Abstract
The great advances in brain imaging techniques over the last few decades have determined a shift in our understanding of chronic pain conditions and opened the door for new opportunities to develop better diagnoses and perhaps better drug treatments. Neuroimaging has helped shape the concept of chronic pain from a disease affecting mainly the somatosensory system, to a condition in which emotional, cognitive and modulatory areas of the brain are affected, in addition to degenerative processes. All these contribute to the development and maintenance of pain symptoms and co-morbid features including alterations in anxiety, depression and cognitive processes. In this paper we review the current understanding of the brain changes in chronic pain and the developments made possible by the use of various brain imaging techniques. We also discuss the possible applications of brain imaging to developing a “pain phenotype” that could aid in diagnostic and treatment choices of chronic pain conditions.
Keywords: Pain, Neuroimaging, Emotion, Cognition, Sensory
The Problem with Pain
Pain, Pain over here, Pain over there, Pain in my heart, pain in my soul, Pain in my mind …..
(Ellen Kang).
The poem epitomizes the problem of chronic pain, a condition that causes millions of individuals to suffer, and captures the notion of the ‘pain affects the brain’. Chronic pain represents an enormous problem to society – at an individual and societal level. The figures from recent epidemiological surveys have identified the level of this crisis. For example, in a recent survey of chronic pain that was conducted in 15 countries in Europe and included Israel (Breivik and others 2006), 34% of respondents had severe pain (Numeric Rating Scale > 8/10 where 0=no pain and 10 = worst pain). One third of chronic pain sufferers were not receiving any treatment, while a majority used non-medication treatments (i.e., acupuncture, massage, physical therapy) and over the counter drugs (e.g., NSAIDS). Only a small percentage of patients used strong opioids, and 40% of the patients reported inadequate control of their pain. These numbers are reflected in other surveys in the US, Canada and Australia (Blyth and others 2001; Elliott and others 1999; Tripp and others 2006). Clearly, we need an improved understanding of chronic pain that could pave the way to the development of improved diagnoses and better treatments. We are a long way from the specificity and efficacy provided by therapies such as antibiotics for bacterial infections.
Remarkable advances in understanding pain and providing improved treatments have come through scientific discoveries, improved training and access to specialized clinics, organizations (e.g., International Association for the Study of Pain (IASP, www.iasp-pain.org), patient advocacy groups (e.g., National Fibromyagia Association, www.fmaware.org) and pain clinics that provide specialized treatment (Lynch and others 2007). However, our clinical armamentarium is relatively limited in providing relief in chronic pain conditions. In the past, the basic therapy has included, for the most part, (a) drugs mostly belonging to three classes – opioids (e.g., morphine), non-steroidals (e.g., Tylenol, aspirin) and local anesthetics (e.g., lidocaine); (b) interventional treatments (e.g., nerve blocks, surgical procedures); and (c) psychological support (e.g., cognitive behavioral therapy). For all of these efforts, the number of outcome studies of non-pharmacological trials is limited, and most pharmacological studies show poor efficacy of treatment in chronic pain (Deyo and others 2009). Pain researchers, pharmaceutical companies and clinicians have struggled to break the barriers of finding treatments for pain that are both specific and efficient and have limited side effects. One reason that makes this task difficult is that there is no clear, widely accepted determination of what represents ‘success in chronic pain treatment outcomes’ (Turk and others 1993). Therapeutic efficacy in well-controlled studies of pharmacological agents show a 30% benefit compared to placebo, and generally the improvement is small, of about two points decrease in pain on a 10-point numeric rating scale (NRS) scale (Schwerla and others 2008), perhaps because of the complexity of chronic pain (Figure 1). These issues point to the desperate need for an objective measure of pain that would redefine how we evaluate and treat patients. This would allow us to better understand what treatments will work most effectively in different patients.
Figure 1.
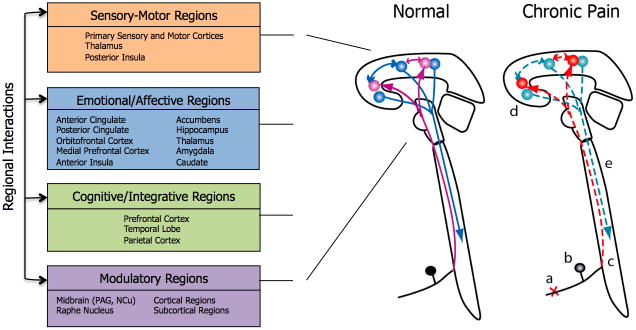
Figure 1 A: Overview of pain pathways and altered neural systems in chronic pain
White arrows: ascending and intracerebral pain pathways; Blue arrows: modulatory descending pathways. A: amygdala; ACC: anterior cingulate cortex; Cer: cerebellum; H: Hypothalamus; Ins: insula; l, m: lateral and medial thalamus; M1: primary motor cortex; NA: nucleus accumbens; PAG: periaqueductal gray; PFC: prefrontal cortex; PPC: posterior parietal cortex; S1, S2: primary and secondary somatosensory cortex; SMA: supplementary motor area.
Figure 1 B: Schematic of anatomical sites and pathways that show changes in chronic pain.
Left: Sensory-motor, emotional/affective, cognitive/integrative and modulatory regions are involved in the complex processing of pain, with some areas being involved in more than one pain domain. One example is insula, which contains a somatotopic representation of pain, and also processes emotional aspects of pain experience. Right: Loss of afferent fibers (a), loss and changes in function in the dorsal root ganglion (b), plasticity in the dorsal horn neurons (c), as well as changes in the brain areas that are processing sensory, emotional, cognitive and modulatory aspects of pain (right panel) (d) and in descending modulatory pathways (e) have been described.
Part of the problem we have faced is a new realization that chronic pain is a disease of the brain. Until recently there has been a lack of ability to measure changes in the brain that are a consequence of chronic pain. Anatomical, functional and chemical neuroimaging have opened the door to new vistas and new opportunities for a better understanding of chronic pain, for better diagnostic possibilities, and perhaps better drug treatments to be developed. While genetic and other molecular approaches in the pain field have shown tremendous advances, only in recent years has brain imaging contributed to the revolution in understanding pain and greatly changed the field of pain research. The major insight that emerged from neuroimaging studies is that chronic pain is a disease of the brain and thus all therapeutic modalities will need to take this into consideration.
The ability to explore the human brain in human volunteers or patients has dramatically changed our understanding of pain. Imaging has the ability to define theoretical constructs of numerous thinkers in the field of brain processing in chronic pain in the human condition. Imaging has allowed unprecedented interrogation of brain systems in terms of brain circuitry, the effects of analgesics on neural networks, transition of acute into chronic pain, definition of brain regions that here-to-for may not have been considered important (e.g., nucleus accumbens, striatal regions), brain plasticity including functional and morphological changes, networks that are involved in the placebo response and alterations in neurochemistry in chronic pain (Figure 1). The magnitude of the ‘imaging revolution’ in the pain field is exemplified by the volume of literature published every year. In a Google Scholar search (scholar.google.com), the number of citations of pain and functional imaging (key words “pain” and “functional imaging”) showed an exponential rise (314 articles during 1993–1996, to 1090 articles between 1997–2000, 2920 between 2001–2004 and 6350 between 2005–2008 (Figure 2)). While there is always an intellectual excitement of new technologies that may advance the pursuit of academic questions the real question is: How has or could functional imaging of pain make a difference in the lives of chronic pain patients now or in the future? We explore the rapid development of functional imaging in the pain field and try to put this question in context. It is now increasingly understood that pain represents a multifaceted process shaped by a multitude of factors (somatosensory, emotional, cognitive, genetic) and in turn affecting behavioral responses as well as producing an altered brain state. In addition, imaging may allow us to provide an objective measure of pain – one that may be complex and require taking into account sensory, emotional and modulatory processes in the context of expectations and life experiences. Imaging pain has already produced far reaching changes in the way we think about chronic pain (Apkarian and others 2009; Borsook and Becerra 2007; Tracey 2008; Tracey and Mantyh 2007) and defining a signature of changes in the brain that contribute or are part of the chronic pain syndrome, which will eventually result in better pain treatments. Indeed, the number of studies investigating the effects of therapy using imaging methods has also shown an increasing trend since 1993, reaching more than 6000 studies published between 2005 and 2008 (source: Google Scholar). ~ Insert Figure 2 here ~
Figure 2. Imaging Publications on Pain.
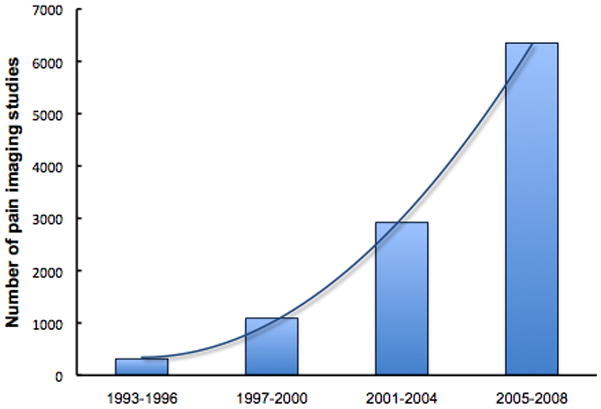
We used Google Scholar (scholar.google.com) to search for articles containing the keywords “pain” and “functional magnetic resonance imaging” during 4-year intervals. Even though the total number of articles may be overestimated by this method, similar exponential increasing trends were seen with more stringent search terms.
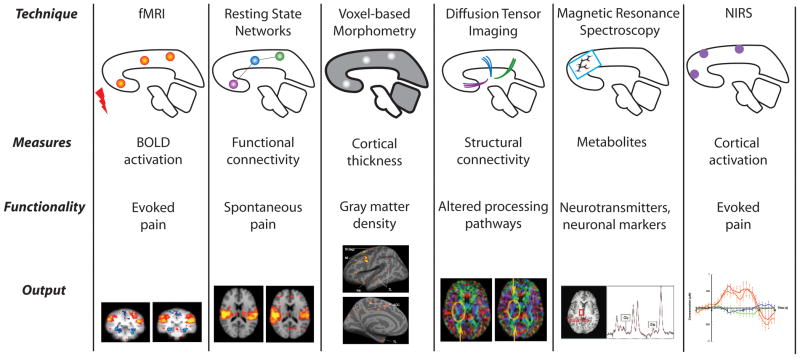
Pain Imaging: Methods 101 (Figure 3)
Figure 3. Imaging Methods.
(Sava et al., 2009, Mol Pain, Permission Pending)
The development of a number of non-invasive magnetic resonance imaging (MRI) methods, including morphological/anatomical imaging of gray matter (voxel based morphometry - VBM), white matter tract connectivity (diffusion tensor imaging - DTI), functional magnetic resonance imaging (fMRI), and magnetic resonance spectroscopy (MRS), has paved the way to an unprecedented boom in brain research. MRI methods, as well as other techniques like magnetic encephalography (MEG) and near infrared spectroscopy (NIRS) are rapidly evolving, as novel analytical methods and more sophisticated equipment become available. Because their non-invasive nature allows in vivo longitudinal studies of the dynamic structural and functional changes in the brain as a result of pain, these approaches (described in Figure 3) have produced a shift in our understanding of chronic pain. From the original definition as an “unpleasant sensory and emotional condition”, chronic pain is now understood to be a multidimensional “disease affecting the central nervous system”, influenced by a variety of biological and psychosocial factors, such as genetics, hormones, emotions, memories, or social expectations (Borsook and Becerra 2007; Borsook and Becerra 2006; Borsook and others 2007b). Application of combined novel research approaches (i.e., brain imaging and genetic and molecular studies) will likely have a great impact on the pain field by improving clinical evaluation methods (disease phenotype) and treatment of pain conditions.
Anatomical Imaging
Voxel-based morphometry (VBM)
VBM measures the local concentration of gray matter in different brain voxels. In the pain field, VBM has been used to measure changes in the volume of subcortical structures including the hippocampus, basal ganglia, thalamus and amygdala (Jovicich and others 2009). Most recently, techniques that allow measurement of small changes in cortical thickness have been developed (http://surfer.nmr.mgh.harvard.edu). These techniques will allow documentation of alterations of gray matter that occur in chronic pain conditions.
Diffusion Tensor Imaging
DTI investigates white matter tract integrity by measuring microstructural changes in directional water diffusion in the brain (Alexander and others 2007; Mukherjee and others 2008). In the pain field, the technique has been used in fibromyalgia to document alterations in the brain’s microcircuitry in the thalamus, insula, amygdala, hippocampus and frontal and anterior cingulate cortex (Lutz and others 2008). By overlaying DTI and fMRI brain maps, future studies may help advance our understanding of functional anatomical mapping in chronic pain conditions.
Functional Imaging
Blood oxygen level dependent (BOLD) fMRI measures changes in the local concentration of deoxyhemoglobin, and provides an indirect index of neuronal activity. Several BOLD methods have been applied to pain research and have revealed the neural correlates of pain perception and modulation by characterizing the brain response to evoked stimuli (e.g., pain, allodynia), task driven responses, or drugs (phMRI).
Evoked Stimuli fMRI
Evoked Stimuli fMRI has been commonly used in the pain field due to the relative ease of presenting well-characterized objective stimuli during the imaging session (i.e., cold and hot temperatures, somatosensory stimulation). Functional imaging has helped uncover the neural circuitry involved in pain processing and modulation, and described the brain areas that reflect sensory, cognitive and affective dimensions of pain (May 2007).
Resting State Networks (RSN) and Functional Connectivity
This approach uses low frequency BOLD signal fluctuations to evaluate the functional brain connectivity during resting states as opposed to task performance. These default mode networks are consistent across healthy subjects (Damoiseaux and others 2006) and can be used to define disease phenotypes by differentiating disease states (i.e., chronic back pain (Baliki and others 2008)) from healthy states. Simultaneous imaging of structural and functional connectivity may provide a better understanding of pathological processes by uncovering changes in specific brain networks as a result of disease.
Pharmacological MRI (phMRI)
Pharmacological MRI (phMRI) investigates the functional effects of drugs on the brain and links levels of drug exposure to the changes in evoked responses or RSN activity. More recently, arterial spin labeling (ASL) methodology, that measures blood flow changes with improved contrast and signal to noise ratio through magnetization of the blood, has been used to measure the regional dose-related effects of drugs on brain function (Detre and others 2009). These measures can be used to monitor the functional effects of drug receptor binding and the dose-relationship of central responses and provide objective indices of therapeutic efficacy in pain conditions.
Chemical CNS Measures
Magnetic Resonance Spectroscopy (MRS)
Magnetic Resonance Spectroscopy (MRS) is used to non-invasively assess different metabolites and neurotransmitters in the brain (Soares and Law 2009), to characterize the composition of neuronal and synaptic markers (e.g., glutamate, glutamine and gamma-aminobutyric acid [GABA]) in different brain regions, and to identify relationships between disease states and changes in the brain metabolic or chemical composition. MRS techniques have been widely applied in the study of psychiatric diseases as well as pain syndromes (Prescot and others 2009). Recently, analytic technologies such as 13C-based flux analysis have been developed. This fluxometric method allows real-time analysis of metabolic changes in brain networks. While this technology has so far been applied to mammalian cells grown in tissue culture but not to the human brain in vivo, it represents a potentially promising technique that in the future could aid the understanding of the disease-associated metabolic changes in the brain.
Brain Receptor Mapping
A novel approach that could aid the localization of functionally activated brain regions in the brain is mapping of multiple neurotransmitter receptors sites (Zilles and Amunts 2009). While not yet applied to pain conditions, this approach may provide a better understanding of the underlying basis of neurotransmission in healthy and disease states by correlating brain data obtained through different techniques (anatomical, functional) with cytoarchitectonical and molecular brain maps.
Near Infrared Spectroscopy
Near Infrared Spectroscopy (NIRS) or diffuse optical tomography (DOT) is a non-invasive technique that can detect changes in blood hemoglobin concentrations associated with neural activity and therefore assess the brain function through an intact skull in human subjects (Boas and others 2004). NIRS has a great potential in measuring pain effects on the brain. Recently, it had been shown that it is possible to record a pain-specific signal using NIRS, and this signal was similar to that observed in previous fMRI studies (Becerra and others 2008).
Magnetoencephalography
Magnetoencephalography (MEG) is a non-invasive imaging technique that measures the magnetic field produced by synchronized synaptic currents in the brain. Similar to electroencephalography (EEG), MEG directly measures parameters of neuronal activity. A rich literature exists that describes the correlation between neuronal oscillations as recorded by MEG and different brain functions, including attention, visual processing or motor planning (Bandettini 2009). Recently, a growing number of studies have employed both MEG and fMRI, taking advantage of the strengths of each method (i.e., excellent spatial resolution of fMRI and the millisecond temporal resolution of MEG) to help uncover the mechanisms of cortical processing (Auranen and others 2009).
Pain Imaging: Driving the New Revolution in Pain Research (Figure 4)
Figure 4. Broad Applications of fMRI in Pain.
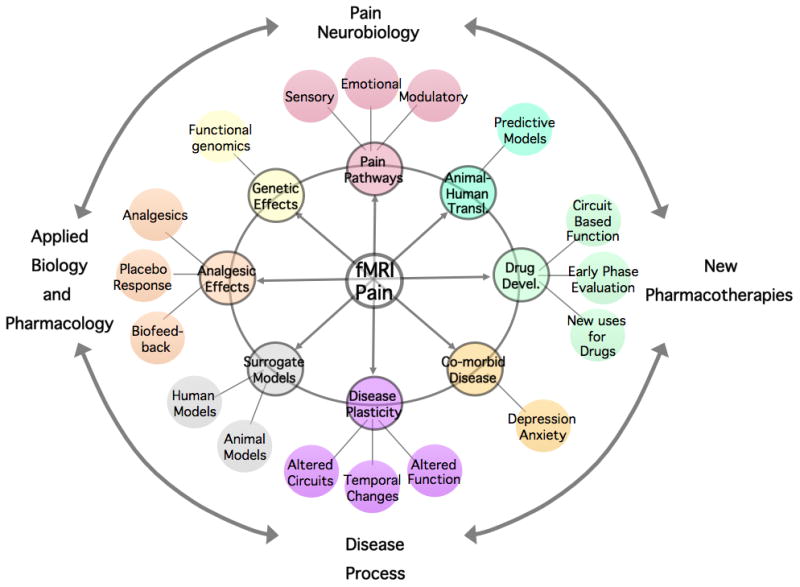
(Borsook and Becerra, 2007 Permission Pending, Current Pain and Headache, 2007)
Imaging Pain in Healthy Brains – The New Neurobiology
The early pain imaging studies used PET and reported on pain responses to noxious heat (see below). Since then, functional imaging studies in healthy volunteers have either confirmed brain regions involved in pain processing (thalamus, somatosensory cortex, anterior cingulate cortex) or added important new components of pain processing (e.g., nucleus accumbens, insula, dorsolateral prefrontal cortex, basal ganglia and cerebellum). Thus, there is a new complexity in understanding brain function in pain that allows for sensory, emotional/affective, modulatory and cognitive responses to pain. Functional imaging research in healthy subjects has provided new insights into these regions and their possible role in pain. As such, these studies have been invaluable in providing a basis to explore changes in the clinical condition and evaluation of analgesic drugs on brain function.
Brain Regions and Pain Function
In the first imaging study of pain, “Multiple representations of pain in the Human Cerebral Cortex”, Talbot and colleagues reported on activation in a number of brain regions in response to noxious heat, including the contralateral anterior cingulate cortex and primary and sensory somatosensory cortices (Talbot and others 1991). This study opened up the path for brain imaging of pain, which initially focused on ‘expected areas’ such as the thalamus. What these studies did is raise issues of pain processing in regions beyond the primary somatosensory cortex (Bushnell and others 1999; Treede and others 2000). Subsequently, numerous cortical regions have been shown across pain imaging studies to be activated by painful stimuli (reviewed in (Apkarian and others 2005; Peyron and others 2000; Treede and others 1999). Overall, these meta-analyses reported that pain produced activation in the primary and secondary somatosensory, insular, anterior cingulate and prefrontal cortices and thalamus. Some regions like the cerebellum (Borsook and others 2008), the anterior cingulate and insular cortices, are consistently activated across most functional imaging pain studies, but have remained an enigma as to a specific role in pain processing. More recent studies have begun to dissect the pain-induced brain activation as it relates to specific functions, such as sensory processing, emotional/affective and cognitive processing, and pain modulatory processing. The more focused studies have allowed for a better understanding of these regions in pain function.
Imaging Somatosensory Pain Processing
Somatosensory processing of pain stimuli classically include the thalamus and somatosensory cortices. Other studies have used imaging to trace a pain pathway (trigeminal) from the periphery (ganglion), to the dorsal horn (trigeminal nucleus in the brainstem), and traditional sensory pathways through the thalamus and to the cortex (Borsook and others 2003; DaSilva and others 2002). Insula is a recent addition to brain regions involved in the evaluation of pain intensity, and imaging studies have uncovered the somatosensory representation of pain in the insular cortex (Brooks and others 2005). Still, investigations of the insular functions warrant a broader look at the potential involvement in sensory and emotional evaluative components, as well as interoception (i.e., sensing the physiological condition of the body). As it turns out, this region is intricately involved in complex pain and analgesic processing (see below).
Imaging Emotional Pain Processing
The notion that pain is not only a sensory but also an emotional experience required neuroimaging to help define an brain underlying circuitry that contributes to the emotional processing of pain. Generally, greater acute and chronic pain intensity is associated with higher negative emotional state. The experience of pain is able to trigger emotional responses, and the emotional state can also affect the perception of pain. Studies indicated that the classic reward circuitry, that includes regions such as the amygdala, nucleus accumbens and orbitofrontal lobe were all activated by noxious heat (Becerra and others 2001; Becerra and others 2004). Specific connectivity between entorrhinal cortex and cingulate regions involved in anxiety and anticipation of pain was also described (Ploghaus and others 2001). Such studies contributed to the characterization of a brain network that could underlie, in addition to emotional responses to pain, the placebo and nocebo responses (Craggs and others 2007; Scott and others 2008), as well as empathy of pain in others (Danziger and others 2009). This network has also been involved in the development of co-morbid symptoms (e.g., depression, anxiety) frequently associated with pain (Borsook and others 2007a). A recent review suggested that the reciprocal effects of pain on emotional state could be explained by the common anatomical brain network shared by these two processes (Duquette and others 2007). Many brain regions have been discussed in previous reviews (Bruehl and others 2009). Perhaps the dorsolateral prefrontal (DLPFC) and medioprefrontal (mPFC) cortices, the cingulate cortex (CC) and basal ganglia are worthy of further mention because of their relative importance in understanding chronic pain. Besides their role in the emotional pain processing, DLPFC, mPFC and the CC are also part of the modulatory networks that can alter pain perception, as well as networks involved in cognitive processing (see below). The anatomical overlap of these neuronal networks and the known roles of the frontal cortical regions in emotion and cognition may explain the wide effects that pain has on multiple brain functions.
Pain and analgesia are at opposite ends of the reward-aversion spectrum. However, the neural circuits that support these functions are similar (Leknes and Tracey 2008). Endogenous systems including opioidergic and dopaminergic may provide useful models for evaluating these opponent processes in chronic pain and nocebo and placebo responses (Scott and others 2008).
Cognitive Processing and Pain
Pain can affect cognitive processing, but the neural substrates of this interaction are not well elucidated. Given that acute pain has an adaptive role of signaling injury to the body, it represents a stimulus that can induce rapid emotional learning involving the prefrontal-limbic circuitry (amygdala, insula, the anterior cingulate and orbitofrontal cortex; (Sehlmeyer and others 2009)). However, patients with chronic pain however often complain of attention and memory deficits. It has been postulated that pain modulates an attention-specific network which includes the DLPFC, anterior and posterior cingulate cortices (ACC and PCC), posterior parietal cortex and medial frontal cortex (Seminowicz and Davis 2007).
Imaging Modulatory Circuits
Understanding modulatory pain processing (both pro- and antinociceptive effects) has enormous implications for evaluating alterations in disease state and analgesic drug effects (Porreca and others 2002). The basic neurobiology of modulatory circuits had been defined previously (Basbaum and Fields 1984). A network of subcortical and cortical regions (predominantly frontal areas) has been involved in endogenous pain modulation. Functional imaging studies have been able to evaluate descending modulatory processes in experimental pain and have shown a direct participation of well-described regions such as the periaqueductal gray (PAG) (Becerra and others 2001) and less well understood regions such as the nucleus cuneiformis (NCF) in pain processing. Among cortical areas, the medial prefrontal cortex (mPFC) exerts an inhibitory effect on the perception of pain (Seifert and others 2009a). The DLPFC, orbitofrontal cortex (OFC) and cingulate cortex were also found to have key roles in cortical mechanisms of pain modulation. The pain-modulating roles of the frontal cortices might be mediated by cognitive interference during nociceptive stimulation (Wager and others 2004), since patients with chronic pain show increased ‘vigilance’ towards pain and pain-related information. Recent studies have proposed that an altered interaction of pain-inhibitory and pain-facilitatory mechanisms may contribute to the development or maintenance of chronic pain states (Bingel and others 2007).
Applied Neurobiology – From Theory to Function
Imaging Analgesic Effect in Healthy Volunteers with pharmacological MRI (phMRI)
Although many drugs used as analgesics influence CNS function, little is known about the direct effects of these agents on the brain or the mechanisms through which they provide analgesia in humans. PhMRI studies use two approaches: evaluating the brain regions that show activation by the drug and the drug effect on the modulation of pain processing in the brain. In healthy volunteers, studies have evaluated opioids including morphine (Becerra and others 2006a) and remifentanil (Wagner and others 2007; Wise and others 2002), naloxone (Borras and others 2004), ketamine (Rogers and others 2004; Sprenger and others 2006) local anesthetics (Seifert and others 2009a), cox inhibitors (Maihofner and others 2007b) and drugs used in neuropathic pain including gabapentin, and imipramine (Borsook and Becerra 2006; Gottrup and others 2004; Iannetti and others 2005). Overall these types of studies have provided a basis to investigate pharmacological effects on brain systems (Borsook and others 2006) as provided by the following three examples. (i) Morphine, a well characterized drug behaviorally, affects neural circuits that are expected to define these behavioral features of the drug (e.g., sedation, reward, analgesia) (Becerra and others 2006a). (ii) Gabapentin, while having a measurable antinociceptive effect on activation patterns, it has a more profound antihyperalgesic effect, suggesting that the drug may be more effective in modulating pain when central sensitization is present (Iannetti and others 2005). (iii) Activation patterns in specific brain regions such mPFC were shown to inversely correlate with individual extent of central hyperalgesia and predicts individual pharmacological antihyperalgesic treatment response (Seifert and others 2009a). Although remarkable progress has been made, the real advances are still to come in using fMRI on drug development to evaluate new drugs (Borsook and others 2006; Wise and Tracey 2006).
Imaging Gender Differences
Given that many chronic pain conditions predominantly affect women [e.g., complex regional pain syndrome - CRPS (Birklein and others 2000), fibromyalgia (Hooten and others 2007), temporomandibular disease (Dao and LeResche 2000), irritable bowel syndrome (Chial and Camilleri 2002), headache (Silberstein 1992)], it is possible that gender differences exist in pain processing. While some of the gender variability in pain thresholds and pain may result from genetic differences at loci on the sex chromosomes, current data on pain sensitivity in women (Fillingim and others 2009; Wiesenfeld-Hallin 2005) and brain activation studies suggest a hormonal contribution as well, supported by the finding that differences in the response to pain are found during the follicular and luteal phase (Choi and others 2006). Still, many pain-imaging studies do not account for the menstrual phase in women who are selected as part of a cohort. In addition, menstrual phase alters reward related functions (Dreher and others 2007) as well as chemistry in cortical regions (Epperson and others 2002) that may have an impact in chronic pain conditions where there is a hedonic deficit syndrome. Importantly, the data suggests that differences in pain responses in women as a result of the modulatory effects of sex hormones may have important implications for therapy.
Imaging and Surrogate Pain Models
One of the big questions in pain research is the translation of surrogate models to the clinical condition. Since brain circuits can be measured in both the surrogate model (e.g., capsaicin induced central sensitization) as well as in human models, imaging may help define the utility of such models for testing analgesic efficacy or understanding brain processes that contribute to the chronic pain condition.
Imaging the Placebo Response
Imaging has allowed for a better understanding of the biological mechanisms systems that determine the placebo response (Benedetti and others 2005; Zubieta and Stohler 2009). The placebo response in pain is based on beliefs, expectations and anticipation of pain. The neural mechanisms of this effect relate to brain regions involved in expectancies (cognitive processing) and also reward related functions that include dopaminergic (Wise 2004) and opioidergic systems (Henriksen and Willoch 2008). Both improvement (placebo/therapeutic response), or worsening (nocebo/adverse response) of pain may result from altered brain processing. Functional imaging has focused on a few approaches that investigate the placebo response as provided by the three examples that follow. (i) Altered expectations in the response to painful stimuli: Functional MRI experiments using manipulation of expectations in healthy volunteers showed that placebo analgesia resulted in a decrease in activation in thalamus, insula and ACC with a corresponding increase in activity in the prefrontal cortex in anticipation of pain (Wager and others 2004). Studies such as this one provided evidence for the involvement of pain related structures in the placebo response. (ii) Alterations related to opioidergic and dopaminergic function: Prior studies had evaluated the role of endogenous opioid systems in the placebo response using PET (Petrovic and others 2002). The data indicated that both opioid and placebo analgesia are associated with increased activity in the rostral ACC and in the brainstem, suggesting that placebo-induced analgesia affects the pain circuitry. More recently, this approach has been extended to include evaluation of underlying endogenous chemical processes. Two neurotransmitter systems, one involved in analgesia (opioidergic) and another in reward (dopaminergic), were examined for their potential role in placebo and nocebo responses (Scott and others 2008). The results of this study are highly significant in furthering our understanding of these responses. Consistent with the Petrovic study, opioid neurotranmission was increased in the ACC, Gob, insular cortex, nucleus accumbens (NAc), amygdala and PAG, while dopaminergic neurotransmission was increased in the basal ganglia with the placebo response. Placebo effects correlated with increased opioidergic and dopaminergic responses in the NAc, while nocebo effects were associated with decreased response of both neurotransmitters. (iii) Placebo responses in diseases where expectations may be altered: In patient populations, the study of the placebo responses and how they may be affected by disease has enormous implications for treatments and clinical trials, as the loss of expectation makes analgesic therapies less effective (Benedetti and others 2006).
Acupuncture
Imaging has advanced acupuncture from ‘a difficult to understand’ process to a more plausible process that has scientific underpinnings. While the basis for neural systems involved in acupuncture has not clearly been differentiated from placebo response, some new data adds important information on the underlying CNS processes involved in acupuncture-induced pain control. One such study, using PET, differentiates traditional acupuncture from sham acupuncture based on differences in effects on opioid receptors (Harris and others 2009) suggesting a basis for mediation of analgesic effects in real acupuncture. In a similar vein, differentiation of acupuncture analgesia and expectancy evoked placebo analgesia seems to involve different brain networks (Kong and others 2009).
Imaging, Genetics and Pain
Given the tendency of chronic pain syndromes to aggregate within families, these differences might be genotypically-mediated, even though no definitive pain-susceptibility genes have been identified thus far. Even though the influence of shared environmental factors can not be excluded, recent twin studies highlight the genetic contribution to pain sensitivity (Nielsen and others 2005). The most notable imaging and genetic studies to date have evaluated the contribution of the catecholaminergic and opioidergic systems to pain (Oertel and others 2008; Zubieta and others 2003).
Imaging Chronic Pain - Disease Process and New Discoveries
Imaging patients with chronic pain has been a greater challenge than imaging healthy volunteers, owing to a number of confounding factors like current treatments and duration of disease. Nevertheless, advances have been made in imaging of a number of chronic pain conditions including chronic back pain (Apkarian and others 2001), complex regional pain syndrome (Lebel and others 2008; Maihofner and others 2004), neuropathic pain (Becerra and others 2006b; Geha and Apkarian 2005), fibromyalgia, gastrointestinal disease (Kwan and others 2005). Such studies are attempting to define specific brain phenotypes for different chronic pain conditions, and they could greatly advance diagnostic methods and therapy, with the final goal of developing disease-modifying treatments. A number of important and novel insights related to pain processing and treatment effects have been described in these patient populations.
Functional Reorganization
Functional reorganization and chronic pain was most clearly defined in a report on patients with upper extremity amputation and pain (Flor and others 1995). Since then, a number of papers have described how plasticity occurs in neural circuits in a number of chronic pain conditions, including complex regional pain syndrome (Maihofner and others 2007a; Maihofner and others 2006). Most importantly, it seems as if appropriate treatments “reconstitute” or normalize brain activation patterns concomitant with remission of pain (Flor 2003).
Altered Pain Modulation
Chronic pain patients may have decreased opioid receptor availability (Harris and others 2007) as well as enhanced pain responses or impairment of antinociceptive modulatory processes (Jensen and others 2009; Seifert and others 2009b). An alteration in the tone of inhibitory vs. facilitatory systems may underlie the unmasking or exacerbation of chronic pain syndromes. In this type of data, imaging has helped define specific regions that show abnormal activation patterns and provided a method to determine if effective therapies alter these abnormal patterns.
Altered Morphometry
In recent years a number of laboratories have reported on decreased cortical and subcortical gray matter (using voxel based morphometry) in chronic pain in a variety of conditions, including chronic back pain (Apkarian and others 2004) neuropathic pain (DaSilva and others 2008) and fibromyalgia (Kuchinad and others 2007). These changes in brain structure seem to be related to the chronicity of the pain, and have redefined chronic pain as a degenerative disorder. While the precise mechanism of altered brain volume is not fully described, some studies have pointed to the potential loss of neurons and dendritic spines as potential contributors (Metz and others 2009). As such, our treatment approaches to chronic pain should be radically redefined to include methods of preventing neuronal degeneration and promoting neuronal survival.
Altered Chemistry
Chemical measures using MRS have shown altered neurotransmitters in chronic back pain (Grachev and others 2000), migraine (Prescott and others 1993), CRPS (Grachev and others 2002) and fibromyalgia (Harris and others 2008).
Brain Measures of Spontaneous Pain
The spontaneous component of chronic pain is a critical component of pain symptomatology, and neuroimaging is exploring CNS circuits that are involved in spontaneous or ongoing pain. A few reports have evaluated spontaneous pain in diabetic neuropathy (Cauda and others 2009) and treatment effects of ketamine (Becerra and others 2009). Default mode resting states are disrupted in chronic pain (Baliki and others 2008). While still early, this approach holds the promise of defining new evaluative processes for disease state and therapeutic effect.
Pain Imaging: Clinical Relevance and Future Clinical Applications
Our understanding of the brain changes in chronic pain and the brain responses to pharmacological or other therapeutic interventions has been significantly changed as a result of developments in neuroimaging of the CNS (Borsook and Becerra 2006; Borsook and others 2007b; Casey and others 2003; Moisset and Bouhassira 2007). These domains have already changed the way in which we think of pain - it should now be considered an altered brain state in which there may be altered functional connections or systems concurrent with degenerative aspects of the CNS. In addition, future developments will inevitably lead to major progress in a number of areas (see below) that will enhance our understanding of pain and eventually have significant impact in the clinic.
Going Beyond Subjective Ratings
Experimental studies have shown that identical stimuli applied to research volunteers elicit widely variable pain responses (i.e., thresholds, tolerance) and psychophysical ratings (Nielsen and others 2005). Interestingly, the individual differences in pain ratings correlate with cortical activation differences observed on fMRI studies, but not with thalamic activation, suggesting that even though the afferent input is similar at thalamic level, it is being modified at the cortical level resulting in the subject-specific experience of pain (Nielsen and others 2005). Imaging methods should allow us to go beyond visual or verbal analogue scale evaluations of pain for a number of reasons. First, subjective measures are highly variable and we usually evaluate these along a single scale (e.g., pain intensity or sensory experience), while imaging is clearly more objective and also provides an assay of potential function in multiple brain regions involved in the pain experience. Second, imaging provides evidence for changes that may have affected the brain over time. Third, imaging provides the ability to define changes across different brain measures, from functional to anatomical integrity and chemical changes. Pain is obviously a chronic disease and the ability to take a snapshot that provides so much additional information should be useful in the clinical domain.
Brain ‘Pain’ Phenotype: An objective biomarker for Pain and Analgesia
In clinical practice, as noted above, the use of drugs for treatments pain diseases is frequently empirical. The defining of an objective brain phenotype for “Drug Effect” or “Disease State” would obviously be a major step forward in understanding and discovering new treatments. Defining a brain pain phenotype or biomarker will allow for a correlation of brain activity with pain measures (i.e., duration, intensity, frequency) and the changes in brain activity in response to therapeutic interventions that lead to pain alleviation. This will surely transform the way we understand pain and allow researchers to use the measures of brain function as an intermediate phenotype for studying pain processing and for developing new therapies. For drug development, defining a pain phenotype through imaging might translate into potential regulatory acceptance of using fewer subjects in FDA approved trails.
Analgesic Drug Development
Currently, few effective treatments for pain are available. Translation from preclinical to the clinical domain has proven to be highly inefficient with most analgesic drug candidates failing because of bothersome side effects or low efficacy. PhMRI and functional imaging may provide early readouts for ‘go’ or ‘no go’ decisions in drug development (Borsook and others 2006).
Application in the Clinic
Given that a brain “engram” provided by imaging could provide information on (i) diagnosis; (ii) measure of changes in sensory, emotional and modulatory circuits; (iii) measures of morphological change; (iv) measures of chemical changes; (v) drug effects – both in terms of symptomatic treatment and disease modification; and (vi) underlying brain changes that may precede subjective changes, the opportunity for the “pain clinic of the future” could parallel the fMRI application in neurosurgery (Chakraborty and McEvoy 2008). In addition, segregation of addiction vs. analgesic effects may also be possible, affording better therapy in those patients who may need addictive medications to control their pain.
Transition from Acute to Chronic Pain
It is still unknown why some individuals develop chronic pain after an injury or disease process. This represents a great clinical challenge. Some examples include surgery (even relatively minor surgery such as third molar tooth extraction) leading to chronic neuropathic pain (Katz and Seltzer 2009) or acute to chronic migraine (Lipton 2009). Some studies have suggested that genetic (Tegeder and Lotsch 2009) and other premorbid factors (Young Casey and others 2008) may contribute to the development of chronic pain. Imaging studies have only recently begun to address the process of pain ‘chronicization’, with the expectation that having an early readout of this process would allow interventional therapeutic trials to be conducted.
Imaging and Patient Evaluation for Disability
While there is great interest in objective measures of pain-related disability from insurance companies and the law, we have some ground to cover before brain imaging could be used as a diagnostic tool, because it first has to meet several criteria to be validated and accepted (Borsook and Becerra 2005). These issues are being addressed from different perspectives, including neuroethics. If objective valid processes can be established for detecting/defining pain, this would have enormous implications for the the insurance industry and legal field, as a significant number of cases relate to pain, suffering and disability (Kolber A.J. American Journal of Law & Medicine (Brain Imaging & The Law Symposium), Vol. 33, p. 433, 2007 San Diego Legal Studies Paper No. 07-9). Also, it will provide patients with objective evidence of their condition and its change over time.
Going Forward – The Challenges
Evidence from imaging studies in humans points to the pain experience as being complex and involving not only somatosensory pathways but also brain systems that regulate the processing of emotion, motivation and memory. Individual expectations and even perception of social roles can shape the way subjects perceive pain. Consequently, the way we approach pain management should shift from treating a symptom to treating a disease that greatly affects the brain. Because a multitude of factors contribute to the pain experience, it is expected that successful therapies will produce normalization across multiple pain domains and limit the development of long-term consequences.
Acknowledgments
Supported by NINDS grant K24 064050 (DB).
References
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–29. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87(2):81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Krauss BR, Fredrickson BE, Szeverenyi NM. Imaging the pain of low back pain: functional magnetic resonance imaging in combination with monitoring subjective pain perception allows the study of clinical pain states. Neurosci Lett. 2001;299(1–2):57–60. doi: 10.1016/s0304-3940(01)01504-x. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24(46):10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auranen T, Nummenmaa A, Vanni S, Vehtari A, Hamalainen MS, Lampinen J, et al. Automatic fMRI-guided MEG multidipole localization for visual responses. Hum Brain Mapp. 2009;30(4):1087–99. doi: 10.1002/hbm.20570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28(6):1398–403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA. What’s new in neuroimaging methods? Ann N Y Acad Sci. 2009;1156:260–93. doi: 10.1111/j.1749-6632.2009.04420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–38. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32(5):927–46. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Becerra L, Harris W, Joseph D, Huppert T, Boas DA, Borsook D. Diffuse optical tomography of pain and tactile stimulation: activation in cortical sensory and emotional systems. Neuroimage. 2008;41(2):252–9. doi: 10.1016/j.neuroimage.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Harter K, Gonzalez RG, Borsook D. Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volunteers. Anesth Analg. 2006a;103(1):208–16. doi: 10.1213/01.ane.0000221457.71536.e0. table of contents. [DOI] [PubMed] [Google Scholar]
- Becerra L, Iadarola M, Borsook D. CNS activation by noxious heat to the hand or foot: site-dependent delay in sensory but not emotion circuitry. J Neurophysiol. 2004;91(1):533–41. doi: 10.1152/jn.00326.2003. [DOI] [PubMed] [Google Scholar]
- Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, et al. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci. 2006b;26(42):10646–57. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Schwartzman RJ, Kiefer RT, Rohr P, Moulton EA, Wallin D, et al. CNS Measures of Pain Responses Pre- and Post-Anesthetic Ketamine in a Patient with Complex Regional Pain Syndrome. Pain Med. 2009 doi: 10.1111/pme.12939. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Arduino C, Costa S, Vighetti S, Tarenzi L, Rainero I, et al. Loss of expectation-related mechanisms in Alzheimer’s disease makes analgesic therapies less effective. Pain. 2006;121(1–2):133–44. doi: 10.1016/j.pain.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK. Neurobiological mechanisms of the placebo effect. J Neurosci. 2005;25(45):10390–402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel U, Schoell E, Buchel C. Imaging pain modulation in health and disease. Curr Opin Neurol. 2007;20(4):424–31. doi: 10.1097/WCO.0b013e328259c34d. [DOI] [PubMed] [Google Scholar]
- Birklein F, Riedl B, Sieweke N, Weber M, Neundorfer B. Neurological findings in complex regional pain syndromes--analysis of 145 cases. Acta Neurol Scand. 2000;101(4):262–9. doi: 10.1034/j.1600-0404.2000.101004262x./. [DOI] [PubMed] [Google Scholar]
- Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ. Chronic pain in Australia: a prevalence study. Pain. 2001;89(2–3):127–34. doi: 10.1016/s0304-3959(00)00355-9. [DOI] [PubMed] [Google Scholar]
- Boas DA, Dale AM, Franceschini MA. Diffuse optical imaging of brain activation: approaches to optimizing image sensitivity, resolution, and accuracy. Neuroimage. 2004;23(Suppl 1):S275–88. doi: 10.1016/j.neuroimage.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Borras MC, Becerra L, Ploghaus A, Gostic JM, DaSilva A, Gonzalez RG, et al. fMRI measurement of CNS responses to naloxone infusion and subsequent mild noxious thermal stimuli in healthy volunteers. J Neurophysiol. 2004;91(6):2723–33. doi: 10.1152/jn.00249.2003. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L. Functional imaging of pain and analgesia--a valid diagnostic tool? Pain. 2005;117(3):247–50. doi: 10.1016/j.pain.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L. Phenotyping central nervous system circuitry in chronic pain using functional MRI: considerations and potential implications in the clinic. Curr Pain Headache Rep. 2007;11(3):201–7. doi: 10.1007/s11916-007-0191-7. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Carlezon WA, Jr, Shaw M, Renshaw P, Elman I, et al. Reward-aversion circuitry in analgesia and pain: implications for psychiatric disorders. Eur J Pain. 2007a;11(1):7–20. doi: 10.1016/j.ejpain.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra L, Hargreaves R. A role for fMRI in optimizing CNS drug development. Nat Rev Drug Discov. 2006;5(5):411–24. doi: 10.1038/nrd2027. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra LR. Breaking down the barriers: fMRI applications in pain, analgesia and analgesics. Mol Pain. 2006;2:30. doi: 10.1186/1744-8069-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, DaSilva AF, Ploghaus A, Becerra L. Specific and somatotopic functional magnetic resonance imaging activation in the trigeminal ganglion by brush and noxious heat. J Neurosci. 2003;23(21):7897–903. doi: 10.1523/JNEUROSCI.23-21-07897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Moulton EA, Schmidt KF, Becerra LR. Neuroimaging revolutionizes therapeutic approaches to chronic pain. Mol Pain. 2007b;3:25. doi: 10.1186/1744-8069-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Moulton EA, Tully S, Schmahmann JD, Becerra L. Human cerebellar responses to brush and heat stimuli in healthy and neuropathic pain subjects. Cerebellum. 2008;7(3):252–72. doi: 10.1007/s12311-008-0011-6. [DOI] [PubMed] [Google Scholar]
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27(1):201–9. doi: 10.1016/j.neuroimage.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Burns JW, Chung OY, Chont M. Pain-related effects of trait anger expression: neural substrates and the role of endogenous opioid mechanisms. Neurosci Biobehav Rev. 2009;33(3):475–91. doi: 10.1016/j.neubiorev.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci U S A. 1999;96(14):7705–9. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KL, Lorenz J, Minoshima S. Insights into the pathophysiology of neuropathic pain through functional brain imaging. Exp Neurol. 2003;184(Suppl 1):S80–8. doi: 10.1016/j.expneurol.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Cauda F, Sacco K, Duca S, Cocito D, D’Agata F, Geminiani GC, et al. Altered resting state in diabetic neuropathic pain. PLoS ONE. 2009;4(2):e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A, McEvoy AW. Presurgical functional mapping with functional MRI. Curr Opin Neurol. 2008;21(4):446–51. doi: 10.1097/WCO.0b013e32830866e2. [DOI] [PubMed] [Google Scholar]
- Chial HJ, Camilleri M. Gender differences in irritable bowel syndrome. J Gend Specif Med. 2002;5(3):37–45. [PubMed] [Google Scholar]
- Choi JC, Park SK, Kim YH, Shin YW, Kwon JS, Kim JS, et al. Different brain activation patterns to pain and pain-related unpleasantness during the menstrual cycle. Anesthesiology. 2006;105(1):120–7. doi: 10.1097/00000542-200607000-00021. [DOI] [PubMed] [Google Scholar]
- Craggs JG, Price DD, Verne GN, Perlstein WM, Robinson MM. Functional brain interactions that serve cognitive-affective processing during pain and placebo analgesia. Neuroimage. 2007;38(4):720–9. doi: 10.1016/j.neuroimage.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger N, Faillenot I, Peyron R. Can we share a pain we never felt? Neural correlates of empathy in patients with congenital insensitivity to pain. Neuron. 2009;61(2):203–12. doi: 10.1016/j.neuron.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Dao TT, LeResche L. Gender differences in pain. J Orofac Pain. 2000;14(3):169–84. discussion 184–95. [PubMed] [Google Scholar]
- DaSilva AF, Becerra L, Makris N, Strassman AM, Gonzalez RG, Geatrakis N, et al. Somatotopic activation in the human trigeminal pain pathway. J Neurosci. 2002;22(18):8183–92. doi: 10.1523/JNEUROSCI.22-18-08183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva AF, Becerra L, Pendse G, Chizh B, Tully S, Borsook D. Colocalized structural and functional changes in the cortex of patients with trigeminal neuropathic pain. PLoS ONE. 2008;3(10):e3396. doi: 10.1371/journal.pone.0003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA, Wang J, Wang Z, Rao H. Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Curr Opin Neurol. 2009;22(4):348–55. doi: 10.1097/WCO.0b013e32832d9505. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22(1):62–8. doi: 10.3122/jabfm.2009.01.080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A. 2007;104(7):2465–70. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquette M, Roy M, Lepore F, Peretz I, Rainville P. Cerebral mechanisms involved in the interaction between pain and emotion. Rev Neurol (Paris) 2007;163(2):169–79. doi: 10.1016/s0035-3787(07)90388-4. [DOI] [PubMed] [Google Scholar]
- Elliott AM, Smith BH, Penny KI, Smith WC, Chambers WA. The epidemiology of chronic pain in the community. Lancet. 1999;354(9186):1248–52. doi: 10.1016/s0140-6736(99)03057-3. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59(9):851–8. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H. Cortical reorganisation and chronic pain: implications for rehabilitation. J Rehabil Med. 2003;41(Suppl):66–72. doi: 10.1080/16501960310010179. [DOI] [PubMed] [Google Scholar]
- Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375(6531):482–4. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- Geha PY, Apkarian AV. Brain imaging findings in neuropathic pain. Curr Pain Headache Rep. 2005;9(3):184–8. doi: 10.1007/s11916-005-0060-1. [DOI] [PubMed] [Google Scholar]
- Gottrup H, Juhl G, Kristensen AD, Lai R, Chizh BA, Brown J, et al. Chronic oral gabapentin reduces elements of central sensitization in human experimental hyperalgesia. Anesthesiology. 2004;101(6):1400–8. doi: 10.1097/00000542-200412000-00021. [DOI] [PubMed] [Google Scholar]
- Grachev ID, Fredrickson BE, Apkarian AV. Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. Pain. 2000;89(1):7–18. doi: 10.1016/S0304-3959(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Grachev ID, Thomas PS, Ramachandran TS. Decreased levels of N-acetylaspartate in dorsolateral prefrontal cortex in a case of intractable severe sympathetically mediated chronic pain (complex regional pain syndrome, type I) Brain Cogn. 2002;49(1):102–13. doi: 10.1006/brcg.2001.1489. [DOI] [PubMed] [Google Scholar]
- Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27(37):10000–6. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RE, Sundgren PC, Pang Y, Hsu M, Petrou M, Kim SH, et al. Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in fibromyalgia. Arthritis Rheum. 2008;58(3):903–7. doi: 10.1002/art.23223. [DOI] [PubMed] [Google Scholar]
- Harris RE, Zubieta JK, Scott DJ, Napadow V, Gracely RH, Clauw DJ. Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on mu-opioid receptors (MORs) Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen G, Willoch F. Imaging of opioid receptors in the central nervous system. Brain. 2008;131(Pt 5):1171–96. doi: 10.1093/brain/awm255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooten WM, Townsend CO, Decker PA. Gender differences among patients with fibromyalgia undergoing multidisciplinary pain rehabilitation. Pain Med. 2007;8(8):624–32. doi: 10.1111/j.1526-4637.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Zambreanu L, Wise RG, Buchanan TJ, Huggins JP, Smart TS, et al. Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans. Proc Natl Acad Sci U S A. 2005;102(50):18195–200. doi: 10.1073/pnas.0506624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, et al. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain. 2009;144(1–2):95–100. doi: 10.1016/j.pain.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46(1):177–92. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9(5):723–44. doi: 10.1586/ern.09.20. [DOI] [PubMed] [Google Scholar]
- Kong J, Kaptchuk TJ, Polich G, Kirsch I, Vangel M, Zyloney C, et al. Expectancy and treatment interactions: a dissociation between acupuncture analgesia and expectancy evoked placebo analgesia. Neuroimage. 2009;45(3):940–9. doi: 10.1016/j.neuroimage.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27(15):4004–7. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan CL, Diamant NE, Pope G, Mikula K, Mikulis DJ, Davis KD. Abnormal forebrain activity in functional bowel disorder patients with chronic pain. Neurology. 2005;65(8):1268–77. doi: 10.1212/01.wnl.0000180971.95473.cc. [DOI] [PubMed] [Google Scholar]
- Lebel A, Becerra L, Wallin D, Moulton EA, Morris S, Pendse G, et al. fMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain. 2008;131(Pt 7):1854–79. doi: 10.1093/brain/awn123. [DOI] [PubMed] [Google Scholar]
- Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9(4):314–20. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- Lipton RB. Tracing transformation: chronic migraine classification, progression, and epidemiology. Neurology. 2009;72(5 Suppl):S3–7. doi: 10.1212/WNL.0b013e3181974b19. [DOI] [PubMed] [Google Scholar]
- Lutz J, Jager L, de Quervain D, Krauseneck T, Padberg F, Wichnalek M, et al. White and gray matter abnormalities in the brain of patients with fibromyalgia: a diffusion-tensor and volumetric imaging study. Arthritis Rheum. 2008;58(12):3960–9. doi: 10.1002/art.24070. [DOI] [PubMed] [Google Scholar]
- Lynch ME, Campbell FA, Clark AJ, Dunbar MJ, Goldstein D, Peng P, et al. Waiting for treatment for chronic pain - a survey of existing benchmarks: toward establishing evidence-based benchmarks for medically acceptable waiting times. Pain Res Manag. 2007;12(4):245–8. doi: 10.1155/2007/891951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maihofner C, Baron R, DeCol R, Binder A, Birklein F, Deuschl G, et al. The motor system shows adaptive changes in complex regional pain syndrome. Brain. 2007a;130(Pt 10):2671–87. doi: 10.1093/brain/awm131. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Handwerker HO, Neundorfer B, Birklein F. Cortical reorganization during recovery from complex regional pain syndrome. Neurology. 2004;63(4):693–701. doi: 10.1212/01.wnl.0000134661.46658.b0. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Neundorfer B, Birklein F, Handwerker HO. Mislocalization of tactile stimulation in patients with complex regional pain syndrome. J Neurol. 2006;253(6):772–9. doi: 10.1007/s00415-006-0117-z. [DOI] [PubMed] [Google Scholar]
- Maihofner C, Ringler R, Herrndobler F, Koppert W. Brain imaging of analgesic and antihyperalgesic effects of cyclooxygenase inhibition in an experimental human pain model: a functional MRI study. Eur J Neurosci. 2007b;26(5):1344–56. doi: 10.1111/j.1460-9568.2007.05733.x. [DOI] [PubMed] [Google Scholar]
- May A. Neuroimaging: visualising the brain in pain. Neurol Sci. 2007;28(Suppl 2):S101–7. doi: 10.1007/s10072-007-0760-x. [DOI] [PubMed] [Google Scholar]
- Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A. 2009;106(7):2423–8. doi: 10.1073/pnas.0809897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisset X, Bouhassira D. Brain imaging of neuropathic pain. Neuroimage. 2007;37(Suppl 1):S80–8. doi: 10.1016/j.neuroimage.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Berman JI, Chung SW, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. AJNR Am J Neuroradiol. 2008;29(4):632–41. doi: 10.3174/ajnr.A1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen CS, Price DD, Vassend O, Stubhaug A, Harris JR. Characterizing individual differences in heat-pain sensitivity. Pain. 2005;119(1–3):65–74. doi: 10.1016/j.pain.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Oertel BG, Preibisch C, Wallenhorst T, Hummel T, Geisslinger G, Lanfermann H, et al. Differential opioid action on sensory and affective cerebral pain processing. Clin Pharmacol Ther. 2008;83(4):577–88. doi: 10.1038/sj.clpt.6100441. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia-- imaging a shared neuronal network. Science. 2002;295(5560):1737–40. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30(5):263–88. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21(24):9896–903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25(6):319–25. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Prescot A, Becerra L, Pendse G, Tully S, Jensen E, Hargreaves R, et al. Excitatory neurotransmitters in brain regions in interictal migraine patients. Mol Pain. 2009;5:34. doi: 10.1186/1744-8069-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott DM, Charles HC, Sostman HD, Page RL, Thrall DE, Moore D, et al. Manipulation of intra- and extracellular pH in spontaneous canine tumours by use of hyperglycaemia. Int J Hyperthermia. 1993;9(5):745–54. doi: 10.3109/02656739309032061. [DOI] [PubMed] [Google Scholar]
- Rogers R, Wise RG, Painter DJ, Longe SE, Tracey I. An investigation to dissociate the analgesic and anesthetic properties of ketamine using functional magnetic resonance imaging. Anesthesiology. 2004;100(2):292–301. doi: 10.1097/00000542-200402000-00018. [DOI] [PubMed] [Google Scholar]
- Schwerla F, Bischoff A, Nurnberger A, Genter P, Guillaume JP, Resch KL. Osteopathic treatment of patients with chronic non-specific neck pain: a randomised controlled trial of efficacy. Forsch Komplementmed. 2008;15(3):138–45. doi: 10.1159/000132397. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65(2):220–31. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, et al. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS ONE. 2009;4(6):e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert F, Bschorer K, De Col R, Filitz J, Peltz E, Koppert W, et al. Medial prefrontal cortex activity is predictive for hyperalgesia and pharmacological antihyperalgesia. J Neurosci. 2009a;29(19):6167–75. doi: 10.1523/JNEUROSCI.4654-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert F, Kiefer G, DeCol R, Schmelz M, Maihofner C. Differential endogenous pain modulation in complex-regional pain syndrome. Brain. 2009b;132(Pt 3):788–800. doi: 10.1093/brain/awn346. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD. Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. J Neurophysiol. 2007;97(5):3651–9. doi: 10.1152/jn.01210.2006. [DOI] [PubMed] [Google Scholar]
- Silberstein SD. The role of sex hormones in headache. Neurology. 1992;42(3 Suppl 2):37–42. [PubMed] [Google Scholar]
- Soares DP, Law M. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clin Radiol. 2009;64(1):12–21. doi: 10.1016/j.crad.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Sprenger T, Valet M, Woltmann R, Zimmer C, Freynhagen R, Kochs EF, et al. Imaging pain modulation by subanesthetic S-(+)-ketamine. Anesth Analg. 2006;103(3):729–37. doi: 10.1213/01.ane.0000231635.14872.40. [DOI] [PubMed] [Google Scholar]
- Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991;251(4999):1355–8. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Lotsch J. Current evidence for a modulation of low back pain by human genetic variants. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I. Imaging pain. Br J Anaesth. 2008;101(1):32–9. doi: 10.1093/bja/aen102. [DOI] [PubMed] [Google Scholar]
- Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–91. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Treede RD, Apkarian AV, Bromm B, Greenspan JD, Lenz FA. Cortical representation of pain: functional characterization of nociceptive areas near the lateral sulcus. Pain. 2000;87(2):113–9. doi: 10.1016/S0304-3959(00)00350-X. [DOI] [PubMed] [Google Scholar]
- Treede RD, Kenshalo DR, Gracely RH, Jones AK. The cortical representation of pain. Pain. 1999;79(2–3):105–11. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- Tripp DA, VanDenKerkhof EG, McAlister M. Prevalence and determinants of pain and pain-related disability in urban and rural settings in southeastern Ontario. Pain Res Manag. 2006;11(4):225–33. doi: 10.1155/2006/720895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk DC, Rudy TE, Sorkin BA. Neglected topics in chronic pain treatment outcome studies: determination of success. Pain. 1993;53(1):3–16. doi: 10.1016/0304-3959(93)90049-U. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wagner KJ, Sprenger T, Kochs EF, Tolle TR, Valet M, Willoch F. Imaging human cerebral pain modulation by dose-dependent opioid analgesia: a positron emission tomography activation study using remifentanil. Anesthesiology. 2007;106(3):548–56. doi: 10.1097/00000542-200703000-00020. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. 2005;2(3):137–45. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RG, Rogers R, Painter D, Bantick S, Ploghaus A, Williams P, et al. Combining fMRI with a pharmacokinetic model to determine which brain areas activated by painful stimulation are specifically modulated by remifentanil. Neuroimage. 2002;16(4):999–1014. doi: 10.1006/nimg.2002.1146. [DOI] [PubMed] [Google Scholar]
- Wise RG, Tracey I. The role of fMRI in drug discovery. J Magn Reson Imaging. 2006;23(6):862–76. doi: 10.1002/jmri.20584. [DOI] [PubMed] [Google Scholar]
- Young Casey C, Greenberg MA, Nicassio PM, Harpin RE, Hubbard D. Transition from acute to chronic pain and disability: a model including cognitive, affective, and trauma factors. Pain. 2008;134(1–2):69–79. doi: 10.1016/j.pain.2007.03.032. [DOI] [PubMed] [Google Scholar]
- Zilles K, Amunts K. Receptor mapping: architecture of the human cerebral cortex. Curr Opin Neurol. 2009;22(4):331–9. doi: 10.1097/WCO.0b013e32832d95db. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299(5610):1240–3. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Stohler CS. Neurobiological mechanisms of placebo responses. Ann N Y Acad Sci. 2009;1156:198–210. doi: 10.1111/j.1749-6632.2009.04424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]