Abstract
The vaccine strains against influenza virus A/H3N2 for the 2010-2011 season and influenza virus B for the 2009-2010 and 2010-2011 seasons in Japan are a high-growth reassortant A/Victoria/210/2009 (X-187) strain and an egg-adapted B/Brisbane/60/2008 (Victoria lineage) strain, respectively. Hemagglutination inhibition (HI) tests with postinfection ferret antisera indicated that the antisera raised against the X-187 and egg-adapted B/Brisbane/60/2008 vaccine production strains poorly inhibited recent epidemic isolates of MDCK-grown A/H3N2 and B/Victoria lineage viruses, respectively. The low reactivity of the ferret antisera may be attributable to changes in the hemagglutinin (HA) protein of production strains during egg adaptation. To evaluate the efficacy of A/H3N2 and B vaccines, the cross-reactivities of postvaccination human serum antibodies against A/H3N2 and B/Victoria lineage epidemic isolates were assessed by a comparison of the geometric mean titers (GMTs) of HI and neutralization (NT) tests. Serum antibodies elicited by the X-187 vaccine had low cross-reactivity to both MDCK- and egg-grown A/H3N2 isolates by HI test and narrow cross-reactivity by NT test in all age groups. On the other hand, the GMTs to B viruses detected by HI test were below the marginal level, so the cross-reactivity was assessed by NT test. The serum neutralizing antibodies elicited by the B/Brisbane/60/2008 vaccine reacted well with egg-grown B viruses but exhibited remarkably low reactivity to MDCK-grown B viruses. The results of these human serological studies suggest that the influenza A/H3N2 vaccine for the 2010-2011 season and B vaccine for the 2009-2010 and 2010-2011 seasons may possess insufficient efficacy and low efficacy, respectively.
INTRODUCTION
Protection from infection with influenza virus is largely mediated by antibodies directed against the major viral surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA) (12). The antibodies can be elicited by vaccination with live attenuated vaccine (6, 17, 41) or inactivated trivalent vaccine consisting of influenza A viruses (H1N1pdm09 and H3N2 subtypes) and an influenza B virus (12). To prepare effective vaccines, it is critical that the vaccine viruses antigenically match the epidemic virus as closely as possible. Fortunately, in the 2009-2010 and 2010-2011 seasons, the influenza A/H1N1pdm09, A/H3N2, and B viruses that circulated worldwide were antigenically and genetically closely related to the vaccine viruses (69, 70) and a high efficacy of influenza vaccine was expected.
Embryonated hen eggs (here referred to as eggs) have been a useful substrate for the propagation of virus used in influenza vaccine production. Consequently, the high growth ability of vaccine viruses in eggs and the yield of the viral HA and NA antigens are important issues. A high yield of viruses in eggs is achieved through the use of reassortant viruses, which are prepared by simultaneously infecting eggs with a selected prevalent wild-type virus and an egg-adapted donor virus, such as A/PR/8/34 virus for influenza A vaccine (4, 5, 31) and B/Lee/40 virus for influenza B vaccine (68). The reassortant viruses must carry the HA and NA genes of the prevalent wild-type virus and have the high-growth phenotype of the donor virus. Propagation of influenza A and B viruses in eggs, however, is known to select HA that differs antigenically and structurally from the original epidemic virus isolated in mammalian cells, such as Madin-Darby canine kidney (MDCK) cells (26, 27, 54, 55). The differences in the egg-grown viruses are mainly attributable to amino acid substitutions around the receptor binding site in HA (16). For influenza B virus, egg adaptation is accompanied by the loss of the N-linked glycosylation site (N-X-T/S) from HA at amino acid residues 196 to 198 (B/Yamagata/16/88 lineage) or 197 to 199 (B/Victoria/2/87 lineage), resulting in a significant change in antigenicity (55, 56, 58). Consequently, the antigenic change in the vaccine virus by egg adaptation is a major obstacle in the production of effective influenza vaccines, even if the vaccine viruses are properly selected by the World Health Organization (WHO) on the basis of the antigenic and genetic characteristics of viruses circulating in the world.
The components of trivalent inactivated ether-split vaccine used in Japan are two egg-adapted, high-growth reassortant viruses, A/California/7/2009 (H1N1)pdm09 (X-179A) and A/Victoria/210/2009 (H3N2) (X-187), for the 2010-2011 season and an egg-adapted B/Brisbane/60/2008 (Victoria lineage) virus for the 2009-2010 and 2010-2011 seasons. We recently found that the vaccine production strains of X-187 and B/Brisbane/60/2008 were antigenically distinguishable from the original wild-type viruses by hemagglutination inhibition (HI) tests with postinfection ferret antisera, although the X-179A vaccine virus of A/H1N1pdm09 retained its original antigenicity to wild-type virus A/California/7/2009. We anticipated that the antigenic changes of these vaccine viruses would affect the vaccine efficacy. Therefore, we evaluated the efficacy of A/H3N2 and B vaccines on the basis of the cross-reactivity of human serum antibodies elicited by the vaccination against epidemic A/H3N2 and B viruses isolated in MDCK cells and eggs.
MATERIALS AND METHODS
Viruses.
The influenza viruses used in the present study were egg-grown viruses propagated in the allantoic cavities of 10-day-old embryonated chicken eggs at 34°C for 48 h and MDCK-grown viruses propagated at 34°C for 72 h. The egg-grown A/H3N2 viruses were A/Victoria/210/2009 (X-187), A/Victoria/210/2009, A/Perth/16/2009, A/Rhode Island/01/2010, and A/Taiwan/839/2009. The MDCK-grown A/H3N2 viruses were A/Akita/10/2010, A/Fukuoka-C/35/2010, A/Okinawa/72/2010, A/Mie/19/2010, A/Hiroshima/49/2010, A/Kobe/357/2010, A/Yokohama/96/2010, A/Kanagawa/79/2010, and A/Ehime/39/2010. The egg-grown B viruses were B/Brisbane/60/2008, B/Hiroshima/9/2010, B/Mie/6/2010, B/Taiwan/9/2010, B/Taiwan/71/2010, and B/Chongqing-Yuzhong/1362/2010. The MDCK-grown B viruses were B/Brisbane/60/2008, B/Hiroshima/9/2010, B/Hiroshima/10/2010, B/Mie/6/2010, B/Hiroshima-C/2/2010, B/Kobe/406/2010, and B/Saitama/7/2011.
Pre- and postvaccination human sera.
For A/H3 vaccine evaluation, we collected pre- and postvaccinated human sera administered with the 2010-2011 season vaccine [A/California/7/2009 (H1N1)pdm09 (X-179A), A/Victoria/210/2009 (H3N2) (X-187), and B/Brisbane/60/2008] from an adult group (n = 24; age range, 21 to 40 years; mean, 30.8 years), a middle-aged group (n = 24; age range, 41 to 61 years; mean, 51.5 years), and an elderly group (n = 24; age range, 61 to 98 years; mean, 86.0 years). For B vaccine evaluation, we collected pre- and postvaccinated human sera administered with the 2009-2010 season vaccine [A/Brisbane/59/2007 (IVR-148), A/Uruguay/716/2007 (X-175C), and B/Brisbane/60/2008] from an adult group (n = 24; age range, 20 to 38 years; mean, 28.4 years), a middle-aged group (n = 21; age range, 42 to 60 years; mean age, 51.4 years), and an elderly group (n = 24; age range, 69 to 103 years; mean, 87.3 years). All individuals had been vaccinated with one dose of a standard commercial trivalent influenza split vaccine, containing 15 μg each of the hemagglutinin proteins of vaccine viruses.
Hemagglutination inhibition assays.
Serum samples were treated with RDEII (Denka Seiken Co.) to remove nonspecific inhibitors. A 1:10 dilution of treated sera was prepared with phosphate-buffered saline (PBS). Twofold serial dilutions of sera were mixed with 4 HA units of antigen virus per well and preincubated for 60 min in 96-well plates at room temperature. Because recent A/H3N2 viruses do not agglutinate blood cells of avian species (chicken and turkey), guinea pig red blood cells were used for HI tests of A/H3N2 viruses. The guinea pig red blood cells (1%) were added, and the plate was incubated for 60 min at room temperature. For HI tests of B viruses, 0.5% turkey red blood cells were added, and the plate was incubated for 45 min at room temperature. HI titers of sera were determined as the highest dilution that did not display hemagglutinating activity.
Neutralization assay.
Human serum samples were treated with RDEII to remove inhibitors of influenza virus replication. One hundred 50% tissue culture infectious doses (TCID50) of A/H3N2 and B viruses were preincubated with 2-fold serial dilutions of treated sera for 30 min and then inoculated into MDCK cells. Cytopathic effects on the MDCK cells were measured on day 4 to determine the neutralizing activity of the test sera.
Statistical analysis.
In the HI and NT tests, differences between pre- and postvaccination sera were determined using the two-sided Student t test. P values of < 0.05 were considered statistically significant.
Molecular modeling of influenza virus HA.
The crystal structures of influenza A virus [A/X-31(H3N2)] HA at a resolution of 2.50 Å (PDB code, 2VIU [15]) and influenza B virus (B/Hong Kong/8/1973) HA at a resolution of 2.80 Å (PDB code, 3BT6 [67]) were retrieved from the RCSB Protein Data Bank (14). Three-dimensional (3-D) models of influenza A/H3 virus HA [A/Victoria/210/2009 and A/Victoria/210/2009 (X-187)] and influenza B virus HA (B/Brisbane/60/2008 E4/E4 and B/Brisbane/60/2008 MDCKx/1) were constructed by the homology modeling technique using “MOE-Align” and “MOE-Homology” in Molecular Operating Environment (MOE) version 2008.1002 (Chemical Computing Group Inc., Quebec, Canada) as previously described (59, 60). We obtained 25 intermediate models per homology model in MOE, and we selected the 3-D models with the best scores according to the generalized Born/volume integral methodology (32). In the case of influenza B virus HA, a high-mannose-type asparagine-linked sugar chain was attached at 197N. The final 3-D models were thermodynamically optimized by energy minimization with an AMBER99 force field (51) combined with the generalized Born model of aqueous solvation implemented in MOE (48). Physically unacceptable local structures of the optimized 3-D models were further refined on the basis of evaluation by a Ramachandran plot using MOE.
RESULTS
Antigenicity of the A/H3N2 and B vaccine viruses. (i) A/H3N2 X-187.
A/H3N2 vaccine virus X-187 is a high-growth reassortant virus with HA and NA genes from A/Victoria/210/2009, which possesses antigenicity similar to that of the WHO-recommended vaccine virus A/Perth/16/2009 (70), and internal genes derived from the high-growth donor strain A/PR/8/34 virus. To confirm that the X-187 virus retains antigenicity similar to that of the original wild-type A/Victoria/210/2009 virus and that of the prototype A/Perth/16/2009 virus, which represent the majority of current epidemic viruses, HI tests with postinfection ferret antisera raised against A/Victoria/210/2009, A/Perth/16/2009, and A/Niigata/403/2009 (A/Perth/16-like reference virus of Japan) were performed (Table 1). The X-187 virus reacted well with the three antisera with the same or within-2-fold-different HI titers from the homologous titers of each antiserum, indicating that the X-187 vaccine virus retained antigenicity similar to those of the original wild-type and prototype viruses by one-way HI test.
Table 1.
Cross-reactivities of postinfection ferret antisera raised to A/Victoria/210/09 (X-187) and reference viruses (H3N2)
| Influenza A virus strains | Passage historya | HI titerb of postinfection ferret antisera |
||||
|---|---|---|---|---|---|---|
| Uruguay/716/07 (egg) | Victoria/210/09 (egg) | Victoria/210/09 (X-187) (egg) | Perth/16/09 (egg) | Niigata/403/09 (cell) | ||
| Reference antigens | ||||||
| A/Uruguay/716/2007 | C1 E3 + 2 | 1,280 | 80 | 20 | 40 | 40 |
| A/Victoria/210/2009 | E2 + 2 | 10 | 640 | 640 | 160 | 320 |
| A/Victoria/210/2009 (X-187)c | E7/E2 + 1 | 10 | 1,280 | 2,560 | 320 | 640 |
| A/Perth/16/2009 | E3 + 2 | 10 | 320 | 80 | 160 | 320 |
| A/Niigata/403/2009 | C2 + 2 | 20 | 640 | 80 | 320 | 640 |
| Test antigens | ||||||
| A/Kobe/357/2010 | C1 + 1 | 10 | 1,280 | 640 | 1,280 | 1,280 |
| A/Akita/10/2010 | C0 + 1 | 20 | 1,280 | 320 | 160 | 640 |
| A/Fukuoka-C/35/2010 | C1 + 1 | 20 | 640 | 640 | 640 | 1,280 |
| A/Rhode Island/01/2010 | E3 + 1 | 40 | 640 | 80 | 160 | 160 |
| A/Taiwan/839/2009 | E3/E1 + 1 | 20 | 320 | 40 | 160 | 160 |
| A/Yokohama/96/2010 | C2 + 1 | 40 | 320 | 40 | 80 | 160 |
| A/Hiroshima/49/2010 | C2 + 1 | 20 | 320 | 40 | 80 | 160 |
| A/Ehime/39/2010 | C1 + 1 | 20 | 160 | 40 | 80 | 80 |
| A/Kanagawa/79/2010 | C2 + 1 | 20 | 160 | 40 | 80 | 80 |
| A/Okinawa/72/2010 | C1 + 1 | 20 | 160 | 20 | 40 | 80 |
| A/Mie/19/2010 | C1 + 1 | 10 | 160 | 20 | 40 | 80 |
C and E, passage in MDCK cells and eggs, respectively; numbers indicate the numbers of passages.
1% guinea pig red blood cells were used for the HI test. Underlining indicates the homologous titers of antisera.
Vaccine production virus.
We next examined the reactivity of X-187 ferret antiserum against representative A/H3N2 epidemic viruses isolated in MDCK cells and in eggs (Table 1). The antiserum poorly inhibited most test viruses except for high and broad reactors A/Kobe/357/2010, A/Akita/10/2010, and A/Fukuoka-C/35/2010, and the HI titers of test viruses were 32- to 128-fold lower than the homologous titer of X-187 virus. For HI tests of A/H3N2 viruses in the present study, we used guinea pig red blood cells rather than turkey red blood cells, which are widely used for HI tests of influenza A and B viruses, because recent A/H3N2 isolates do not agglutinate the blood cells of avian species due to changes in the receptor-binding properties (37, 45, 46). In the test viruses that were still able to agglutinate turkey red blood cells, similar results were obtained from HI tests performed with turkey red blood cells and HI tests performed with guinea pig red blood cells (data not shown). Therefore, the decreased HI titers of isolates with X-187 ferret antiserum were not caused by the use of guinea pig red blood cells for the HI test. These data raised concerns that the antibody induced by X-187 vaccine may not efficiently inhibit epidemic viruses; therefore, we anticipated that the efficacy of X-187 vaccine would be low.
(ii) B/Brisbane/60/2008.
Since the vast majority of influenza B viruses that circulated worldwide in the 2009-2010 and 2010-2011 seasons consisted of B/Victoria/2/87 lineage virus, the influenza B vaccine virus was selected from this lineage (69, 70). The majority of circulating viruses from this lineage consisted of B/Brisbane/60/2008-like viruses (69, 70). Therefore, the egg-adapted B/Brisbane/60/2008 virus, whose growth ability and yield of HA protein were improved by multiple passages in eggs, was selected for vaccine production in Japan. Antigenically changed variant virus is selected when influenza B virus is passaged in eggs, whereas virus propagated in mammalian cells is structurally and antigenically identical to circulating epidemic viruses (26, 27, 54, 55). To confirm whether B/Brisbane/60/2008 vaccine virus retained the original antigenicity, we compared the cross-reactivities of ferret antisera raised to egg- and MDCK-grown B/Brisbane/60/2008 viruses against epidemic B viruses isolated in eggs and MDCK cells (Table 2). The antiserum to egg-grown B/Brisbane/60/2008 virus reacted well to egg-grown epidemic viruses with the same HI titers as the homologous titer; however, it poorly inhibited MDCK-grown viruses, including MDCK-grown original B/Brisbane/60/2008 virus, with 4- to 32-fold-reduced HI titers from the homologous titer. The most strikingly different reactivity was seen between two paired viruses of egg- and MDCK-grown B/Hiroshima/9/2010 and B/Mie/6/2010. In contrast, antiserum to MDCK-grown B/Brisbane/60/2008 broadly reacted to both MDCK- and egg-grown viruses, although some of the egg-grown viruses, B/Taiwan/9/2010, B/Chongqing-Yuzhong/1362/2010, and B/Mie/6/2010, had 4-fold-reduced HI titers compared to the homologous titer. From the HI data, it was obvious that the antigenicity of B vaccine virus was greatly changed from the original wild-type virus by egg adaptation, so that the efficacy of influenza B vaccine was anticipated to be remarkably decreased.
Table 2.
Cross-reactivities of postinfection ferret antisera raised to egg- and MDCK-grown B/Brisbane/60/2008
| Influenza B virus strains | Passage historya | HIb titers of postinfection ferret antisera |
|||
|---|---|---|---|---|---|
| Malaysia/2506/04 (egg) | Brisbane/60/08 (egg) | Brisbane/60/08 (cell) | Fujian Gulou/1272/08 (egg) | ||
| Reference antigens | |||||
| B/Malaysia/2506/2004 | E3/E1 + 2 | 1,280 | 320 | <10 | 160 |
| B/Brisbane/60/2008c | E4 + 1 | 640 | 320 | 80 | 160 |
| B/Brisbane/60/2008 | Cx/1 + 2 | 10 | 20 | 160 | 10 |
| B/Fujian Gulou/1272/2008 | E1/E2 + 1 | 640 | 320 | 10 | 320 |
| Test antigens | |||||
| B/Hiroshima/9/2010 | E1 + 1 | 320 | 320 | 80 | 80 |
| B/Taiwan/71/2010 | E3 + 1 | 320 | 320 | 80 | 80 |
| B/Taiwan/9/2010 | E3 + 1 | 160 | 320 | 40 | 40 |
| B/Chongqing-Yuzhong/1362/2010 | E1 + 1 | 320 | 320 | 40 | 80 |
| B/Mie/6/2010 | E1 + 1 | 640 | 320 | 40 | 160 |
| B/Mie/6/2010 | C1 + 1 | 40 | 80 | 80 | 40 |
| B/Hiroshima/10/2010 | C1 + 1 | 10 | 20 | 160 | 10 |
| B/Kobe/406/2010 | C1 + 1 | 10 | 20 | 160 | 10 |
| B/Hiroshima/9/2010 | C1 + 1 | 20 | 10 | 80 | <10 |
| B/Saitama/7/2011 | C2 + 1 | 20 | 10 | 80 | <10 |
C and E, passage in MDCK cells and eggs, respectively; numbers indicate the numbers of passages.
0.5% turkey red blood cells were used for HI test. Underlining indicates the homologous titers of antisera.
Vaccine production virus.
Comparison of three-dimensional (3-D) molecular models of the HA proteins of A/H3 and B viruses.
To understand the structural differences in HA proteins from egg-adapted vaccine virus and original wild-type virus, the spatial locations of amino acid changes on 3-D models of the HA protein were determined based on multiple alignments of the deduced amino acid sequences of vaccine and wild-type viruses, as described in Materials and Methods.
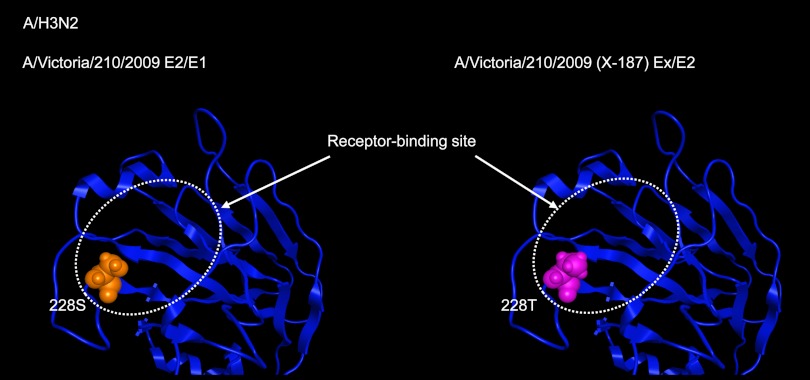
Between the HAs of A/Victoria/210/2009 and X-187 vaccine virus, one amino acid change from Ser to Thr at residue 228 (Ser228Thr) was observed. The Ser228Thr substitution in X-187 virus was located in the receptor-binding site of the HA molecule (Fig. 1) and was deduced to cause structural change in the receptor-binding pocket. This change may result in the production of antiserum with altered reactivity to the original wild-type virus and epidemic viruses (Table 1).
Fig 1.
3-D models of the receptor-binding site of the HA molecules of A/Victoria/210/2009 (vaccine original virus) and X-187 (vaccine virus). The 3-D model structures of the virus HAs were constructed by homology modeling as described in Materials and Methods. Between the A/Victoria/210/2009 and X-187 viruses, only one amino acid at residue 228 located in the receptor-binding site was different.
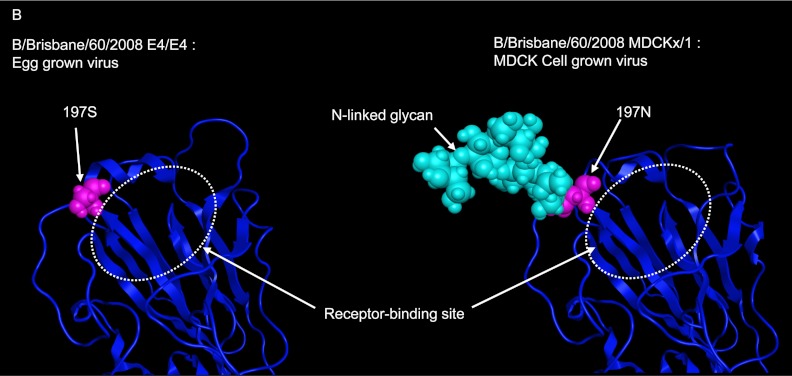
A comparison of amino acid alignments between the HAs of MDCK-grown and egg-adapted B/Brisbane/60/2008 viruses revealed one amino acid difference from Asn to Ser at residue 197. Residues 197 to 199 of MDCK-grown B/Brisbane/60/2008 virus were Asn-Glu-Thr, so that the site was N-glycosylated as reported previously (55, 56). The change from Asn to Ser at residue 197 of egg-adapted B/Brisbane/60/2008 virus resulted in the loss of the N-glycosylation site (Fig. 2). Because residue 197 is in antigenic site B (42, 43, 64), the loss of the N-glycan would be expected to greatly influence antigenic change in the egg-grown B/Brisbane/60/2008 virus (Table 2).
Fig 2.
Comparison of 3-D models of the potential N-glycosylation site in the HA molecules of egg- and MDCK-grown Brisbane/60 viruses. The Brisbane/60 E4/E4 (egg-grown) virus had one amino acid substitution at residue 197 in the HA protein by egg adaptation, resulting in the loss of an N-glycosylation site that exists in the original MDCK-grown wild-type virus.
Cross-reactivity of human serum antibodies elicited by A/Victoria/210/2009 (H3N2) (X-187) vaccine.
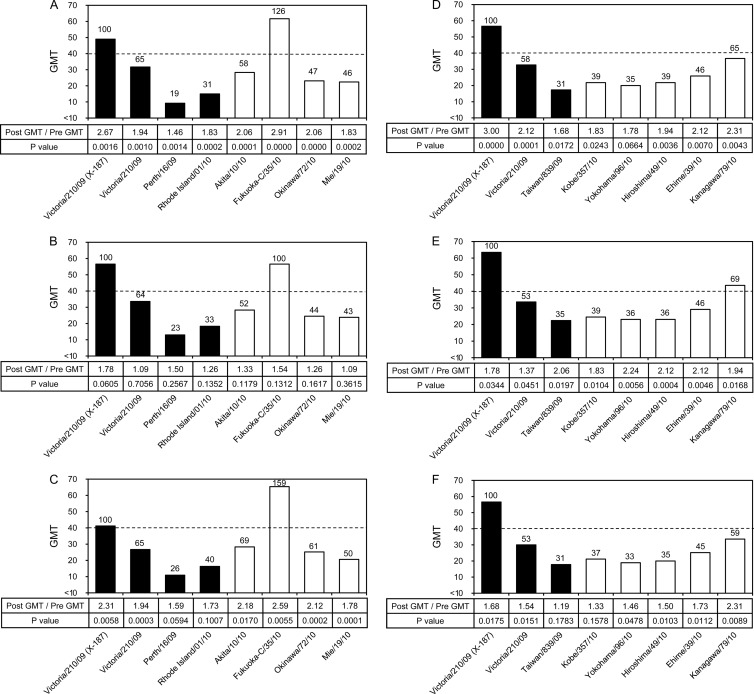
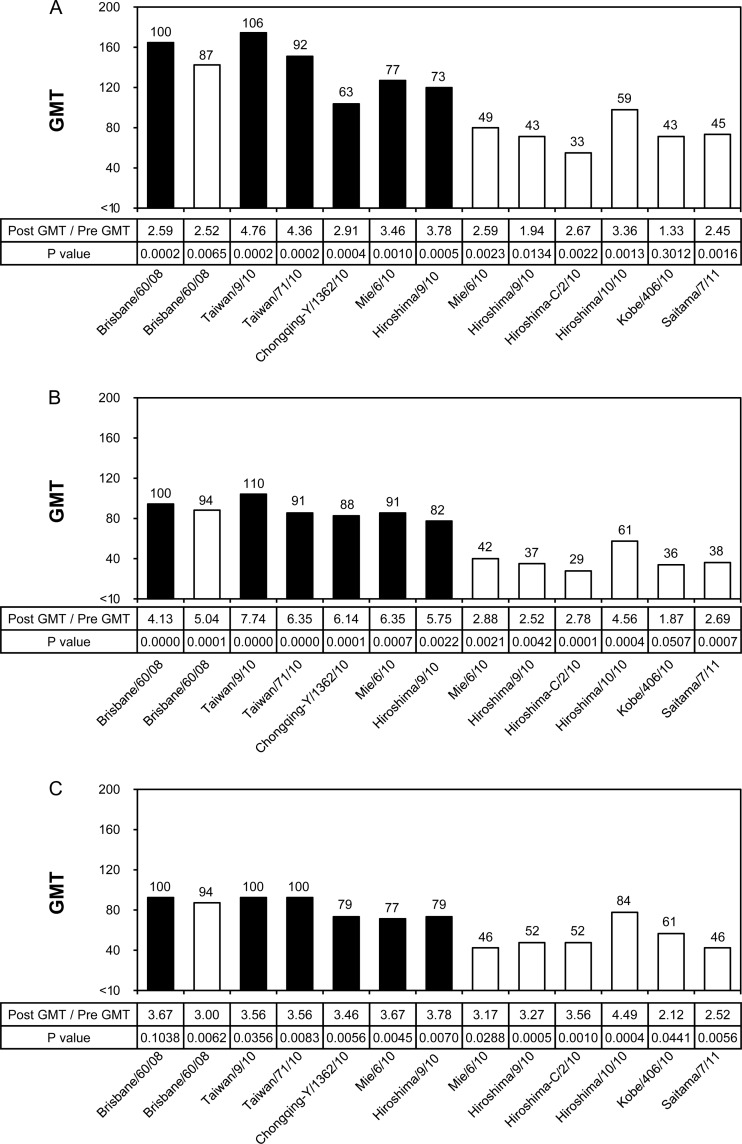
To assess the cross-reactivity of human serum antibodies elicited by the X-187 vaccine, the geometric mean titers (GMTs) evaluated by HI and neutralization (NT) tests against vaccine virus X-187, its wild-type virus A/Victoria/210/2009, and epidemic A/H3N2 viruses isolated in eggs and in MDCK cells were compared. In HI tests, the X-187 vaccine satisfied one of the criteria specified by the Committee for Medicinal Products for Human Use (CHMP) (11) that are required for vaccine licensing so that a sufficient level of immunogenicity can be achieved (Table 3). Since hemagglutination-inhibiting (HI) antibody titers of 40 are associated with at least a 50% reduction in the risk of influenza infection in a population (3), we assessed how many test viruses had GMTs that exceeded the GMT of 40. In the adult group (Fig. 3A and D), only one virus (A/Fukuoka-C/35/2010) had a GMT greater than 40. Most test viruses showed relatively low GMTs, especially A/Perth/16/2009, A/Rhode Island/1/2010, and A/Taiwan/839/2009 egg-grown viruses, even though most viruses tested were antigenically similar to vaccine virus A/Victoria/210/2009 (Table 1). The serum samples of the middle-aged (Fig. 3B and E) and elderly (Fig. 3C and F) groups showed GMT profiles for each test virus essentially similar to the profiles observed with adults, suggesting that the X-187 vaccine may not induce broadly reactive serum HI antibody.
Table 3.
Human serum HI antibody responses induced by vaccines used in the 2009-2010 and 2010-2011 seasons against homologous vaccine antigensa
| Age group | No. of sera | Mean age (yr) | Antigen | GMT |
% of HI tests with GMT ≥40 |
% of HI titers with 4-fold rise | ||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||||
| Adult | 24 | 30.8 | A/Victoria/210/2009 (X-187) | 18.3 | 49.0 | 29.2 | 79.2 | 41.7 |
| 24 | 28.4 | B/Brisbane/60/2008 | 9.4 | 17.0 | 9.5 | 19.0 | 23.8 | |
| Middle-aged | 24 | 51.5 | A/Victoria/210/2009 (X-187) | 31.7 | 56.6 | 41.7 | 70.8 | 29.2 |
| 21 | 51.6 | B/Brisbane/60/2008 | 9.4 | 17.0 | 9.5 | 19.0 | 23.8 | |
| Elderly | 24 | 86.0 | A/Victoria/210/2009 (X-187) | 17.8 | 41.2 | 20.8 | 66.7 | 33.3 |
| 24 | 87.3 | B/Brisbane/60/2008 | 9.4 | 20.6 | 0.0 | 37.5 | 25.0 | |
Pre, prevaccination; Post, postvaccination.
Fig 3.
Cross-reactivity of postvaccination human serum antibodies elicited by X-187 vaccine against various A/H3N2 viruses analyzed by HI test. Geometric mean titers (GMTs) of HI tests to egg-grown (black bars) and MDCK-grown (white bars) viruses are shown. The broken line indicates an HI GMT of 40. Numbers above the columns indicate percentages compared with the GMTs of the vaccine virus. Human serum samples were collected from the adult group (n = 24; age range, 21 to 40 years; mean, 30.8 years) (A and D), middle-aged group (n = 24; age range, 41 to 60 years; mean, 51.5 years) (B and E), and elderly group (n = 24; age range, 61 to 98 years; mean, 86.0 years) (C and F). The HI tests of panels D, E, and F were performed on different days from those of panels A, B, and C.
In NT tests, serum samples of the three age groups exhibited statistically significantly increased GMTs after vaccination (Fig. 4), although the overall GMTs of test viruses in the elderly group (Fig. 4C) were relatively lower than the GMTs in the adult (Fig. 4A) and middle-aged (Fig. 4B) groups. As observed by HI tests, three egg-grown viruses, A/Perth/16/2009, A/Rhode Island/1/2010, and A/Taiwan/839/2009, showed remarkably low GMTs by NT test in all age groups, whereas three MDCK-grown viruses, A/Akita/10/2010, A/Fukuoka-C/35/2010, and A/Kobe/357/2010, showed 1.1- to 2.6-fold-higher GMTs than the GMTs of X-187. A/Fukuoka-C/35/2010 virus also exhibited higher GMTs by HI tests in all age groups (Fig. 3). Since there is no cutoff value for protection for neutralizing antibody titers, unlike the serum HI antibody titer of 40 (3), we evaluated the percent reduction of GMT of each test virus compared with the GMT of X-187. Five of 13 test viruses showed greater than 50% decreased GMTs in all age groups, while 3 of 13 and 1 of 13 test viruses showed 30% to 50% decreased GMTs in the adult and middle-aged groups, respectively (Fig. 4). The results of NT tests together with those of HI tests suggest that human serum antibodies induced by the X-187 vaccine did not have broad reactivity to epidemic viruses and that the X-187 vaccine would possess insufficient efficacy.
Fig 4.
Cross-reactivity of postvaccination human serum antibodies elicited by the X-187 vaccine against various A/H3N2 viruses analyzed by NT test. GMTs of NT tests to egg-grown (black bars) and MDCK-grown (white bars) viruses are shown. Numbers above the columns indicate percentages compared with the GMTs of vaccine virus. The serum samples used were the same as those described in the legend for Fig. 3. (A) Adult group; (B) middle-aged group; (C) elderly group.
Cross-reactivity of human serum antibodies elicited by B/Brisbane/60/2008 vaccine.
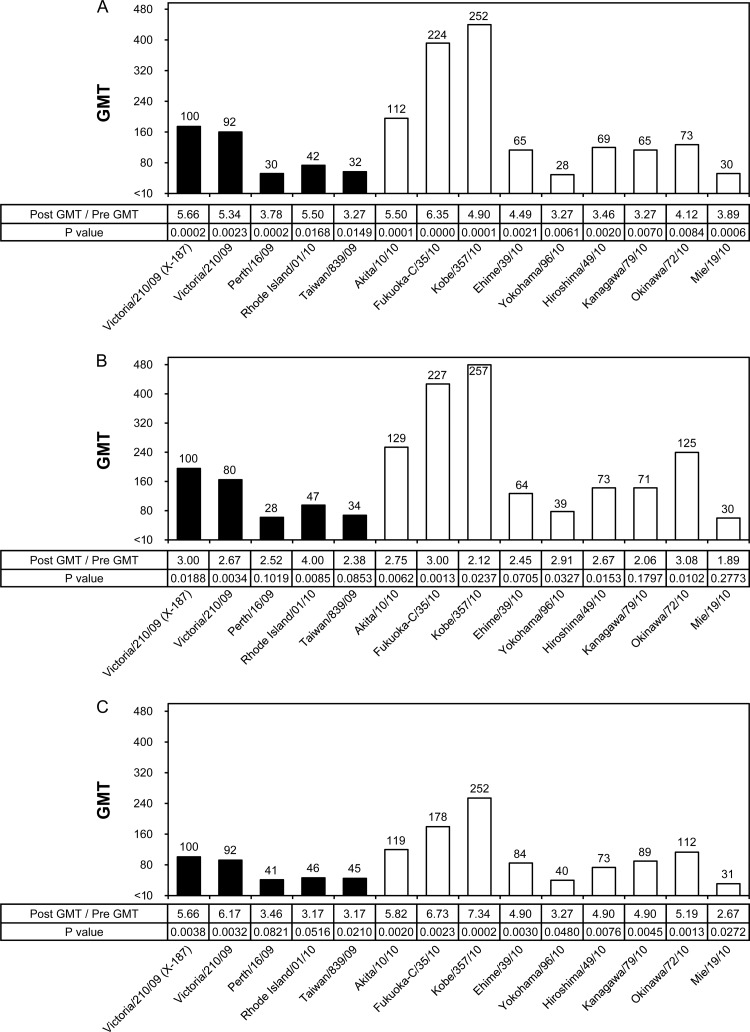
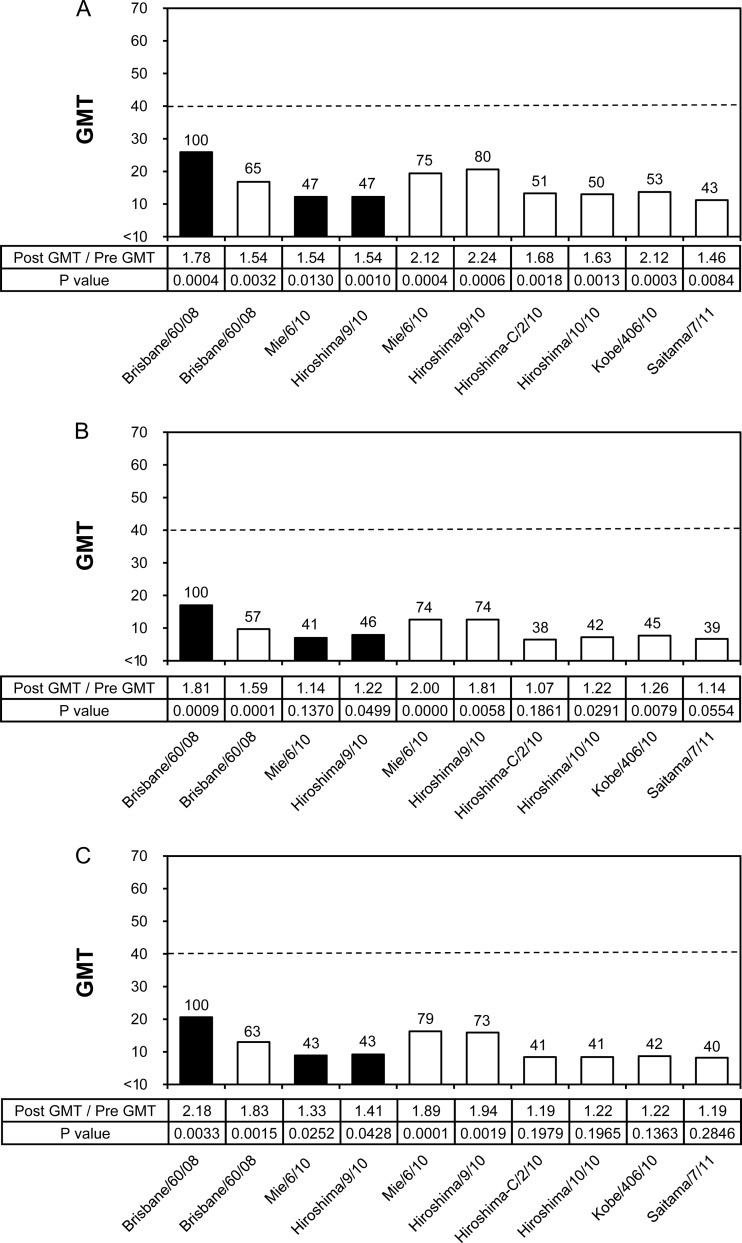
Similarly, the cross-reactivity and efficacy of the B/Brisbane/60/2008 vaccine were evaluated by HI and NT tests (Fig. 5 and 6). In HI tests, the GMTs to homologous vaccine virus B/Brisbane/60/2008 as well as to all test viruses were remarkably low; in particular, the GMTs of most test viruses in the middle-aged and elderly groups were below the marginal level of 1:10 (Fig. 5B and C). Moreover, the B/Brisbane/60/2008 vaccine did not satisfy any of the three criteria of CHMP in the adult and middle-aged groups, and it barely satisfied one criterion, a 2.0 GMT increase between pre- and postvaccination, in the elderly group (Table 3). There were no test viruses, including the B/Brisbane/60/2008 vaccine virus, that exceeded the GMT of 40. Because of the poor ability of the B/Brisbane/60/2008 vaccine to induce serum HI antibodies, the efficacy of the vaccine could not be precisely evaluated by HI tests.
Fig 5.
Cross-reactivity of postvaccination human serum antibodies elicited by the B/Brisbane/60/2008 vaccine against various B/Victoria lineage viruses analyzed by HI test. GMTs of HI tests to egg-grown (black bars) and MDCK-grown (white bars) viruses are shown. The broken line indicates an HI GMT of 40. Numbers above the columns indicate percentages compared with the GMTs of the vaccine virus. Human serum samples were collected in 2009 from an adult group (n = 24; age range, 21 to 40 years; mean, 28.4 years) (A), a middle-aged group (n = 21; age range, 42 to 60 years; mean, 51.4 years) (B), and an elderly group (n = 24; age range, 69 to 103 years; mean, 87.3 years) (C).
Fig 6.
Cross-reactivity of postvaccination human serum antibodies elicited by the B/Brisbane/60/2008 vaccine against various B/Victoria lineage viruses analyzed by NT test. GMTs of NT tests to egg-grown (black bars) and MDCK-grown (white bars) viruses are shown. Numbers above the columns indicate percentages compared with the GMTs of the vaccine virus. The serum samples used were the same as those described in the legend for Fig. 5. (A) Adult group; (B) middle-aged group; (C) elderly group.
By NT test, on the other hand, GMTs to homologous vaccine virus and epidemic test viruses were high enough for evaluation (Fig. 6), although the middle-aged and elderly groups had 1.5-fold-lower GMTs than the adult group (Fig. 6B and C). Unlike GMTs induced by A/H3N2 vaccine, GMTs of egg-grown viruses were relatively high; the viruses induced 63% to 106% GMTs of B/Brisbane/60 vaccine virus in the adult group, 88% to 110% GMTs in the middle-aged group, and 77% to 100% GMTs in the elderly group. In contrast, GMTs of MDCK-grown test viruses were remarkably low, and most viruses, except for the original wild-type B/Brisbane/60 virus, exhibited 50% to 70% decreased GMTs from the GMT of B/Brisbane/60 vaccine virus in all age groups. Although B/Hiroshima/10/2010 showed the highest GMT in MDCK-grown viruses, its GMT in each age group was 59% to 84% of the GMT of B/Brisbane/60 vaccine virus. It is noteworthy that the paired viruses B/Mie/6/2010 and B/Hiroshima/9/2010 isolated from eggs or MDCK cells showed dramatically different GMTs, as observed in the HI test with ferret antisera (Table 2). These findings by NT test clearly demonstrate that human serum NT antibodies induced by the B/Brisbane/60 vaccine were less cross-reactive to epidemic B viruses isolated from MDCK cells than to those from eggs.
DISCUSSION
The assessment of vaccine efficacy is generally based on the European regulatory requirements for annual licensing of influenza vaccine, i.e., >40% seroconversion (4-fold increase of HI titer), >70% seroprotection (HI titer, ≥40), and >2.5 GMT increase of HI titer between pre- and postvaccination sera (11). These criteria mainly cover the immunogenicity of vaccines against the homologous vaccine viruses, but not the cross-reactivity or protective efficacy of serum antibodies against circulating epidemic viruses. The most important criterion in the assessment of vaccine efficacy is the evaluation of whether the vaccine can produce high titers of broad-spectrum HI and NT antibodies against circulating epidemic viruses, including antigenically variant viruses. In Japan, the licensing process for the clinical use of influenza vaccine is based on the antigen protein contents; the requirements include a minimum content of HA protein (greater than 15 μg/dose), a maximum total viral protein content (less than 240 μg/ml), and some safety assessments of the vaccine. However, neither immunogenicity testing nor preclinical studies of vaccines in volunteers are required for vaccine licensing in Japan. Consequently, low-immunogenic B vaccine, which cannot satisfy any of the CHMP criteria required for use in European countries (Table 3), can be distributed in Japan and has been used in clinics. Moreover, we have recently observed by HI tests that the ferret antisera immunized by X-187 (A/H3N2) and egg-adapted B/Brisbane/60/2008 vaccine strains exhibited greatly decreased cross-reactivity to recent circulating epidemic viruses (Tables 1 and 2), and we anticipated that the A/H3N2 and B vaccines for the 2010-2011 season could be less effective against circulating viruses. Although studies have assessed immunogenicity against vaccine viruses after the administration of vaccines (3, 22, 24, 65, 71), studies assessing cross-reactivity of postvaccinated human serum antibodies against epidemic viruses are quite limited (2, 13, 18, 33, 50). Consequently, in the present study we assessed the cross-reactivity of serum HI and NT antibodies of adult, middle-aged, and elderly individuals who received the 2010-2011 season vaccine.
During egg adaptation of the X-187 vaccine virus, an amino acid substitution, Ser228Thr, in the globular head of the HA molecule occurred; this substitution is evident in a comparison to the wild-type HA of A/Victoria/210/2009 virus. Amino acid position 228 of H3 subtypes of influenza HA has been implicated as a critical residue for receptor specificity and host range restriction (25, 49, 66). Three-dimensional modeling analysis showed that the substitution is located at a site where it could cause critical structural changes in the receptor-binding surface due to changes in the amino acid side chain (Fig. 1). Such structurally changed X-187 HA protein would induce antibodies with reduced reactivity to the original wild-type A/Victoria/210/2009 virus and antigenically similar prototype viruses A/Perth/16/2009 and A/Niigata/403/2009 as well as many epidemic viruses (Table 1). Human serum antibodies induced by the X-187 vaccine also showed decreased HI titers to the majority of test viruses in all age groups (Fig. 3). Many studies have shown that susceptibility to influenza virus infection is inversely related to the initial titer of serum IgG HI antibody (38, 40, 52). Most results indicate that, following immunization with inactivated virus vaccines, HI antibody titers of approximately 1:30 to 1:40 represent the 50% protective level of antibody (3, 20, 22, 53). Similarly, the NT antibodies induced in human sera exhibited narrow cross-reactivity against epidemic A/H3N2 viruses in all age groups, since half of the test viruses showed greater than 50% reduction of GMT from the GMT of the vaccine virus (Fig. 4). These results suggest that the X-187 vaccine possesses a small inhibitory effect against circulating epidemic A/H3N2 viruses, resulting in the decreased protective efficacy of the A/H3N2 vaccine. However, three MDCK-grown viruses (A/Kobe/357/2010, A/Akita/10/2010, and A/Fukuoka-C/35/2010) showed higher NT antibody titers than did the homologous X-187 vaccine virus. These three viruses were also relatively better inhibited in HI tests with all reference ferret antisera raised to vaccine-like viruses (Table 1). Although these three viruses did not possess the consensus amino acid substitutions in the HA protein that are suggestive of broad reactors, other epitopes could be recognized by NT antibody and result in elevated reactivity in the NT test.
Generally, the antibody response to influenza B vaccine is significantly lower than the antibody response to influenza A vaccine (7, 8, 24). Our routine serology studies of human sera for vaccine strain selection by WHO have also indicated the low immunogenicity of influenza B vaccine (data not shown). A recent report by Xie et al. (71) indicated that the B/Brisbane/60/2008 vaccine used for the 2009-2010 influenza season was less immunogenic than the two influenza A vaccine components. By HI tests in the present study, human serum antibodies induced by B/Brisbane/60/2008 vaccine included in the vaccine composition of the 2009-2010 season showed overall very low GMTs against all test B viruses, including homologous vaccine virus, particularly for middle-aged and elderly individuals, and the titers were as low as the marginal level (Fig. 5). Moreover, no test viruses, including vaccine virus, exceeded the GMT of 40, indicating a poor protective efficacy of the vaccine. Similarly, for the adult group (mean age, 38.1 years) and elderly group (mean age, 82.3 years), serum antibodies induced by B/Brisbane/60/2008 vaccine included in the vaccine composition of the 2010-2011 season revealed remarkably low immunogenicity and low GMTs to test viruses isolated in the 2010-2011 season (data not shown). We therefore concluded that the efficacy of the influenza B vaccine should not be assessed by HI test.
HI antibody substantially recognizes the receptor-binding region in the HA1 molecule and inhibits virus binding to the receptor of host cells, while NT antibody includes not only HI antibody but also the antibodies recognizing other regions outside antigenic sites in the HA1 molecule (23, 29, 47, 63) and plays a substantial role in the protective efficacy of vaccines. Consequently, the proper assessment of vaccine efficacy for human use should be done by NT test. NT tests of human serum antibodies showed a sufficient level of GMTs against homologous vaccine virus like the GMTs of H3 vaccine (Fig. 6), so that the cross-reactivity of the serum antibody induced by B/Brisbane/60/2008 vaccine was able to be assessed by NT test. The overall reactivity of the serum antibodies in all age groups was high with egg-grown B viruses, whereas the reactivity against MDCK-grown B viruses, except for the original wild-type virus B/Brisbane/60/2008, was significantly lower (Fig. 6). In particular, a marked difference was seen between the pair of egg- and MDCK-grown viruses of B/Hiroshima/9/2010 and B/Mie/6/2010. Our results were consistent with the previous report that egg-grown influenza B vaccine virus induced narrow-spectrum serum antibodies that were more specific to homologous egg-grown viruses (1). From the assessment by NT test, it was strongly suggested that the efficacy of the B/Brisbane/60/2008 vaccine for the 2009-2010 and 2010-2011 seasons was significantly decreased.
Previous studies have demonstrated that egg passage of B/Victoria/2/87 lineage viruses is associated with the loss of an N-glycosylation site (Asn-X-Thr/Ser) at residues 197 to 199 of the HA protein in mammalian-cell isolates, resulting in the antigenic change from the original wild-type virus (55, 56, 58). Our amino acid alignment of the egg-adapted B/Brisbane/60/2008 vaccine strain and all other egg-grown isolates used in the present study confirmed the change from Asn or an Asn/Ser mixture to Ser at residue 197 or from Thr or a Thr/Ile mixture to Ile at residue 199, resulting in the loss of the N-glycosylation site. Between the HAs of egg- and MDCK-grown B/Brisbane/60/2008 viruses, only one amino acid at residue 197 was different, and the difference was reflected in the absence or presence of N-glycan in antigenic site B (Fig. 2). Attachment of N-glycan at the antigenic site of HA protein could hide the epitope and affect immune recognition by the antibody induced by non-N-glycosylated virus. In fact, the reactivity of egg-grown B/Brisbane/60/2008 ferret antiserum against the glycosylated MDCK-grown B/Brisbane/60/2008 virus was dramatically affected (Table 2). However, such a striking change of B/Brisbane/60/2008 virus was not crucial for NT antibody, since MDCK-grown B/Brisbane/60/2008 virus showed high cross-reactivity in the NT test (Fig. 6). It is likely that the NT antibodies react with another homologous part of the HA protein in MDCK-grown B/Brisbane/60/2008 virus to restore cross-reactivity. On the other hand, other MDCK-grown test viruses possessed 3 to 4 amino acid differences in the HA1 region from B/Brisbane/60/2008 vaccine virus together with the retained N-glycan at residues 197 to 199. This heterogeneity of HA protein might not be covered by the NT serum antibodies induced by B/Brisbane/60/2008 vaccine. Several studies point to the importance of the second most abundant surface influenza glycoprotein neuraminidase (NA) in conferring cross-reactive immunity (10, 36, 57). Anti-N2 serum antibodies provide protection against antigenically distinct viruses belonging to the same subtype (10). However, the NA gene sequences of A/Victoria/210/2009 and X-187 vaccine virus and MDCK-grown and egg-adapted B/Brisbane/60/2008 viruses were identical, respectively. Therefore, we conclude that anti-NA antibodies had no effect on the different reactivities of these viruses.
Egg-grown virus vaccine has been suggested to produce less cross-reactive antibody and inferior protective efficacy compared to MDCK-grown virus vaccine (1). Unfortunately, this was the case for the A/H3N2 X-187 vaccine virus as assessed by our serology study. In addition, recent A/H3N2 viruses have become extremely difficult to isolate in eggs because of the change in receptor-binding properties related to amino acid substitutions at residue 156, 186, 194, or 196 (16, 19, 21, 28, 30, 35, 39, 61). Such a viral change causes difficulties in the development of a high-growth reassortant vaccine virus and in the timely supply of vaccine seed virus to manufacturers so that the vaccine can be produced before the annual outbreak of influenza. Moreover, most B viruses of both B/Victoria/2/87 lineage and B/Yamagata/16/88 lineage lose the N-glycosylation site in their important antigenic site by egg adaptation (55, 56, 58). Although an amino acid substitution from Gly to Arg at residue 141 in the HA protein of B virus can stabilize the glycosylation site without affecting antigenicity (9), the production of influenza vaccine in eggs has encountered difficult problems, and these problems will continue to arise in every influenza season. Many studies of influenza vaccine efficacy have been conducted on the basis of virological and statistical studies (13, 34, 44, 62). However, the possibility that altered antigenicity caused by egg adaptation during the vaccine production process has harmed the efficacy of influenza vaccine (as shown in our present study) has not been well explored. Ideally, any new influenza vaccine for humans should be evaluated for potential alteration of immunogenicity prior to use, as described in this study. However, manufacturers may not have sufficient time to conduct exhaustive clinical trials. Therefore, it is important that HI and NT tests using ferret antisera and HA gene sequencing of vaccine viruses be performed to (i) confirm that the vaccines have not suffered any modifications that decrease their efficacy and (ii) confirm that the vaccine viruses are comparable to the circulating viruses or viruses grown in MDCK cells. Consequently, to solve the problems of egg adaptation, the development and supply of influenza vaccines grown in MDCK cells or other surrogate cells should be globally propelled to replace current egg-grown virus vaccines.
ACKNOWLEDGMENTS
We thank Y. Ami and Y. Suzaki, Division of Experimental Animal Research, National Institute of Infectious Diseases, for their excellent support in the production of ferret antisera, Y. Shu, National Institute for Viral Disease Control and Prevention China CDC, for providing Chinese isolates, and H. Sugawara, R. Ito, T. Doi, N. Kim, A. Sato, and M. Ejima for their technical assistance.
This study was supported by Grants-in-Aid for Emerging and Reemerging Infectious Diseases (H21-Shinko-Ippan 005, H22-Shinko-Ippan 005) from the Ministry of Health, Labor and Welfare of Japan.
We declare no potential conflict of interest relevant to this article.
Footnotes
Published ahead of print 4 April 2012
REFERENCES
- 1. Alymova IV, et al. 1998. Immunogenicity and protective efficacy in mice of influenza B virus vaccines grown in mammalian cells or embryonated chicken eggs. J. Virol. 72:4472–4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ansaldi F, et al. 2012. Intanza(®) 15 intradermal influenza vaccine elicits cross-reactive antibody responses against heterologous A(H3N2) influenza viruses. Vaccine 30:2908–2913 [DOI] [PubMed] [Google Scholar]
- 3. Arden NH, et al. 1986. Safety and immunogenicity of a 45-microgram supplemental dose of inactivated split-virus influenza B vaccine in the elderly. J. Infect. Dis. 153:805–806 [DOI] [PubMed] [Google Scholar]
- 4. Beare AS, Kendal AP, Cox NJ, Scholtissek C. 1980. Human trials with wild-type H1N1 and recombinant H3N2-H1N1 influenza A viruses of 1977–1978. Infect. Immun. 28:753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beare AS, Schild GC, Craig JW. 1975. Trials in man with live recombinants made from A/PR/8/34 (H0 N1) and wild H3 N2 influenza viruses. Lancet ii:729–732 [DOI] [PubMed] [Google Scholar]
- 6. Belshe RB, et al. 2007. Live attenuated versus inactivated influenza vaccine in infants and young children. N. Engl. J. Med. 356:685–696 [DOI] [PubMed] [Google Scholar]
- 7. Beyer WE, et al. 1990. Effect of immunomodulator thymopentin on impaired seroresponse to influenza vaccine in patients on haemodialysis. Nephron 54:296–301 [DOI] [PubMed] [Google Scholar]
- 8. Camilloni B, et al. 2010. An influenza B outbreak during the 2007/2008 winter among appropriately immunized elderly people living in a nursing home. Vaccine 28:7536–7541 [DOI] [PubMed] [Google Scholar]
- 9. Chen Z, Aspelund A, Jin H. 2008. Stabilizing the glycosylation pattern of influenza B hemagglutinin following adaptation to growth in eggs. Vaccine 26:361–371 [DOI] [PubMed] [Google Scholar]
- 10. Chen Z, et al. 2000. Cross-protection against a lethal influenza virus infection by DNA vaccine to neuraminidase. Vaccine 18:3214–3222 [DOI] [PubMed] [Google Scholar]
- 11.Committee for Medicinal Products for Human Use (CHMP) 1997. Note for guidance on harmonization of requirements for influenza vaccines. European Agency for the Evaluation of Medicinal Products, Brussels, Belgium [Google Scholar]
- 12. Couch RB, Douglas RG, Jr, Fedson DS, Kasel JA. 1971. Correlated studies of a recombinant influenza-virus vaccine. 3. Protection against experimental influenza in man. J. Infect. Dis. 124:473–480 [DOI] [PubMed] [Google Scholar]
- 13. de Jong JC, Beyer WE, Palache AM, Rimmelzwaan GF, Osterhaus AD. 2000. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J. Med. Virol. 61:94–99 [PubMed] [Google Scholar]
- 14. Deshpande N, et al. 2005. The RCSB Protein Data Bank: a redesigned query system and relational database based on the mmCIF schema. Nucleic Acids Res. 33:D233–D237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fleury D, Wharton SA, Skehel JJ, Knossow M, Bizebard T. 1998. Antigen distortion allows influenza virus to escape neutralization. Nat. Struct. Biol. 5:119–123 [DOI] [PubMed] [Google Scholar]
- 16. Gambaryan AS, Robertson JS, Matrosovich MN. 1999. Effects of egg-adaptation on the receptor-binding properties of human influenza A and B viruses. Virology 258:232–239 [DOI] [PubMed] [Google Scholar]
- 17. Gorse GJ, et al. 1995. Increased anti-influenza A virus cytotoxic T cell activity following vaccination of the chronically ill elderly with live attenuated or inactivated influenza virus vaccine. J. Infect. Dis. 172:1–10 [DOI] [PubMed] [Google Scholar]
- 18. Greenberg SB, Couch RB, Kasel JA. 1974. An outbreak of an influenza type A variant in a closed population: the effect of homologous and heterologous antibody on infection and illness. Am. J. Epidemiol. 100:209–215 [DOI] [PubMed] [Google Scholar]
- 19. Gubareva LV, et al. 1994. Codominant mixtures of viruses in reference strains of influenza virus due to host cell variation. Virology 199:89–97 [DOI] [PubMed] [Google Scholar]
- 20. Hannoun C, Megas F, Piercy J. 2004. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 103:133–138 [DOI] [PubMed] [Google Scholar]
- 21. Hardy CT, Young SA, Webster RG, Naeve CW, Owens RJ. 1995. Egg fluids and cells of the chorioallantoic membrane of embryonated chicken eggs can select different variants of influenza A (H3N2) viruses. Virology 211:302–306 [DOI] [PubMed] [Google Scholar]
- 22. Hobson D, Curry RL, Beare AS, Ward-Gardner A. 1972. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J. Hyg. (Lond.) 70:767–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Imai M, Sugimoto K, Okazaki K, Kida H. 1998. Fusion of influenza virus with the endosomal membrane is inhibited by monoclonal antibodies to defined epitopes on the hemagglutinin. Virus Res. 53:129–139 [DOI] [PubMed] [Google Scholar]
- 24. Iorio AM, et al. 2006. An influenza A/H3 outbreak during the 2004/2005 winter in elderly vaccinated people living in a nursing home. Vaccine 24:6615–6619 [DOI] [PubMed] [Google Scholar]
- 25. Ito T, et al. 2000. Recognition of N-glycolylneuraminic acid linked to galactose by the alpha2,3 linkage is associated with intestinal replication of influenza A virus in ducks. J. Virol. 74:9300–9305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Katz JM, Wang M, Webster RG. 1990. Direct sequencing of the HA gene of influenza (H3N2) virus in original clinical samples reveals sequence identity with mammalian cell-grown virus. J. Virol. 64:1808–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katz JM, Webster RG. 1992. Amino acid sequence identity between the HA1 of influenza A (H3N2) viruses grown in mammalian and primary chick kidney cells. J. Gen. Virol. 73(Pt 5):1159–1165 [DOI] [PubMed] [Google Scholar]
- 28. Katz JM, Webster RG. 1989. Efficacy of inactivated influenza A virus (H3N2) vaccines grown in mammalian cells or embryonated eggs. J. Infect. Dis. 160:191–198 [DOI] [PubMed] [Google Scholar]
- 29. Kida H, Brown LE, Webster RG. 1982. Biological activity of monoclonal antibodies to operationally defined antigenic regions on the hemagglutinin molecule of A/Seal/Massachusetts/1/80 (H7N7) influenza virus. Virology 122:38–47 [DOI] [PubMed] [Google Scholar]
- 30. Kilbourne ED, et al. 1993. Influenza A virus haemagglutinin polymorphism: pleiotropic antigenic variants of A/Shanghai/11/87 (H3N2) virus selected as high yield reassortants. J. Gen. Virol. 74(Pt 7):1311–1316 [DOI] [PubMed] [Google Scholar]
- 31. Kilbourne ED, Murphy JS. 1960. Genetic studies of influenza viruses. I. Viral morphology and growth capacity as exchangeable genetic traits. Rapid in ovo adaptation of early passage Asian strain isolates by combination with PR8. J. Exp. Med. 111:387–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Labute P. 2008. The generalized Born/volume integral implicit solvent model: estimation of the free energy of hydration using London dispersion instead of atomic surface area. J. Comput. Chem. 29:1693–1698 [DOI] [PubMed] [Google Scholar]
- 33. Larson HE, Tyrrell DA, Bowker CH, Potter CW, Schild GC. 1978. Immunity to challenge in volunteers vaccinated with an inactivated current or earlier strain of influenza A(H3N2). J. Hyg. (Lond.) 80:243–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Legrand J, Vergu E, Flahault A. 2006. Real-time monitoring of the influenza vaccine field effectiveness. Vaccine 24:6605–6611 [DOI] [PubMed] [Google Scholar]
- 35. Lu B, Zhou H, Chan W, Kemble G, Jin H. 2006. Single amino acid substitutions in the hemagglutinin of influenza A/Singapore/21/04 (H3N2) increase virus growth in embryonated chicken eggs. Vaccine 24:6691–6693 [DOI] [PubMed] [Google Scholar]
- 36. Marcelin G, et al. 2011. A contributing role for anti-neuraminidase antibodies on immunity to pandemic H1N1 2009 influenza A virus. PLoS One 6:e26335 doi:10.1371/journal.pone.0026335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Medeiros R, Escriou N, Naffakh N, Manuguerra JC, van der Werf S. 2001. Hemagglutinin residues of recent human A(H3N2) influenza viruses that contribute to the inability to agglutinate chicken erythrocytes. Virology 289:74–85 [DOI] [PubMed] [Google Scholar]
- 38. Meiklejohn G, Kempe CH, Thalman WG, Lennette EH. 1952. Evaluation of monovalent influenza vaccines. II. Observations during an influenza a-prime epidemic. Am. J. Hyg. (Lond.) (55):12–21 [DOI] [PubMed] [Google Scholar]
- 39. Meyer WJ, et al. 1993. Influence of host cell-mediated variation on the international surveillance of influenza A (H3N2) viruses. Virology 196:130–137 [DOI] [PubMed] [Google Scholar]
- 40. Morris JA, Kasel JA, Saglam M, Knight V, Loda FA. 1966. Immunity to influenza to antibody levels. N. Engl. J. Med. 274:527–535 [DOI] [PubMed] [Google Scholar]
- 41. Murphy BR, Coelingh K. 2002. Principles underlying the development and use of live attenuated cold-adapted influenza A and B virus vaccines. Viral Immunol. 15:295–323 [DOI] [PubMed] [Google Scholar]
- 42. Muyanga J, et al. 2001. Antigenic and genetic analyses of influenza B viruses isolated in Lusaka, Zambia in 1999. Arch. Virol. 146:1667–1679 [DOI] [PubMed] [Google Scholar]
- 43. Nakagawa N, Kubota R, Maeda A, Okuno Y. 2004. Influenza B virus Victoria group with a new glycosylation site was epidemic in Japan in the 2002-2003 season. J. Clin. Microbiol. 42:3295–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nichol KL. 2009. Challenges in evaluating influenza vaccine effectiveness and the mortality benefits controversy. Vaccine 27:6305–6311 [DOI] [PubMed] [Google Scholar]
- 45. Nobusawa E, Ishihara H, Morishita T, Sato K, Nakajima K. 2000. Change in receptor-binding specificity of recent human influenza A viruses (H3N2): a single amino acid change in hemagglutinin altered its recognition of sialyloligosaccharides. Virology 278:587–596 [DOI] [PubMed] [Google Scholar]
- 46. Oh DY, Barr IG, Mosse JA, Laurie KL. 2008. MDCK-SIAT1 cells show improved isolation rates for recent human influenza viruses compared to conventional MDCK cells. J. Clin. Microbiol. 46:2189–2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Okuno Y, Isegawa Y, Sasao F, Ueda S. 1993. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J. Virol. 67:2552–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Onufriev A, Bashford D, Case DA. 2000. Modification of the generalized Born model suitable for macromolecules. J. Phys. Chem. B 104:3712–3720 [Google Scholar]
- 49. Pekosz A, Newby C, Bose PS, Lutz A. 2009. Sialic acid recognition is a key determinant of influenza A virus tropism in murine trachea epithelial cell cultures. Virology 386:61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pereira MS, Chakraverty P, Schild GC, Coleman MT, Dowdle WR. 1972. Prevalence of antibody to current influenza viruses and effect of vaccination on antibody response. Br. Med. J. 4:701–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ponder JW, Case DA. 2003. Force fields for protein simulations. Adv. Protein Chem. 66:27–85 [DOI] [PubMed] [Google Scholar]
- 52. Potter CW, Jennings R, Nicholson K, Tyrrell DA, Dickinson KG. 1977. Immunity to attenuated influenza virus WRL 105 infection induced by heterologous, inactivated influenza A virus vaccines. J. Hyg. (Lond.) 79:321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Potter CW, Oxford JS. 1979 Determinants of immunity to influenza infection in man. Br. Med. Bull. 35:69–75 [DOI] [PubMed] [Google Scholar]
- 54. Robertson JS, et al. 1987. Structural changes in the haemagglutinin which accompany egg adaptation of an influenza A(H1N1) virus. Virology 160:31–37 [DOI] [PubMed] [Google Scholar]
- 55. Robertson JS, et al. 1985. Alterations in the hemagglutinin associated with adaptation of influenza B virus to growth in eggs. Virology 143:166–174 [DOI] [PubMed] [Google Scholar]
- 56. Saito T, et al. 2004. Antigenic alteration of influenza B virus associated with loss of a glycosylation site due to host-cell adaptation. J. Med. Virol. 74:336–343 [DOI] [PubMed] [Google Scholar]
- 57. Sandbulte MR, et al. 2007. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 4:e59 doi:10.1371/journal.pmed.0040059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shaw MW, et al. 2002. Reappearance and global spread of variants of influenza B/Victoria/2/87 lineage viruses in the 2000-2001 and 2001-2002 seasons. Virology 303:1–8 [DOI] [PubMed] [Google Scholar]
- 59. Shirakawa K, et al. 2008. Phosphorylation of APOBEC3G by protein kinase A regulates its interaction with HIV-1 Vif. Nat. Struct. Mol. Biol. 15:1184–1191 [DOI] [PubMed] [Google Scholar]
- 60. Song H, et al. 2007. A single amino acid of the human immunodeficiency virus type 2 capsid affects its replication in the presence of cynomolgus monkey and human TRIM5alphas. J. Virol. 81:7280–7285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stevens J, et al. 2010. Receptor specificity of influenza A H3N2 viruses isolated in mammalian cells and embryonated chicken eggs. J. Virol. 84:8287–8299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Szilagyi PG, et al. 2008. Influenza vaccine effectiveness among children 6 to 59 months of age during 2 influenza seasons: a case-cohort study. Arch. Pediatr. Adolesc. Med. 162:943–951 [DOI] [PubMed] [Google Scholar]
- 63. Throsby M, et al. 2008. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One 3:e3942 doi:10.1371/journal.pone.0003942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tung CS, Goodman JL, Lu H, Macken CA. 2004. Homology model of the structure of influenza B virus HA1. J. Gen. Virol. 85:3249–3259 [DOI] [PubMed] [Google Scholar]
- 65. Vajo Z, Tamas F, Jankovics I. 2012. A reduced-dose seasonal trivalent influenza vaccine is safe and immunogenic in adult and elderly patients in a randomized controlled trial. Clin. Vaccine Immunol. 19:313–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vines A, et al. 1998. The role of influenza A virus hemagglutinin residues 226 and 228 in receptor specificity and host range restriction. J. Virol. 72:7626–7631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang Q, Cheng F, Lu M, Tian X, Ma J. 2008. Crystal structure of unliganded influenza B virus hemagglutinin. J. Virol. 82:3011–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.World Health Organization 2011. Summary of status of development and availability of influenza B candidate vaccine viruses and potency testing reagents. World Health Organization, Geneva, Switzerland [Google Scholar]
- 69.World Health Organization 2009. Recommended composition of influenza virus vaccines for use in the 2009-2010 influenza season. World Health Organization, Geneva, Switzerland [Google Scholar]
- 70.World Health Organization 2010. Recommended composition of influenza virus vaccines for use in the 2010-2011 northern hemisphere influenza season. World Health Organization, Geneva, Switzerland [Google Scholar]
- 71. Xie H, et al. 2011. Immunogenicity and cross-reactivity of 2009-2010 inactivated seasonal influenza vaccine in US adults and elderly. PLoS One 6:e16650 doi:10.1371/journal.pone.0016650 [DOI] [PMC free article] [PubMed] [Google Scholar]