Abstract
OBJECTIVES
The purpose of this study was to identify and characterize Na+-dependent, high affinity glutamate transporter (GLUT) activity in the hypothalamic paraventricular nucleus (PVN) and to compare GLUT activity in PVN of euhydrated versus water-deprived rats.
METHODS
Sprague-Dawley rats were deprived of water for two days before sacrifice. Control rats received water ad libitum. After sacrifice, PVN and cerebrum were removed and synaptosomes were prepared using standard techniques. Glutamate uptake was measured using [3H]-glutamate as substrate, physiological buffer, approximately 100 mg of synaptosomal tissue per assay and a Brandel cell harvester.
RESULTS
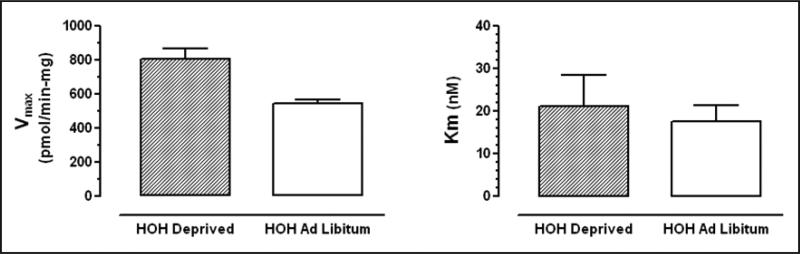
Glutamate uptake was saturable in PVN synaptosomes from euhydrated, control rats with a Vmax of 541 ± 22 pmol/min-mg protein (SEM) and Km of 17.6 ± 3.8 nM (SEM). In contrast, Vmax of glutamate uptake was 808 ± 58 pmol/min-mg protein in PVN of rats deprived of water for 2 days. This was significantly higher than controls (p<0.001). Km was 21.2 ± 7.3 nM and not significantly different from controls (NS).
CONCLUSIONS
Our results suggest that water deprivation of rats results in significantly higher synaptosomal glutamate uptake in PVN. Although the exact mechanism is unknown, increased transcription of the GLUT gene and/or increased cell surface expression of GLUT may contribute to the observed increase of glutamate uptake in dehydrated rats. Increased glutamate uptake may serve to restrict dehydration-induced activation of PVN efferent pathways specifically involved in release of neurohypophysial hormones and activation of sympathetic outflow that operate to maintain body fluid balance and cardiovascular function.
Keywords: Glutamate, glutamate uptake, paraventricular nucleus, dehydration
INTRODUCTION
As one of the primary brain areas responsive to changes in osmolality, the hypothalamic paraventricular nucleus (PVN) plays a central role in regulating fluid homeostasis. This is accomplished through progressive recruitment of neuroendocrine and sympathetic nervous system responses. Discharge of PVN neurons is increased by acute elevations of plasma osmolality and plasma angiotensin II and is decreased by blood volume expansion (Toney et al. 2003; Bains & Ferguson 1995; Chen & Toney 2001; Chen & Toney 2003; Ferguson 1988; Ferguson & Washburn 1998; Lovick & Coote 1988). Water deprivation increases plasma osmolality and circulating angiotensin II and these effects are accompanied by activation of PVN neurons (Stocker et al. 2004). Activation of PVN neurons by water deprivation may also be facilitated by loss of inhibitory input due to the decrease of intravascular volume. Among numerous and varied output projections of the PVN are two that play particularly important roles in maintaining body fluid homeostasis. These are vasopressinergic and oxytocinergic neurons that project to the posterior pituitary and a neurochemically diverse group of neurons with axonal projections to the intermediolateral cell column (IML) of the spinal cord (Pyner & Coote 2000; Saper et al. 1976; Shafton et al. 1998; Swanson & Kuypers 1980) as well as to the rostral ventrolateral medulla (RVLM) (Pyner & Coote 1999; 2000; Shafton et al. 1998). Activity of the premotor neurons of the RVLM maintains sympathetic vasomotor tone and regulates various vasomotor reflexes including activation of the sympathoadrenal system, increased arterial blood pressure, elevated heart rate (Dampney 1994a,b; Guyenet et al. 1996).
Dehydration-induced changes in the PVN includes enhanced synaptic glutamate release, increased synaptic glutamate receptor density and activation and enhanced glutamate receptor-effector coupling (Li & Tasker 2004). Glutamate activity within individual synapses is terminated by uptake of the neurotransmitter via selective glutamate transporters located in neuronal and astrocyte cell membranes (Nicholls & Attwell 1990). An inability to effectively clear glutamate could result in its diffusion to heterosynaptic pathways. Such glutamate “spillover” could result in abnormal activation of functionally unrelated pathways. Pathway specificity is maintained through activation of glutamate uptake via selective glutamate transporters (Tsvetkov et al. 2004; Nie & Weng 2009). Limiting glutaminergic activity to specific synaptic pathways for defined time periods is accomplished both by reducing glutamate release and by reducing the amount of glutamate in the synaptic cleft. The latter is typically accomplished via glutamate diffusion and/or by enhanced transportermediated uptake (Nicholls & Attwell 1990).
Increased glutaminergic drive associated with dehydration leads to enhanced excitability of magnocellular neurosecretory neurons of the PVN and supraoptic nuclei (Hatton 1997; Miyata et al. 1994; Theodosis et al. 1993; Li & Tasker 2004) as well as to increased angiotensin II and glutaminergic drive to pre-autonomic neurons of the PVN (Chen et al. 2001; Freeman & Brook 2007). In this study, we sought to identify and partially characterize Na+-dependent, high affinity glutamate transporter (GLUT) activity in the PVN and to compare GLUT activity in PVN of euhydrated versus water-deprived rats. We also examined effects of angiotensin II on glutamate uptake in the rat PVN.
MATERIALS AND METHODS
Animals and Water Deprivation Study
Adult male Sprague–Dawley rats (Charles River Laboratories) weighing 250–375 g were housed in a temperature-controlled room (22–23 °C) with an automatically controlled cycle of 14 h:10 h light:dark (lights on at 0700 h daily). Control (euhydrated) rats were provided tap water and laboratory chow (Harlan Teklad LM-485, 0.3% NaCl) ad libitum. Experimental (dehydrated) rats were deprived of water but not food for two days before sacrifice in accordance with IACUC approval. All experimental procedures conformed to National Institutes of Health Guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas Health Science Center at San Antonio.
Collection of Periventricular Nucleus Tissue
Rats were decapitated and the brain rapidly removed. The hypothalamus was dissected from the surrounding brain. The paraventricular nucleus (PVN) was identified visually and removed by microdissection. For comparison with PVN glutamate uptake measurements, cerebellum and cerebrum tissues were also collected from some rats.
Preparation of Synaptosomes
Synaptosomes are prepared as previously described (Whittaker & Barker 1972; Robinson et al. 1991) using pooled rat PVN. Synaptosomes were also prepared from rat cerebrum and cerebellum for comparison to the rat PVN. Briefly, fresh 80–90 mg dissections of the PVN from two rats were homogenized by 12 strokes of a fitted Teflon homogenizer in 10 ml of 0.32 M sucrose buffer (pH 7.4) in a glass homogenizer at 4 °C. The supernatant was next centrifuged at 1,000 × g for 10 minutes. The supernatant from this step was further centrifuged at 11,000 × g for 20 minutes at 4 °C. The resultant pellet was gently resuspended in assay buffer to a concentration of 20 mg original tissue weight/mL and stored briefly on ice before use.
Determination of Synaptosomal Glutamate Uptake
Glutamate uptake was measured as previously described (Robinson et al. 1991) with modifications. Briefly, duplicate assays were done in a final volume of 2 mL assay buffer containing (in mM) NaCl (126), KCl (4.8), CaCl2 (1.25), dextrose (11), NaH2PO4 (3.7), Na2HPO4 (12.7), MgSO4 (1.4) at pH 7.4. Nonspecific uptake was determined in a second set of samples to which a 1,000-fold increase in cold glutamate over the expected Km (3–5 μM) was added. Glutamate uptake was initiated by addition of 1 μCi of [3H]L-glutamate and various amounts of carrier L-glutamate (2.5–80 μM). Glutamate uptake was stopped by addition of ice-cold phosphate buffer and placing the tubes in an ice-cold water bath, followed immediately by rapid filtration of the suspension through Whatman GFB glass fiber filters using a Brandel vacuum apparatus. The filters were then washed three times with 0.32 sucrose buffer (pH 7.4) at 4 °C to remove extracellular radioactivity from the filters.
Importantly, we avoided the commonly used polyethylenimine (PEI) during harvest of synaptosomes on the glass fiber filters. PEI is a strongly cationic polymer. Because of its ability to neutralize excess anionic colloidal charge on membranes, PEI is often used to increase synapsome attachment to filters (Cohen & Nadler 1997; Vancha et al. 2004; Whittaker & Barker 1972). However, we found that use of PEI resulted in greater background filter counts in comparison to water washes. Instead, we included five 32 M sucrose washes to remove non-specific 3H-glutamate binding to assay filters.
Filters were placed in 5 mL of cytoscint ES (ICN Biochemicals Inc., Irvine, CA) and radioactivity determined by scintillation spectrometry (Beckman Instruments, LS 6500) at a approximate efficiency of 45%. Protein was determined by the BioRad version of the Bradford protein assay (Bradford 1976). Concentration-dependence of glutamate uptake was determined at six concentrations (2.5, 5, 10, 20, 40 and 80 μM) of glutamate. All values are presented as means ± SEM. Eadie-Hofstee transformations of concentration-dependence data and Hill plots were fitted by linear regression analysis (Prism, Graphpad Software Inc., La Jolla, CA).
Na+-dependent uptake was determined by measuring the difference between radiolabeled glutamate accumulated in Na+ and in choline (absent of any Na+) medium.
Statistics
Displacement curves were analyzed by ANOVA using the Graphpad PRISM 4.0 (Graphpad USA). For comparison of Km and Vmax between groups, two-tailed statistics were used throughout, with preset alpha level of significance of p<0.05. Data in the text are expressed as mean ± standard error of the mean (SEM). Each experiment was repeated six times (n=6).
RESULTS
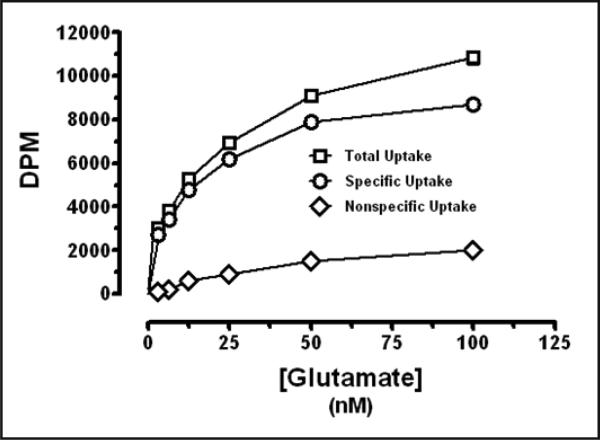
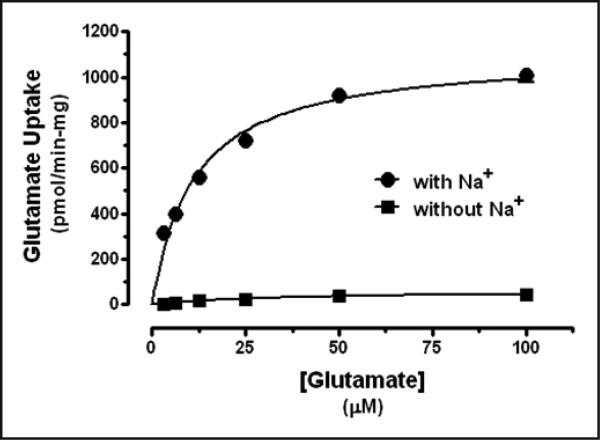
Glutamate uptake was saturable (Figure 1) and Na+-dependent in synaptosomes from the rat PVN. Elimination of Na+ (by substitution with choline) from the uptake assay medium inhibited glutamate uptake (Figure 2). Glutamate uptake was also linear with time through 15 minutes. Metabolism of glutamate was considered negligible. Similar values for Km and Vmax of glutamate uptake were determined for synaptosomes prepared from rat cerebrum and cerebellum (data not shown).
Fig. 1.
Glutamate uptake in rat PVN synaptosomes. Data expressed as mean ± SEM (n=6).
Fig. 2.
Glutamate uptake in rat PVN synaptosomes: Na+ dependence. Data expressed as mean ± SEM (n=6).
Two-days of water deprivation increased Vmax of glutamate uptake but had no effect on Km of gluta-mate uptake in the male rat PVN. Glutamate uptake exhibited a Km of 17.6 ± 3.8 nM (SEM) and a Vmax of 541 ± 22 pmol/min-mg protein (SEM) in euhydrated (control) rats. In rats deprived of water for 3 days, Vmax of glutamate uptake was 808 ± 58 pmol/ min-mg protein, which was significantly higher than controls (p<0.001) whereas Km was 21.2 ± 7.3 nM, not significantly different from controls (NS). Angiotensin II (2 μM) increased Vmax (314.8 ± 10.6 pmol/min-mg protein vs. 254.7 ± 6.6 pmol/min-mg protein for controls/no angiotensin II), but not Km (6.5 ± 0.9 nM vs 4.1 ± 0.5 nM for controls/no angiotensin II), for glutamate uptake in the PVN of control rats.
DISCUSSION
The primary findings of this study are that two-day water deprivation results in increased Vmax, but no difference in Km, for glutamate uptake in the male rat PVN. The Vmax of glutamate uptake was higher in dehydrated rats (808 ± 58 pmol/min-mg protein) than in euhydrated rats (Vmax of 541 ± 22 pmol/min-mg protein). This suggests an increase in the number of available glutamate transporters in the dehydrated rats. In contrast, the Km for glutamate uptake did not differ between dehydrated rats (21.2 ± 7.3 nM) and euhydrated rats (Km of 17.6 ± 3.8 nM), suggesting no change in the affinity of the transporters for glutamate. Glutamate uptake into synaptosomes in the rat PVN was found to be both saturable and Na+ dependent.
PVN neurons play pivotal roles in maintenance of homeostasis by regulating pituitary release of hormones as well as CNS sympathetic outflow during periods of stress and fluid imbalance with associated hypertension (Allen 2002; de Wardener 2001, Miyakubo et al. 2002; Ranson et al. 1998). Glutaminergic, GABAergic, and noradrenergic afferents to the PVN finely tune PVN output (Boudaba et al. 1997; Li et al. 2003) and define pathway specificity during conditions of heightened glutaminergic activity. Dehydration is associated with increased glutaminergic tone and enhanced excitability of magnocellular neurons (Hatton 1997; Miyata et al. 1994; Theodosis et al. 1993; Li & Tasker 2004). Li and Tasker (2004) showed dehydration-induced increases in postsynaptic excitatory potentials interpreted as increased glutamate release sites based on paired-pulse facilitation analysis indicating a lack of change in the probability of glutamate release. Dehydration induced swelling of magnocellular neurons, glial cell retraction away from these neurons and the formation of new glutaminergic and GABAergic synapses within the SON and PVN (Miyata et al. 1994; Tweedle & Hatton 1977). Increased release of glutamate normally induces increased glutamate uptake, either by increases in transporter production and membrane insertion or by translocation of extrasynaptic transporters to synaptic membrane sites (Danbolt 2001; Nicholls & Attwell 1990; Otis et al. 1996b; Takagaki 1976). During dehydration states selectively activated PVN pathways include those projecting to brain stem autonomic centers and to sympathetic preganglionic neurons located within the intermediolateral cell column of the spinal cord (Ranson et al. 1998; Shafton et al. 1998; Toth et al. 1999) and thus sympathetic outflow regulating cardiovascular as well as end organ functions; e.g., renal sodium excretion.
Given the complexity of PVN outflow regulating various autonomic and neuroendocrine functions as well as the ubiquitous distribution of glutamate within and outside the PVN, activation of specific glutaminergic pathways is critical to body fluid regulation. Pathway specificity during conditions of heightened glutaminergic activity may depend on tight control over both duration and synapse-specific glutamate release. An inability to effectively clear such glutamate could result in diffusion of transmitter from the original synapse to heterosynaptic pathways. Such glutamate “spillover” might produce undesirable activation or even eventual synaptic plasticity in those alternate pathways. Because pathway specificity is maintained through activation of glutamate uptake via selective glutamate transporters located in glial and neuronal cell membranes (Tsvetkov et al. 2004; Nie & Weng 2009), an increase in glutamate uptake is predicted to avoid extrasynaptic diffusion of glutamate from the active synapse (Asztely et al. 1997) and thus possible activation of neighboring but otherwise functionally unrelated glutamate synapses and pathways (Asztely et al. 1997). Increases in glutamate uptake may also be important for regulating the duration of postsynaptic pathway activation (Mennerick & Zorumski 1994) and preventing local depletion of the neurotransmitter and/or desensitization of postsynaptic glutamate receptors (Dudel et al. 1988; Otis et al. 1996a; Otis et al. 1996b; Tang et al. 1989; Turecek et al. 2000; Trussell et al. 1989; Trussell & Fischbach 1989). Through such mechanisms, glutamate uptake in dehydration may play a key role in maintaining efficacy of transmission within neuroendocrine and autonomic pathways that preserve cardiovascular function and the ability of animals to forage for sources of water. Our observation of increased Vmax, but not Km, in glutamate uptake within the PVN in dehydrated rats is in keeping with increased glutamate release and maintenance of pathway specificity. Increased gluta-mate uptake thus serves to define pathway specificity between PVN and downstream neurons related to neuroendocrine and sympathetic outflow.
We also found that angiotensin II (2 μM) minimally increases Vmax, but not Km, for glutamate uptake in the rat PVN. Angiotensin II (AT1) receptors are densely distributed in the PVN (Gehlert et al. 1991) with numerous direct synaptic contacts between AT1 receptor expressing fibers and spinally projecting neurons (Li et al. 2003). Sympathetic output induced either by cardiac stimulation or by hyperosmolality can be attenuated by PVN infusion of the selective angiotensin II receptor antagonist losartin (Chen & Toney 2001). The relatively small increases observed in Vmax in response to application of angiotensin II to synaptosomal tissue may be a reflection of suboptimal distribution of angiotensin II in the synaptosomal preparation or because glutamate transporter numbers were already near-maximally increased in the dehydrated animals. Alternatively, G-protein signaling pathways downstream of AT1R receptors that are dominantly expressed in the PVN may be optimal in synaptosomal preparations. Dehydration decreases intravascular volume and increases plasma osmolality and circulating Ang II levels (Stocker et al. 2002; Brooks et al. 2004). Angiotensin II (AT1) receptors are densely distributed in the PVN (Gehlert et al. 1991) with numerous direct synaptic contacts between AT1 receptor expressing fibers and spinally projecting neurons (Li et al. 2003). Angiotensin II has been shown to increase the discharge of PVN neurons in vivo (Ferguson 1988) and in vitro (Cato & Toney 2005; Li et al. 2003). Excitation by angiotensin II involves both presynaptic inhibition of GABAergic synaptic activity (Li et al. 2003) as well as postsynaptically to activate a non-selective cation current (Cato & Toney 2005). Thus, angiotensin II has the potential to act in the PVN to increase sympathetic outflow during dehydration-induced homeostatic stress, as well as during cardiovascular diseases such as heart failure and hyper-tension in which angiotensin II actions are increased in the periphery and CNS. What role angiotensin II might play on glutaminergic tone within the PVN in dehydrated rats remains to be determined.
In summary, we found that two-day water deprivation increased glutamate uptake by PVN synaptosomes reflected as an increased Vmax with no difference in Km. Increased glutamate uptake may be a response to increased glutamatergic activity within the PVN following short-term dehydration. Increased glutamate uptake may serve to maintain pathway specificity within the PVN, thereby preserving relatively selective activation of sympathetic outflow and pituitary neuro-peptide (i.e., vasopressin and oxytocin) release.
Fig. 3.
Glutamate uptake was saturable in PVN synaptosomes from euhydrated, control rats with a Km of 17.6 ± 3.8 nM (SEM) and a Vmax of 541 ± 22 pmol/min-mg protein (SEM). In rats deprived of water for 3 days, Vmax of glutamate uptake was 808 ± 58 pmol/min-mg protein, which was significantly higher than controls (p<0.001). Km was 21.2 ± 7.3 nM which was not significantly different from controls (NS). Data expressed as mean ± SEM (n=6)
ACKNOWLEDGEMENTS
The authors want to acknowledge the technical assistance of Mr. Alfredo S. Calderon, Department of Physiology, UTHSCSA. These studies were funded in part by HL102310 (GMT).
REFERENCES
- 1.Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- 2.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- 3.Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–293. doi: 10.1016/s0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 4.Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am J Physiol Regul Integr Comp Physiol. 1995;268:R625–R633. doi: 10.1152/ajpregu.1995.268.3.R625. [DOI] [PubMed] [Google Scholar]
- 5.Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J Neurophysiol. 1997;77:3396–3400. doi: 10.1152/jn.1997.77.6.3396. [DOI] [PubMed] [Google Scholar]
- 6.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Brooks VL, Freeman KL, O'Donaughy TL. Acute and chronic increases in osmolality increase excitatory amino acid drive of the rostral ventrolateral medulla in rats. Am J Physiol. 2004;287:R1359–R1368. doi: 10.1152/ajpregu.00104.2004. [DOI] [PubMed] [Google Scholar]
- 8.Cato JM, Toney GM. Angiotensin II excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: an in vitro patch-clamp study in brain slices. J Neurophysiol. 2005;93:403–413. doi: 10.1152/jn.01055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen QH, Toney GM. AT(1)-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol. 2001;281:R1844–R1853. doi: 10.1152/ajpregu.2001.281.6.R1844. [DOI] [PubMed] [Google Scholar]
- 10.Chen QH, Toney GM. Identification and characterization of two functionally distinct groups of spinal cord-projecting paraventricular nucleus neurons with sympathetic-related activity. Neurosci. 2003;118:797–807. doi: 10.1016/s0306-4522(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 11.Cohen SM, Nadler JV. Sodium-dependent proline and glutamate uptake by hippocampal synaptosomes during post-natal development. Dev Brain Res. 1997;100:230–233. doi: 10.1016/s0165-3806(97)00045-x. [DOI] [PubMed] [Google Scholar]
- 12.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 13.Dampney RA. The subretrofacial vasomotor nucleus: anatomical, chemical and pharmacological properties and role in cardiovascular regulation. Prog Neurobiol. 1994;42:197–227. doi: 10.1016/0301-0082(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 14.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 15.de Wardener HE. The hypothalamus and hypertension. Physiol Rev. 2001;81:1599–1658. doi: 10.1152/physrev.2001.81.4.1599. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson AV. Paraventricular nucleus neurons projecting to the dorsomedial medulla are influenced by systemic angiotensin. Brain Res Bull. 1988;20:197–201. doi: 10.1016/0361-9230(88)90179-7. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson AV, Washburn DL. Angiotensin II: a peptidergic neurotransmitter in central autonomic pathways. Prog Neurobiol. 1998;54:169–192. doi: 10.1016/s0301-0082(97)00065-8. [DOI] [PubMed] [Google Scholar]
- 18.Freeman KL, Brooks VL. AT(1) and glutamatergic receptors in paraventricular nucleus support blood pressure during water deprivation. Am J Physiol Integr Comp Physiol. 2007;292:R1675–R1682. doi: 10.1152/ajpregu.00623.2006. [DOI] [PubMed] [Google Scholar]
- 19.Gehlert DR, Gackenheimer SL, Schober DA. Autoradio-graphic localization of subtypes of angiotensin II antagonist binding in the rat brain. Neuroscience. 1991;44:501–514. doi: 10.1016/0306-4522(91)90073-w. [DOI] [PubMed] [Google Scholar]
- 20.Guyenet PG, Koshiya N, Huangfu D, Baraban SC, Stornetta RL, Li YW. Role of medulla oblongata in generation of sympathetic and vagal outflows. In: Holstege G, Bandler R, Saper CB, editors. The Emotional Motor System. Prog Brain Res. Vol. 107. Elsevier; Amsterdam: 1996. pp. 127–144. [DOI] [PubMed] [Google Scholar]
- 21.Hatton GI. Function-related plasticity in hypothalamus. Annu Rev Neurosci. 1997;20:375–397. doi: 10.1146/annurev.neuro.20.1.375. [DOI] [PubMed] [Google Scholar]
- 22.Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci. 2003;23:5041–5049. doi: 10.1523/JNEUROSCI.23-12-05041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Tasker JG. Dehydration-induced synaptic plasticity in magnocellular neurons of the hypothalamic supraoptic nucleus. Endocrinology. 2004;145:5141–5149. doi: 10.1210/en.2004-0702. [DOI] [PubMed] [Google Scholar]
- 24.Lovick TA, Coote JH. Effects of volume loading on paraventriculo-spinal neurones in the rat. J. Auton Nerv Syst. 1988;25:135–140. doi: 10.1016/0165-1838(88)90018-5. [DOI] [PubMed] [Google Scholar]
- 25.Mennerick S, Zorumski CF. Glial contributions to excitatory neurotransmission in cultured hippocampal cells. Nature (London) 1994;368:59–62. doi: 10.1038/368059a0. [DOI] [PubMed] [Google Scholar]
- 26.Miyakubo H, Hayashi Y, Tanaka J. Enhanced response of subfornical organ neurons projecting to the hypothalamic paraventricular nucleus to angiotensin II in spontaneously hypertensive rats. Auton Neurosci. 2002;95:131–136. doi: 10.1016/s1566-0702(01)00388-5. [DOI] [PubMed] [Google Scholar]
- 27.Miyata S, Nakashima T, Kiyohara T. Structural dynamics of neural plasticity in the supraoptic nucleus of the rat hypothalamus during dehydration and rehydration. Brain Res Bull. 1994;34:169–175. doi: 10.1016/0361-9230(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 28.Nicholls D, Attwell D. The release and uptake of excitatory amino acids. Trends Pharmacol Sci. 1990;11:462–468. doi: 10.1016/0165-6147(90)90129-v. [DOI] [PubMed] [Google Scholar]
- 29.Nie H, Weng H-R. Glutamate transporters prevent excessive activation of NMDA receptors and extrasynaptic glutamate spillover in the spinal dorsal horn. J Neurophysiol. 2009;101:2041–2051. doi: 10.1152/jn.91138.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otis T, Zhang S, Trussell LO. Direct Measurement of AMPA Receptor Desensitization Induced by glutamatergic synaptic transmission. J Neurosci. 1996a;16:7496–7504. doi: 10.1523/JNEUROSCI.16-23-07496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otis TS, Wu YC, Trussell LO. Delayed clearance of transmitter and the role of glutamate transporters at synapses with multiple release sites. J Neurosci. 1996b;16:1634–1644. doi: 10.1523/JNEUROSCI.16-05-01634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pyner S, Coote JH. Identification of an efferent projection from the paraventricular nucleus of the hypothalamus terminating close to spinally projecting rostral ventrolateral medullary neurons. Neuroscience. 1999;88:949–957. doi: 10.1016/s0306-4522(98)00255-3. [DOI] [PubMed] [Google Scholar]
- 33.Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–556. doi: 10.1016/s0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- 34.Ranson RN, Motawei K, Pyner S, Coote JH. The paraventricular nucleus of the hypothalamus sends efferents to the spinal cord of the rat that closely appose sympathetic preganglionic neurons projecting to the stellate gangion. Exp Brain Res. 1998;120:164–172. doi: 10.1007/s002210050390. [DOI] [PubMed] [Google Scholar]
- 35.Robinson MB, Hunte-Ensor M, Sinor J. Pharmacologically distinct sodium-dependent L-[3H] glutamate transport processes in rat brain. Brain Research. 1991;44:196–202. doi: 10.1016/0006-8993(91)90054-y. [DOI] [PubMed] [Google Scholar]
- 36.Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- 37.Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res. 1998;801:239–243. doi: 10.1016/s0006-8993(98)00587-3. [DOI] [PubMed] [Google Scholar]
- 38.Stocker SD, Stricker EM, Sved AF. Arterial baroreceptors mediate the inhibitory effect of acute increases in arterial blood pressure on thirst. Am J Physiol. 2002;282:R1718–R1729. doi: 10.1152/ajpregu.00651.2001. [DOI] [PubMed] [Google Scholar]
- 39.Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in OVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1172–R1183. doi: 10.1152/ajpregu.00394.2004. [DOI] [PubMed] [Google Scholar]
- 40.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 41.Takagaki G. Properties of the uptake and release of glutamic acid by synaptosomes from rat cerebral cortex. J Neurochem. 1976;27:1417–1425. doi: 10.1111/j.1471-4159.1976.tb02624.x. [DOI] [PubMed] [Google Scholar]
- 42.Theodosis DT, Poulain DA. Activity-dependent neuronalglial and synaptic plasticity in the adult mammalian hypothalamus. Neuroscience. 1993;57:501–535. doi: 10.1016/0306-4522(93)90002-w. [DOI] [PubMed] [Google Scholar]
- 43.Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand. 2003;177:43–55. doi: 10.1046/j.1365-201X.2003.01046.x. [DOI] [PubMed] [Google Scholar]
- 44.Toth ZE, Gallatz K, Fodor M, Palkovits M. Decussations of the descending paraventricular pathways to the brainstem and spinal cord autonomic centers. J Comp Neurol. 1999;414:255–266. [PubMed] [Google Scholar]
- 45.Trussell LO, Fischbach GD. Glutamate receptor desensitization and its role in synaptic transmission. Neuron. 1989;3:209–218. doi: 10.1016/0896-6273(89)90034-2. [DOI] [PubMed] [Google Scholar]
- 46.Trussell LO, Thio LL, Zorumski CF, Fischbach GD. Rapid desensitization of glutamate receptors in vertebrate central neurons. Proc Natl Acad Sci USA. 1988;85:4562–4566. doi: 10.1073/pnas.85.12.4562-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsvetkov E, Ryong-Moon Shin R, Bolshakov VY. Glutamate uptake determines pathway specificity of long-term potentiation in the neural circuitry of fear conditioning. Neuron. 2004;41:139–151. doi: 10.1016/s0896-6273(03)00800-6. [DOI] [PubMed] [Google Scholar]
- 48.Tweedle CD, Hatton GI. Ultrastructural changes in the rat hypothalamic neurosecretory cells and their associated glia during minimal dehydration and rehydration. Cell Tissue Res. 1977;181:59–72. doi: 10.1007/BF00222774. [DOI] [PubMed] [Google Scholar]
- 49.Vancha AR, Govindaraju S, Parsa VL, Jasti M, Gonzalez-Garcia M, Ballestero RP. Use of polyethyleneimine polymer in cell culture as attachment factor and lipofection enhancer. BMC Biotechnology. 2004;4:23–35. doi: 10.1186/1472-6750-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whittaker VP, Barker LA. The subcellular fractionation of brain tissue with special reference to the preparation of synaptosomes and their component organelles. In: Fried R, editor. Methods of Neurochemistry. Vol. 2. Marcel Dekker; New York: 1972. pp. 1–52. [Google Scholar]