopic sinus surgery by a single surgeon (T.K.) between 2009 and 2010 at the University of Colorado Hospital. Patients with cystic fibrosis, ciliary dyskinesia, or age younger than 18 years were excluded from the study. Patient charts were retrospectively reviewed by the primary author (P.M.) for age, sex, number of prior sinus surgeries, preoperative medications, asthma and allergy history, aspirin sensitivity, smoking status, nasal endoscopy findings, and pathological conditions. The diagnosis of asthma was made based on history and pulmonary function testing. The diagnosis of AS was made based on patient history of exacerbated upper and lower airway disease with aspirin use; aspirin challenge was performed in 4 of 6 patients. The diagnosis of CRS was made according to American Academy of Otolaryngology Sinusitis Task Force criteria, with the presence of polyps documented by nasal endoscopy or findings at the time of surgery. Six AS patients with asthma and CRSwNP: designated AS, six AT patients with CRSsNP; designated AT, and six patients with CRS without NP (CRSsNP); designated controls, were included in the study. This study was approved by the Colorado multi-institutional review board through the University of Colorado and National Jewish Health.
Histomorphological Studies
Biopsy specimens were fixed in formalin and embedded in paraffin. Serial 5-μm sections were cut and mounted for each tissue sample. Slides were stained with hematoxylin and eosin and examined by light microscopy. Each slide was evaluated by 2 blinded pathologists, and scored using the method described by Dhong et al11 to identify histomorphological characteristics among patient groups.11 The following elements were examined: basement membrane thickness, goblet cell hyperplasia, subepithelial edema, submucous gland formation, eosinophil infiltration, lymphocyte infiltration, and polymorphonuclear cell infiltration.
Immunofluorescent Staining
Immunofluorescent staining was used to determine the expression of MMP-9 and TIMP-1 in the biopsy specimens. Using this technique, the enzymes can be both quantified for comparison and localized within tissue.
Five-micrometer-thick paraffin-embedded sections were cut and mounted. Sections were deparaffinized and rehydrated in a standard protocol. Specimens were incubated in phosphate-buffered saline with 0.05% saponin (PBS-s) for 10 minutes, then blocked with 10% normal goat serum with PBS-s for 1 hour at room temperature, and immunostained with monoclonal mouse anti-human MMP-9 and polyclonal rabbit anti-human TIMP-1 primary antibodies at concentration 1:50 (Santa-Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. Immunoglobulin G isotype controls were used for each group of tissue samples at same concentration. The slides were washed in PBS-s and labeled with fluorescent tagged goat anti-mouse or goat anti-rabbit secondary antibody (1:100) for 1 hour in darkness at room temperature. Slides were additionally incubated with DAPI (1:200), a fluorescent nuclear stain for improved protein localization. The slides were again washed with PBS-s three times, rinsed with PBS, mounted, and sealed.
The slides were evaluated within 2 hours of staining under an epifluorescence microscope, and representative fluorescent pictures were taken of each slide at 200× for morphometric and comparative analysis. We used a Nikon, Eclipse TE-2000 U microscope with a Coolsnap HQ camera, and an automated station with a z-axis motor linked to a personal computer that is equipped with the Metamorph software, v 6.2 (Molecular Devices, San Diego, California; Fig 1).
Figure 1.
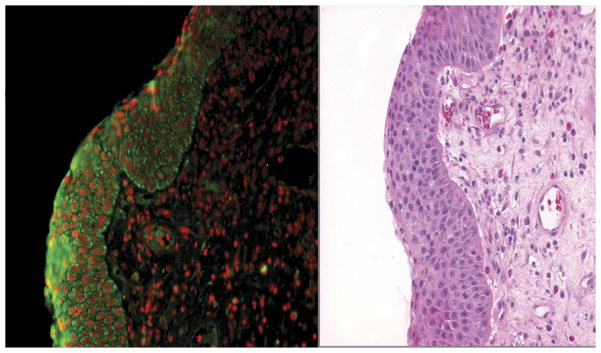
MMP-9 Immunofluorescence of nasal polyp in aspirin-sensitive polyp group (AS). Green is MMP-9 expression, and red is nuclear staining (left). Same tissue sample with hematoxylin and eosin stain (right); 200× magnification.
Subjective Measurements
Subjective measurements were obtained by evaluation from 2 blinded pathologists and an unblinded co-investigator (P.M.). The intensity of immunostaining was scored according to degree of distribution as described by Can et al4:
0(+): No staining
1(+): Staining on surface epithelium or 25% staining
2(+): Staining on surface epithelium, endovascular, perivascular cells, and basement membrane or 25–50% staining
3(+): 2(+) + staining on inflammatory cells or 50–75% staining
4(+): 3(+) + staining on matrix or 75–100% staining
Objective Measurements
For quantification of immunofluorescence results, data from 4 representative epithelium-containing fields per tissue section at magnification of 200× were analyzed and averaged, using Metamorph image analysis software. The same exposure time was used to capture images in all experimental groups. Fluorescent staining-positive areas were defined using the intensity threshold command. The same threshold was applied to all images. Percent thresholded area was defined as the percent of expression measured at this minimal intensity threshold in the full tissue sample or in a selected area of epithelium.
Statistical Analysis
Statistical analysis for histomorphological staining was done with analysis of variance (ANOVA) f test with Tukey HSD post hoc analysis to determine significance in variability between groups. To compare both objective and subjective expression of MMP-9, TIMP-1, and the ratio of MMP-9/TIMP-1 across groups, statistical analysis was done with the ANOVA f test, using the geometric mean. Epithelial expression alone was compared in a similar fashion. The agreement/concordance of objective vs subjective measurements was evaluated using the Kendall-tau correlation coefficient. A P-value < .05 was considered statistically significant.
RESULTS
Patient Information
Patient age ranged from 20 to 62 years, with a mean of 46 years. Eight female and 10 male patients were included. No smokers were in any study groups. Atopy by positive skin testing was present in 5 of the 6 patients in both the AS and AT groups; 2 of the 6 control patients demonstrated positive skin testing. All patients with nasal polyps received preoperative steroid bursts with daily oral prednisone 5 days before surgery and tissue collection. No patients in the control group received preoperative oral steroids. No patients were on other medications known to alter MMP, including antibiotics, at the time of surgery. Fifty-five percent of the patients had at least 1 prior sinus surgery. The time between procedures averaged 2 years for the AS group, 4.5 years for the AT group, and 7 years for the control group.
Histomorphological Studies
The AS group showed greater basement membrane thickening (P <.05), eosinophil (P < .01), lymphocyte (P <.05), and polymorphonuclear cell infiltration (P < .01) than the control patient group. The AT group showed elevated lymphocyte (P <.01) and polymorphonuclear cell infiltration (P < .01) in comparison with control. No significant differences were seen between AT and AS groups (Table 1).
Table 1.
Hemotoxylin and Eosin Staining
| Scale | AS | AT | Control | Pa | |
|---|---|---|---|---|---|
| Thickness of basement membrane | 0–3+ | 2.00 | 1.42 | 0.75 | .024 |
| Goblet cell hyperplasia | 0–3+ | 0.67 | 1.08 | 0.58 | .519 |
| Subepithelial edema | 0–3+ | 1.92 | 1.50 | 1.17 | .136 |
| Submucous gland formation | 0–3+ | 1.00 | 1.50 | 1.50 | .602 |
| Eosinophil infiltration | 0–3+ | 2.83 | 2.25 | 1.00 | .001 |
| Lymphocytre infiltration | 0–4+ | 2.08 | 2.33 | 0.75 | .005 |
| Polymorphonuclear cell infiltration | 0–3+ | 1.25 | 1.25 | 0.25 | .039 |
| Total score | 11.75 | 11.33 | 6.00 | <.001 |
Scale 0 – 4+ as described by Dhong et al, as determined by pathologist review of H&E staining.
Bold values are statistically different from control.
P value based on ANOVA f-test and Tukey HSD post hoc analysis.
Expression of MMP-9, TIMP-1, and MMP-9/TIMP Ratio
Immunofluorescent staining of MMP-9 was diffuse in the surface epithelium, extracellular matrix, vascular basement membrane, endovascular, and perivascular cells. TIMP-1 was more localized to the surface epithelium and basement membrane but was also expressed in the perivascular cells and submucous glands. On visual inspection of immunofluorescent imaging, TIMP-1 expression was significantly less in AS tissue samples than in other study groups; in 3 AS samples, TIMP-1 expression was too minimal to image (Fig 2). MMP-9 expression did not differ between groups. Group subjective scoring measurements for TIMP-1 expression was 0.63 in AS vs 1.35 for AT and 1.20 in control, which was statistically significant (P = .02; Table 2).
Figure 2.
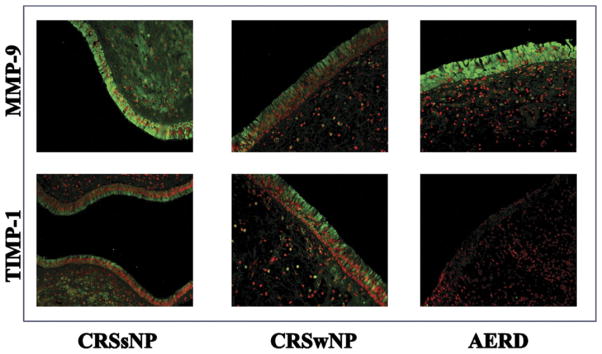
Top row MMP-9 immunofluorescent stain of control, AT, and AS. Green is MMP-9 positive, and red is nuclear stain. Bottom row: TIMP-1 immunofluorescent stain of control, AT, and AS, where green is MMP-9 positive, and red is nuclear stain.
Table 2.
Full Slide Immunofluorescent Staining for Expression of MMP-9 and TIMP-1
| AS | AT | Control | Pa | |
|---|---|---|---|---|
| MMP—subjective | 1.35 (1.92) | 1.35 (1.92) | 1.73 (2.10) | .77 |
| TIMP subjective | 0.63 (1.43) | 1.35 (1.62) | 1.20 (1.57) | .02 |
| MMP/TIMP subjective | 2.14 (1.90) | 1.00 (1.55) | 1.45 (2.05) | .13 |
| MMP objective | 22.65 (1.70) | 18.92 (1.54) | 27.11 (5.37) | .84 |
| TIMP objective | 0.82 (4.48) | 9.78 (1.54) | 13.74 (2.23) | <.001 |
| MMP/TIMP objective | 27.66 (3.53) | 1.93 (1.60) | 1.95 (4.26) | <.001 |
Subjective measurement: 0 – 4+ scale as determined by pathology evaluation of images.
Objective measurements: percent thresholded area of entire tissue area represented as determined using image analysis software (Metamorph, Molecular Devices).
P values are from ANOVA f-test of geometric mean.
In objective measurement of MMP-9 and TIMP-1 immunofluorescence, similar results are seen. Again MMP-9 expression was similar between groups and percent thresholded area in AS (22.65%), AT (18.9%), and control (27.1%) do not significantly differ (P = .84). Objective measurement of TIMP-1 expression in percent thresholded area was statistically lower in the AS group (0.82%) compared with the AT (9.8%) or control (13.7%) groups (P < .001). As a result, the MMP-9/TIMP-1 ratio was statistically higher in AS (27.7) than in AT (1.93) or control (1.95) groups (P < .001). When epithelial expression alone was measured, TIMP-1 expression was again significantly lower in the AS group (9.1%) than in both the AT (50.4%) and control (45.8%) groups (P < .001; Table 3).
Table 3.
Epithelial Immunoflourescence Staining for Expression of MMP-9 and TIMP-1
| AS | AT | Control | Pa | |
|---|---|---|---|---|
| Expression of MMP % area | 61.22 (16.68) | 63.62 (15.04) | 70.18 (32.72) | .78 |
| Expression of TIMP % area | 9.12 (9.16) | 50.42 (22.56) | 45.80 (24.22) | <.001 |
P-values are from ANOVA f-test.
Agreement in Objective and Subjective Measurements of MMP-9 and TIMP-1 Expression
The use of subjective grading scales is widely accepted by pathologists in the field of immunofluorescence staining. The application of computer software analysis is gaining in popularity and in theory should be a more precise measure of protein expression and intensity. We generated agreement measurements to estimate accuracy of our subjective measurements with respect to image analysis data using the percent thresholded area. In grading of MMP-9 expression, 8 of the 18 subjects (44%) demonstrated perfect agreement between the objective and subjective ratings; in the remaining 10 subjects (56%) the objective and subjective ratings differed by 1 category (Table 4).
Table 4.
Agreement of Categorical Scores from Subjective and Objective Measures of MMP-9
| Category | Objective score 1 0–25% threshold area | Objective score 2 26–50% threshold area | Objective score 3 51–75% threshold area | Objective score 4 76–100% threshold area |
|---|---|---|---|---|
| Subjective Score 1 | 6 | 2 | 0 | 0 |
| Subjective Score 2 | 2 | 2 | 1 | 0 |
| Subjective Score 3 | 0 | 3 | 0 | 2 |
| Subjective Score 4 | 0 | 0 | 0 | 0 |
For TIMP-1 expression, perfect agreement was found between objective and subjective ratings in 16 of the 18 subjects (89%). Of the remaining 2 subjects (11%), 1 differed by 1 category and 1 by 2 categories (Table 5).
Table 5.
Agreement of Categorical Scores from Subjective and Objective Measures of TIMP-1
| Category | Objective score 1 0–25% threshold area | Objective score 2 26–50% threshold area | Objective score 3 51–75% threshold area | Objective score 4 76–100% threshold area |
|---|---|---|---|---|
| Subjective score 1 | 15 | 0 | 0 | 0 |
| Subjective score 2 | 1 | 0 | 0 | 0 |
| Subjective score 3 | 1 | 0 | 1 | 0 |
| Subjective score 4 | 0 | 0 | 0 | 0 |
The Kendall-tau correlation coefficient was used to measure concordance between the subjective pathology grading scores and the continuous objective computer image analysis scores. The Kendall-tau coefficient for MMP-9 is 0.61 and for TIMP-1 is 0.69, indicating moderate concordance between scoring methods for both measures.
DISCUSSION
Chronic sinusitis is a prevalent problem affecting more than 30 million Americans.10 Nasal polyps associated with CRS (CRSwNP) are reported to affect up to 5% of the general population.12 In all patients undergoing endoscopic sinus surgery, an estimated 4.8% may have concomitant aspirin sensitivity. This incidence may jump to 26% in patients with both nasal polyps and asthma.13 Aspirin-exacerbated respiratory sensitivity is believed to be an indicator for chronic intractable and extensive sinonasal disease, persistent asthma of greater than average severity, higher than ordinary medication requirements, and treatment challenges.8,14 The pathogenesis of aspirin-sensitive (AS) airway disease is related to abnormal metabolism of arachidonic acid, resulting in excessive leukotriene production associated with low or dysfunctional prostaglandin expression both before and after aspirin challenge. The precise downstream effects leading to nasal polyp formation in these patients, as well as the AT patients, remains poorly understood.15,16 Thus, from a clinical standpoint, management of the AS patient remains frustrating and often unsatisfactory. Interestingly, research into the potential differences in the inflammatory process of AS vs AT patients on the molecular level remains limited.
The field of airway remodeling associated with asthma has been an active area of research. Both MMPs and TIMPs have been shown to be critical factors in ECM homeostasis, with their dysfunction linked to airway remodeling and worse lower airway disease.3,17 Twenty-three structurally related MMPs together function in ECM degradation, tissue morphogenesis, and immune cell recruitment and are involved in chronic inflammation, degenerative diseases, angiogenesis, and tumor biology.5 Investigators have proposed that overexpression of MMPs relative to lower expression of TIMPs leads to basement membrane thickening and progressive respiratory disease.3,6 Thus, the ratio of MMP expression to TIMP expression may be a measure of this degree of “balance” and an important factor in control of airway remodeling. By extension, airway remodeling might be important in the pathogenesis of sinonasal disease, and specifically, in the development of nasal polyps. In support of Tos’ theory of the development of nasal polyps, dysfunctional MMP-TIMP homeostasis may predispose to excessive ECM degradation, leading to epithelial rupture, and ultimately may contribute to nasal polyp formation.2,18
Recent investigation has increasingly focused on the role of MMPs and TIMPs in the development of CRS, nasal polyp development, and upper airway remodeling. Multiple groups have demonstrated increased MMP-9 expression in CRSwNP and CRSsNP patients compared with normal controls.2,4,7,19 In these studies, expression of MMP-9 did not appear to differ significantly between patients with or without nasal polyps. Can et al4 did report differential expression of MMPs, namely, elevated MMP-2 expression in their CRSwNP group and elevated MMP-7 expression in the CRSsNP group. In these studies, investigators did not select for AS status or present results specific for patients with AS disease. In fact, we did not find literature specifically examining the potential influence aspirin status may have on this underlying process.
The TIMPs must be studied in concert with MMP analysis to fully understand their potential impact. The recent literature, however, is less consistent in this area. Can et al4 reported decreased TIMP-1 expression in both CRSwNP and CRSsNP groups compared with normal controls; no difference between the CRS groups was noted, however. Kahveci et al2 also reported decreased expression of TIMP-1 in the nasal polyp group compared with normal controls. In contrast, Chen et al20 found no difference, and Shin et al19 reported increased TIMP-1 expression in patients with allergic rhinitis.19,20 These studies,2,4 in conjunction with literature published in the asthma fields,3,6 point to an alteration in TIMP-1 expression or MMP/TIMP-1 ratio as a factor in CRS with and without nasal polyps and subsequent airway remodeling. In polyps, excessive MMP-9 within the tissue suggests a direct degradative function, whereas upregulation of TIMP-1 over MMP-9 may explain the prominent fibrosis in CRSsNP and chronic asthma.6 This recent work supports the hypothesis that decreased expression of TIMP-1 may lead to “unchecked” MMP-9 expression and significant alterations in ECM homeostasis.
The most significant finding in our study is evidence of decreased TIMP-1 expression in the AS patients compared with both the AT and CRSsNP groups. Using the immunofluorescence technique, the subjective and objective measures support this finding. Although MMP-9 expression was consistent across groups, the MMP-9/TIMP-1 ratio was significantly elevated in the AS group, supporting the work of Can et al4 and Kahveci et al.2 This is further supported by our hematoxylin and eosin staining data showing increased basement membrane thickening in the study groups, although no apparent differences were noted between AS and AT patients. Overall, our data suggest a greater alteration in MMP-9/TIMP-1 homeostasis in CRSwNP patients with aspirin sensitivity. This appears to be the first report specifically investigating MMP-9 and TIMP-1 expression in patients with nasal polyps based on the presence of aspirin-sensitive disease.
Clinically, these results may provide further support of previous hypotheses on the role of novel therapeutic strategies such as MMP inhibition. Targeting MMP-9 and TIMP-1 may prove to be important in restoring balance and regulation for tissue remodeling. Recent data from early clinical trials of specific MMP inhibitors for diseases such as cancer and arthritis have been presented.4,21,22 Several medications commonly used in the management of CRS also have been shown to impact MMP expression. The macrolide antibiotics, tetracycline and doxycycline, are examples of several agents that have been linked with MMP downregulation, suppression of inflammation, and positive therapeutic outcomes in CRS.23–27 Development of novel therapeutic options directed at MMP-9/TIMP-1 “balance” may be of tremendous benefit in the management of CRS and specifically nasal polyposis.
Several potential limitations with our study must be addressed. As a matter of standard of care in our practice, patients with nasal polyps received oral steroids, leading up to their surgical procedure. The CRSsNP group did not; thus, our results may have been altered by this exposure to corticosteroids. Because both the AS and AT patients received oral steroids preoperatively, little or no impact is expected when comparing results between these groups. The opposite effect might be expected when comparing results with the CRSsNP group; namely, one would expect MMP suppression from the oral steroids and less pronounced differences between the polyp and nonpolyp groups. Although an important consideration, a remarkable difference was seen in TIMP-1 expression between the groups that did receive steroids. The role of atopy in alterations in MMP-9 and TIMP-1 is unclear, and although we had more patients with allergy in our 2 study groups as compared with controls, too few samples were available to complete further subgroup analysis. An additional criticism might be our selection of the “control” group. We felt the literature was clear in defining differences in MMP-9 and TIMP-1 expression between CRS and normal groups; thus, we felt no need to repeat this work. Our focus was polyp tissue and the presence of aspirin sensitivity. Thus, we felt CRS without NP would be a logical comparator group. Finally, our sample size is small; thus, we must be careful in our conclusions. This is effectively a pilot study looking at a clinical group (CRSwNP and AS) that had not been extensively studied previously. Therefore, exploring this question with smaller sample size was warranted.
In this study, we evaluated expression of MMP-9 and TIMP-1 in 3 patient populations with CRS: (1) CRSwNP and asthma in the setting of aspirin sensitivity (AS), (2) CRSwNP and tolerance to aspirin (AT), and (3) CRSsNP (control). This is the first published data looking specifically at these enzymes in patients with nasal polyposis based on status of aspirin sensitivity. We found decreased expression of TIMP-1 and elevated MMP-9/TIMP-1 ratio in the AS group. These results in CRSwNP patients with aspirin sensitivity are novel findings not previously reported. Our findings support previous work suggesting the importance in the MMP/TIMP “balance” in airway remodeling and development of nasal polyps. Clearly, additional studies are required to further elucidate the mechanism involved in MMP-9 and TIMP-1 expression and their role in the development of CRS and the potential unique impact on aspirin-sensitive respiratory disease.
Acknowledgments
Funding Sources: This project was supported by divisional funds.
We would like to acknowledge Maxwell Smith, MD, Assistant Professor of Pathology at University of Colorado Denver, for his contribution to manual review and grading of hematoxylin and eosin and immunofluorescence.
Footnotes
Disclosures: Authors have nothing to disclose.
References
- 1.Kelly E, Busse W, Jarjour N. Increased matrix metalloproteinase-9 in the airway after allergen challenge. Am J Respir Crit Care Med. 2000;162:1157–1161. doi: 10.1164/ajrccm.162.3.9908016. [DOI] [PubMed] [Google Scholar]
- 2.Kahveci O, Derekoy F, Yilmaz M, Sertesser M, Altunas A. The role of MMP-9 and TIMP-1 in nasal polyp formation. Swiss Med Wkly. 2008;138:684–688. doi: 10.4414/smw.2008.12459. [DOI] [PubMed] [Google Scholar]
- 3.Ohbayahi H, Shimokata K. Matrix metalloproteinase-9 and airway remodeling in asthma. Current Drug Targets Inflammation & Allergy. 2005;4:177–181. doi: 10.2174/1568010053586246. [DOI] [PubMed] [Google Scholar]
- 4.Can IH, Ceylan K, Caydere M, Samim E, Ustun H, Karasoy D. The expression of MMP-2, MMP-7, MMO-9, and TIMP-1 on chronic rhinosinusitis and nasal polyposis. Otolaryngol Head Neck Surg. 2008;139:211–215. doi: 10.1016/j.otohns.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Kostamo K, Tervahartialal T, Sorsa T, Richardson M, Toskala E. Metalloproteinase function in chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2007;117:638–643. doi: 10.1097/MLG.0b013e318030aca6. [DOI] [PubMed] [Google Scholar]
- 6.Vignola A, Riccobono L, Mirabella A, et al. Sputum metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio correlates with airflow obstruction in asthma and chronic bronchitis. Am J Respir Crit Care Med. 1998;158:1945–1950. doi: 10.1164/ajrccm.158.6.9803014. [DOI] [PubMed] [Google Scholar]
- 7.Bachert C, Van Bruaene N, Toskala E, et al. Important research questions in allergy and related diseases: 3-chronic rhinosinusitis and nasal polyps- a GA2LEN study. Allergy. 2009;64:520–533. doi: 10.1111/j.1398-9995.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 8.Kowalski M. Management of aspirin-sensitive rhinosinusitis asthma syndrome: what role for aspirin desensitization? Allergy Proc. 1992;13:175–184. doi: 10.2500/108854192778817202. [DOI] [PubMed] [Google Scholar]
- 9.Picado C. Aspirin intolerance and nasal polyposis. Curr Allergy Asthma Rep. 2002;2:488–493. doi: 10.1007/s11882-002-0089-8. [DOI] [PubMed] [Google Scholar]
- 10.Smith T, Meddolia-Loffredo S, Loehrl T, Sparapani R, Laud P, Nattinger A. Predictive factors and outcomes in endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope. 2005;115:2199–2205. doi: 10.1097/01.mlg.0000182825.82910.80. [DOI] [PubMed] [Google Scholar]
- 11.Dhong H, Kim H, Cho D. Histopathologic characteristics of chronic sinusitis with bronchial asthma. Acra Otolaryngol. 2005;125:169–176. doi: 10.1080/00016480410015767. [DOI] [PubMed] [Google Scholar]
- 12.Archer S. [Accessed August 5, 2011.];Nasal polyps, non-surgical treatment eMedicine from WebMD. Update June 2009. Available at http://emedicine.medscape.com/article/861353-overview.
- 13.Kim JE, Kountakis SE. The prevalence of Samter’s triad in patients undergoing functional endoscopic sinus surgery. Ear Nose Throat J. 2007;86:396–399. [PubMed] [Google Scholar]
- 14.Awad O, Lee J, Fasano M, Graham S. Sinonasal outcomes after endoscopic sinus surgery in asthmatic patients with nasal polyps: a difference between aspirin-tolerant and aspirin-induced asthma? Laryngoscope. 2008;118:1282–1286. doi: 10.1097/MLG.0b013e318170af1e. [DOI] [PubMed] [Google Scholar]
- 15.Figueiredo C, Silva I, Weckx L. Inflammatory genes in nasal polyposis. Curr Opin Otolaryngology Head Neck Surg. 2008;16:18–21. doi: 10.1097/MOO.0b013e3282f363f1. [DOI] [PubMed] [Google Scholar]
- 16.McManis K, Koutakins S. Medical and surgical considerations in patients with Samter’s triad. Am J Rhinol. 2006;20:573–576. doi: 10.2500/ajr.2006.20.2913. [DOI] [PubMed] [Google Scholar]
- 17.Katial R, Strand M, Prasertsuntarasai T, Leung R, Zheng W, Alam R. The effect of aspirin desensitization on novel biomarkers in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2010;126:738–744. doi: 10.1016/j.jaci.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 18.Tos M. Early stages of polyp formation. In: Septattipane G, Lund V, Bernstein J, Tos M, editors. Nasal Polyps: Epidemiology, Pathogenesis, and Treatment. Providence, RI: Oceanside Publications; 1997. pp. 65–72. [Google Scholar]
- 19.Shin HW, Han DH, Lim YS, et al. Nonasthmatic nasal polyposis patients with allergy exhibit greater epithelial MMP positivity. Otolaryngol Head Neck Surg. 2009;141:442–447. doi: 10.1016/j.otohns.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y-S, Langhammer T, Westhofen M, Lorenzen J. Relationship between matrix metalloproteinases MMP-2, MMP-9, tissue inhibitor of matrix metalloproteinases-1 and IL-5, IL-8 in nasal polyps. Allergy. 2007;62:66–72. doi: 10.1111/j.1398-9995.2006.01255.x. [DOI] [PubMed] [Google Scholar]
- 21.Hoekstra R, Eskens F, Verweij J. Matrix metalloproteinase inhibitors: current developments and future perspectives. Oncologist. 2001;6:415–427. doi: 10.1634/theoncologist.6-5-415. [DOI] [PubMed] [Google Scholar]
- 22.French J, Midwinter M, Bennett M, Manas D, Charnley R. A matrix metalloproteinase inhibitor to treat unresectable cholangiocarcinoma. HPB. 2005;7:289–291. doi: 10.1080/13651820510042246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvey R, Wallwork B, Lund V. Anti-inflammatory effects of macrolides: applications in chronic rhinosinusitis. Immunol Allergy Clin North Am. 2009;29:689–703. doi: 10.1016/j.iac.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Wallwork B, Coman W, Mackay-Sim A, Greiff L, Cervin A. A double-blind, randomized, placebo-controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope. 2006;116:189–193. doi: 10.1097/01.mlg.0000191560.53555.08. [DOI] [PubMed] [Google Scholar]
- 25.Kani K, Asano K, Hisamitsu T, Suzaki H. Suppression of matrix metalloproteinase production for nasal fibroblasts by macrolide antibiotics in vitro. Eur Respir J. 2004;23:671–678. doi: 10.1183/09031936.04.00057104. [DOI] [PubMed] [Google Scholar]
- 26.Van Zele T, Gevaert P, Holtappels G, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. Allergy Clin Immunol. 2010;125:1069–1076.e4. doi: 10.1016/j.jaci.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Saglam F, Celik A, Tayfur D, et al. Original article: decrease in cell proliferation by an matrix metalloproteinase inhibitor, doxycycline, in a model of immune-complex nephritis. Nephrology. 2010;15:560–567. doi: 10.1111/j.1440-1797.2010.01289.x. [DOI] [PubMed] [Google Scholar]


