Abstract
Vibrio cholerae O1 causes cholera, a dehydrating diarrheal disease. We have previously shown that V. cholerae-specific memory B cell responses develop after cholera infection, and we hypothesize that these mediate long-term protective immunity against cholera. We prospectively followed household contacts of cholera patients to determine whether the presence of circulating V. cholerae O1 antigen-specific memory B cells on enrollment was associated with protection against V. cholerae infection over a 30-day period. Two hundred thirty-six household contacts of 122 index patients with cholera were enrolled. The presence of lipopolysaccharide (LPS)-specific IgG memory B cells in peripheral blood on study entry was associated with a 68% decrease in the risk of infection in household contacts (P = 0.032). No protection was associated with cholera toxin B subunit (CtxB)-specific memory B cells or IgA memory B cells specific to LPS. These results suggest that LPS-specific IgG memory B cells may be important in protection against infection with V. cholerae O1.
INTRODUCTION
Vibrio cholerae is a noninvasive enteric pathogen that causes an estimated yearly 3 million to 5 million cases of secretory diarrhea with 120,000 deaths (30, 31). Two serogroups cause epidemic disease in humans, V. cholerae O1 and V. cholerae O139. V. cholerae O1 causes the majority of disease worldwide and can be further divided into two serotypes, Inaba and Ogawa (20).
V. cholerae O1 causes disease through the elaboration of cholera toxin (CT), which activates adenylate cyclase in enterocytes, resulting in secretion of fluid and electrolytes into the gut lumen, causing profuse watery diarrhea and rapid dehydration (4, 20, 26). Although the mortality of severe cholera can be reduced to <1% under optimal conditions (24), a significant burden of disease due to cholera remains, particularly in resource-limited settings in Asia, Africa, and most recently Haiti (10, 31). This highlights the need for more effective preventive strategies.
There are currently two WHO-prequalified, commercially available, licensed oral cholera vaccines, Dukoral (Crucell; Sweden) and Shanchol (Shantha Biotechnic; India). Both are killed oral multistrain vaccines. Dukoral is licensed in more than 50 countries. In efficacy studies for a predecessor of Dukoral, vaccination conferred 67% protection for the first 2 years, but protection was shown to drop to 17% 3 years after a three-dose vaccine regimen (6). This more limited duration of vaccine efficacy contrasts with natural infection, in which mathematical models suggest that immunity following infection with V. cholerae O1 begins to decline after 3 years but that substantial protective immunity may persist for 3 to 7 years after infection (2, 15).
A major obstacle to the development of cholera vaccines with a duration of efficacy comparable to that of infection is the lack of information on the immunologic mechanism(s) responsible for long-term protection from disease. The vibriocidal antibody, a complement-dependent bactericidal antibody, is the best-characterized immunologic marker of protection against cholera. However, the vibriocidal response is most likely a surrogate marker for an undefined protective mucosal immune response, since persons without detectable circulating vibriocidal antibodies may be immune and there is no threshold titer above which complete protection is achieved (19, 25). Previously, we demonstrated that the presence of cholera toxin B subunit (CtxB)- and lipopolysaccharide (LPS)-specific IgA antibodies is also associated with protection from V. cholerae infection (11). However, these serum immune responses wane quickly in the 6 to 9 months following natural infection, suggesting that they may not themselves mediate long-term protection and may also be surrogate markers (9).
Since cholera is a mucosal infection, direct measurement of immune responses in mucosa rather than the circulation may better correlate with protection. We have previously studied duodenal biopsy specimens from cholera patients at intervals over 1 year of follow-up to examine mucosal immune responses to V. cholerae antigens. Antibody levels in duodenal extracts peak by day 30 and are not significantly different from the baseline by day 180. These data suggest that preformed antibodies at the mucosal surface are also unlikely to mediate protection against V. cholerae (28).
Memory B cells (MBC) develop following a variety of natural infections and immunizations and could be a mechanism by which long-term immunity to cholera is mediated (13). These cells are responsible for anamnestic antibody responses following reexposure to antigen, which stimulates the differentiation of memory B cells into antibody-secreting cells (ASC). In previous studies, we have demonstrated the presence of antigen-specific IgG and IgA memory B cells in cholera patients for up to 1 year following infection (9), and we have hypothesized that anamnestic responses of V. cholerae-specific memory B cells might correlate with long-lasting protection on reexposure (20).
To date, no studies have been carried out to directly examine the possible correlation of memory B cells present in the circulation on exposure and subsequent protection from infection. In this study, we examined the relationship between circulating memory B cell and plasma antibody levels on study entry and the risk of subsequent infection in household contacts of cholera patients.
MATERIALS AND METHODS
Study design and enrollment of participants.
Patients hospitalized with cholera at the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) hospital in Dhaka, Bangladesh, and their household contacts were enrolled in the study. Cholera cases were confirmed microbiologically by a stool culture growing V. cholerae O1 as the sole pathogen. Household contacts were defined as individuals who shared a cooking pot with the index case for three or more days preceding the cholera episode in the index case (11). Consenting contacts were enrolled within 24 h of presentation of the index patient (day 2). On days 2 to 10 following presentation of the index case, contacts were questioned about diarrheal symptoms and rectal swabs were obtained for V. cholerae O1 culture.
Blood samples were obtained from patients on the second day of hospitalization and again, following discharge, on days 7 and 30 following onset of illness. Blood was also collected from contacts on days 2, 7, and 30. At each time point, plasma was assayed for vibriocidal antibodies and IgG and IgA antibodies to cholera toxin B subunit (CtxB) and the homologous serotype of V. cholerae O1 lipopolysaccharide (LPS). Upon enrollment, antigen-specific IgG and IgA memory B cells were measured in household contacts as well.
This study was approved by the Research Review Committee and Ethical Review Committee of the icddr,b and the Institutional Review Board of the Massachusetts General Hospital. Informed written consent was obtained from all participants.
Vibriocidal antibody assay.
Vibriocidal antibody responses in plasma were assessed as previously described using V. cholerae O1 Ogawa (X-25049) or Inaba (19479) as the target organism (23). We defined the vibriocidal titer as the reciprocal of the highest dilution resulting in >50% reduction of the optical density compared to that of control wells without plasma.
CtxB- and LPS-specific IgG and IgA antibodies in plasma.
CtxB- and LPS-specific IgG and IgA responses in plasma were measured using standardized enzyme-linked immunosorbent assay protocols (22, 23). We read the plates kinetically at 450 nm for 5 min, and the maximal rate of change in optical density was measured as milli-absorbance units per minute (mabs/min). ELISA units were normalized by calculating the ratio of the test sample to a standard of pooled convalescent-phase plasma from previously infected cholera patients included as a positive control on each plate.
Memory B-cell detection by enzyme-linked immunosorbent spot (ELISPOT) assay.
Memory B-cell assays were performed as previously described (1, 7). Briefly, peripheral blood mononuclear cells (PBMC) were isolated by centrifugation using a Ficoll Isopaque gradient (Pharmacia, Piscataway, NJ). We placed 5 × 105 PBMC/well in cell culture plates (BD Biosciences, San Jose, CA) containing RPMI 1640 and 10% fetal bovine serum (FBS). To stimulate antigen-independent proliferation and differentiation of memory B cells into antibody-secreting cells (ASC), a mixture of three B-cell mitogens containing 6 μg/ml CpG oligonucleotide (Operon, Huntsville, AL), a 1/100,000 dilution of crude pokeweed mitogen extract, and a 1/10,000 dilution of fixed Staphylococcus aureus Cowan (Sigma, St. Louis, MO) were added to all wells except those being used as negative controls, to which only medium was added. Plates were incubated at 37°C in 5% CO2 for 6 days, after which the cells were harvested and washed.
For the memory B cell ELISPOT assay, we coated nitrocellulose-bottom plates (MSHAN-4550; Millipore, Bedford, MA) with GM1 ganglioside (3 nmol/ml) followed by recombinant CtxB (2.5 μg/ml), LPS (25 μg/ml), 5 μg/ml affinity-purified goat anti-human immunoglobulin (Jackson Immunology Research, West Grove, PA), or 2.5 μg/ml keyhole limpet hemocyanin (KLH) (Pierce Biotechnology, Rockford, IL). After the plates were blocked with RPMI 1640 containing 10% FBS, 20% of the cells from each culture plate well were added for detection of total ASC, and 80% were used for detection of antigen-specific ASC. Plates were incubated for 5 h at 37°C in 5% CO2, after which they were washed, and horseradish peroxidase-conjugated mouse anti-human IgG and IgA (Hybridoma Reagent Laboratory, Baltimore, MD) was added. Following an overnight incubation at 4°C, plates were developed with 3-amino-9-ethyl carbazole (AEC). We excluded data from analysis for the following previously described reasons (16): (i) the total population of memory B cells did not show appropriate stimulation, i.e., a 4-fold or greater increase in the number of total Ig memory cells in the stimulated wells compared to that in the unstimulated wells, (ii) the study subject specimens had three or more antigen-specific spots in the unstimulated wells, or (iii) samples had three or more spots to the negative-control antigen KLH. The number of memory B cell ASC per well were counted independently by two reviewers in a sample-blinded fashion using a stereomicroscope, and the counts were averaged. Results were expressed as the percentage of antigen-specific memory B cells per total pool of isotype-specific memory B cells.
Exclusion criteria and definition of outcomes for household contacts.
Among household contacts, infection was defined as at least one rectal swab culture positive for V. cholerae O1 during the 9-day follow-up period and/or a 4-fold or greater increase in vibriocidal antibody titer between day 2 and day 7. Absence of infection was defined as no V. cholerae-positive rectal swabs, no significant changes in vibriocidal antibody titer, and no symptoms of diarrhea. If contacts did not meet either set of criteria, they were referred to as “unclassified” and excluded from immunologic analysis (Fig. 1B). Index patients with symptoms of diarrhea for more than 24 h prior to presentation were excluded from immunologic analysis to ensure immune measurements corresponded as closely as possible to baseline levels; application of this criterion excluded 24% of enrolled patients (Fig. 1A). Household contacts with symptoms of diarrhea in the week before study entry were also excluded from immunological analysis (so that their day 2 immunologic measurements were done prior to the onset of symptomatic infection), as were contacts who did not complete the first 9 days of follow-up. Contacts were also excluded if they developed infection with V. cholerae of a serogroup or serotype that did not correspond to that of the index case.
Fig 1.
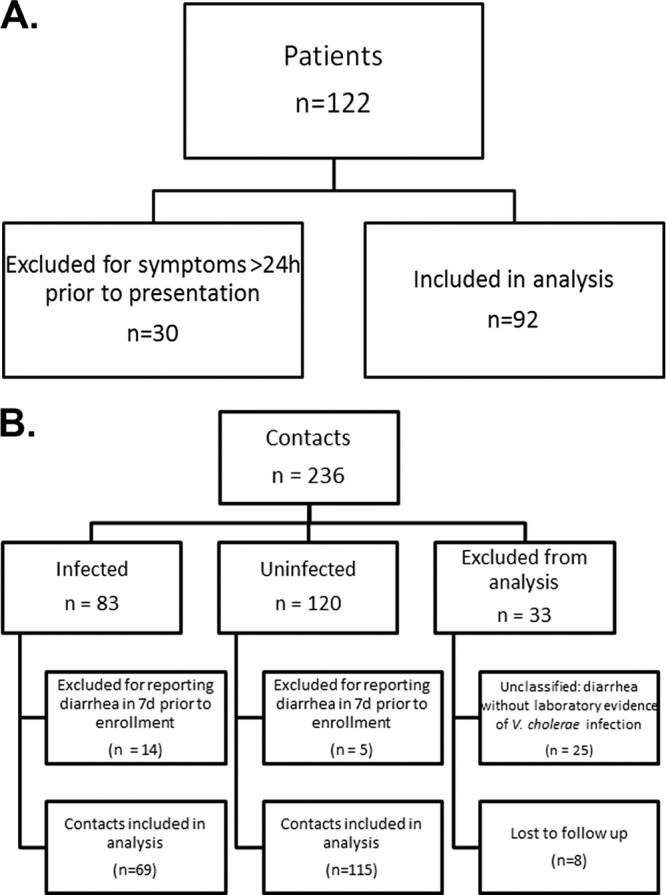
(A and B) Enrollment and classification of study participants. Contacts were classified as “infected” if one or more rectal swabs were positive for V. cholerae and/or they mounted a 4-fold or greater increase in vibriocidal titer between days 2 and 7. Uninfected contacts were classified as such if they had no positive rectal swab, no 4-fold or greater increase in vibriocidal titer, and no reported diarrhea on days 2 to 10.
Statistical analysis.
Baseline characteristics were analyzed using the χ2 test for categorical variables and the Mann-Whitney U test for continuous variables. All reported P values are two-tailed, and statistical significance was defined as a P value of ≤0.05. Statistical analyses were performed using the software programs SPSS Statistics 17.0 (SPSS Inc., Chicago, IL) and GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Study population.
A total of 236 household contacts of 122 index patients with cholera were enrolled in this study between December 2006 and January 2011. There were 206 contacts of 102 index cases infected with V. cholerae O1 Ogawa and 30 contacts of 20 index cases infected with V. cholerae O1 Inaba. Classification of study participants is shown in Fig. 1. Of the 122 patients enrolled, 92 reported symptoms for 24 h or less prior to presentation to the icddr,b hospital for treatment and were included in the immunologic analysis. Of 236 contacts enrolled, 184 were included in the analysis (Fig. 1). Of those included, 37.5% demonstrated evidence of infection. Demographic characteristics of contacts and patients included in the analysis are compared in Table 1. On average, infected contacts were younger than both patients (P > 0.05) and uninfected contacts (P = 0.027).
Table 1.
Demographic features of patients and contacts enrolled in this study
| Feature | Value for groupb |
||
|---|---|---|---|
| Patients | Infected contacts | Uninfected contacts | |
| Age (yrs), mean ± SDa | 24.9 ± 14.5 | 21.9 ± 14.6 | 26.5 ± 14.3 |
| No. (%) female | 40 (43.5) | 40 (58.0) | 52 (45.2) |
| No. (%) with blood group | |||
| O | 40 (43.5) | 31 (44.9) | 50 (43.5) |
| Non-O | 52 (56.5) | 38 (55.1) | 65 (56.5) |
| No. (%) with V. cholerae serotype | |||
| Inaba | 17 (18.5) | 7 (10.1) | 18 (15.7) |
| Ogawa | 75 (8l.5) | 62 (89.9) | 97 (84.3) |
A significant difference between infected and uninfected contacts was observed for age (P = 0.027, Mann-Whitney U test). No significant difference in gender or blood group was observed between contacts. No significant differences were observed between patients and either group of contacts in age, gender, or blood group.
For patients, n = 92; for infected contacts, n = 69; for uninfected contacts, n = 115.
Vibriocidal responses.
V. cholerae O1 Ogawa and Inaba serotype-specific vibriocidal titers were measured in samples from contacts and patients on days 2, 7, and 30. On day 2, following enrollment, samples from uninfected contacts demonstrated the highest vibriocidal antibody titers, which were significantly higher than those for both patients and contacts who became infected (P < 0.001 for both; Fig. 2).
Fig 2.
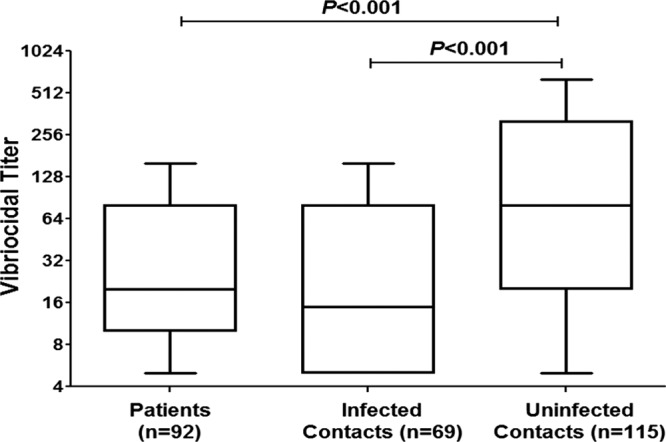
Plasma vibriocidal antibody levels on study day 2. Tukey box plots with bars extending to 1.5× interquartile range from the first and third quartiles are shown. P values for statistically significant differences determined by Mann-Whitney U test are shown.
CtxB- and LPS-specific antibody responses.
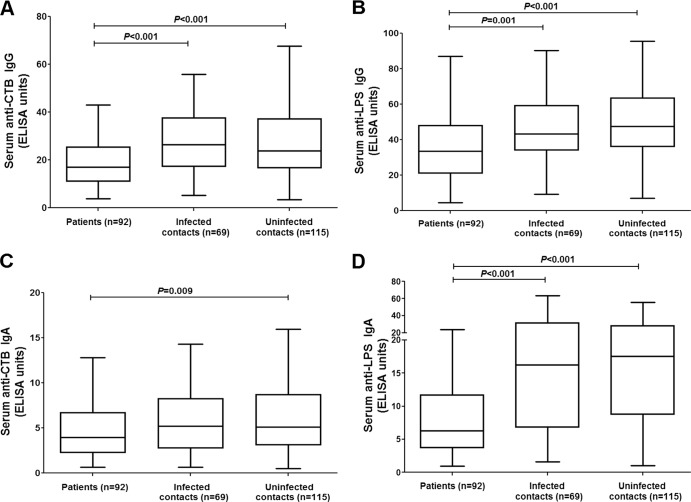
CtxB- and LPS-specific antibody levels were measured in samples from contacts and patients on days 2, 7, and 30; day 2 results are presented in Fig. 3. Patients displayed the lowest V. cholerae antigen-specific antibody levels at day 2, and there were no significant differences in either IgG or IgA levels specific for either CtxB or LPS between infected and uninfected contacts on day 2 (Fig. 3).
Fig 3.
Cholera toxin subunit B (CTB)- and LPS-specific antibody levels on study day 2. Tukey box plots with bars extending to 1.5× interquartile range from the first and third quartiles for CT IgG (A), LPS IgG (B), CT IgA (C), or LPS IgA (D) are shown. P values for statistically significant differences determined by Mann-Whitney U test are shown.
Association between antigen-specific IgG and IgA memory B cell responses and protection against V. cholerae infection.
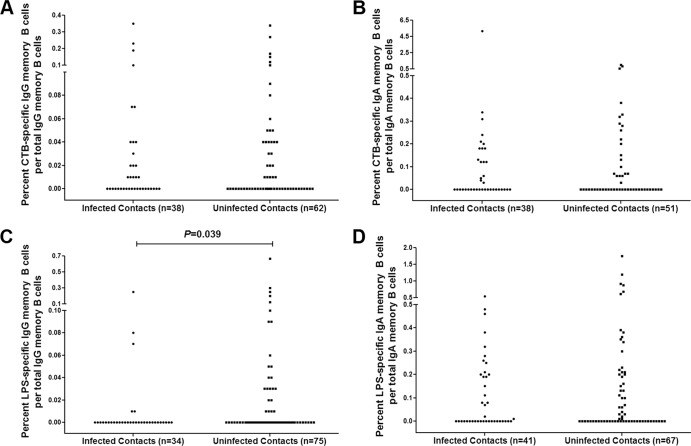
Memory B cell responses were measured at day 2 for contacts (Fig. 4). No significant differences were detected in the percentages of CtxB-specific IgG or IgA memory B cells between infected and uninfected contacts (P = 0.982 and P = 0.919, respectively) (Fig. 4A and B). There was also no significant difference seen in the percentage of LPS-specific IgA memory B cells between infected and uninfected contacts (Fig. 4D). However, uninfected contacts had a significantly higher median percentage of LPS-specific IgG memory B cells on study enrollment than did the contacts who developed infection (P = 0.039) (Fig. 4C).
Fig 4.
(A to D) Cholera toxin subunit B (CTB)- and LPS-specific memory B cells in different contact groups on study day 2. P values for statistically significant differences determined by Mann-Whitney U test are shown for CT IgG (A), CT IgA (B), LPS IgG (C), or LPS IgA (D).
In addition to comparing the median percentages of baseline V. cholerae antigen-specific memory B cells in infected and uninfected contacts, we also measured the association between protection against V. cholerae infection and the presence of detectable IgG or IgA memory B cells specific for CtxB or LPS on study enrollment (Fig. 5). Once again, only the presence of detectable LPS-specific IgG memory B cells was associated with a significantly decreased risk of subsequent infection; the presence of these memory B cells was associated with a 68% decrease in risk (Table 2).
Fig 5.
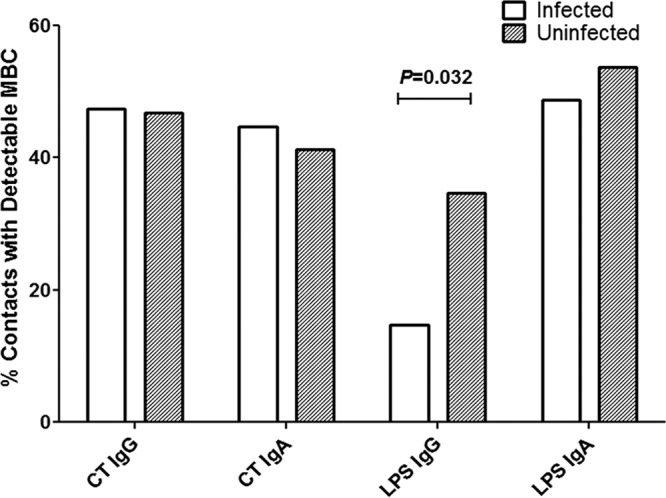
Percentage of infected versus uninfected contacts with detectable cholera toxin subunit B- and LPS-specific memory B cells on study day 2. P values for statistically significant differences determined by Mann-Whitney U test are shown.
Table 2.
Risk of infection for contacts with detectable CtxB- and LPS-specific MBC relative to those without detectable MBC
| MBC detecteda | Risk ratio | 95% CIc | P value |
|---|---|---|---|
| CtxB IgG | |||
| All contacts | 1.02 | 0.46–2.30 | 0.954 |
| Contacts with d2 vibriocidal titer < 160 | 0.66 | 0.257–1.71 | 0.398 |
| CtxB IgA | |||
| All contacts | 1.16 | 0.50–2.70 | 0.737 |
| Contacts with d2 vibriocidal titer < 160 | 1.29 | 0.47–3.55 | 0.619 |
| LPS IgG | |||
| All contactsb | 0.32 | 0.11–0.94 | 0.032 |
| Contacts with d2 vibriocidal titer < 160 | 0.37 | 0.12–1.16 | 0.081 |
| LPS IgA | |||
| All contacts | 0.82 | 0.38–1.79 | 0.617 |
| Contacts with d2 vibriocidal titer < 160 | 0.77 | 0.31–1.89 | 0.570 |
d, day.
A significant decrease in risk was seen in contacts with detectable LPS-specific IgG MBC by Mann-Whitney U test.
CI, confidence interval.
To assess whether the association between baseline V. cholerae LPS-specific IgG memory B cells and protection against V. cholerae infection was independent of the association between high vibriocidal titers and protection seen in previous studies (11, 25), contacts with high vibriocidal titers upon study enrollment (≥160) were excluded, and a subanalysis of protection associated with the presence of detectable antigen-specific memory B cell responses was repeated. In this subanalysis, no protection was associated with having detectable CtxB-specific IgG or IgA and LPS-specific IgA memory B cell responses (P = 0.398, 0.619, and 0.570, respectively) (Table 2). Despite the smaller sample size after excluding contacts with high baseline vibriocidal antibody titers, suggestive of recent infection, we observed a trend suggesting that the presence of LPS-specific IgG memory B cells at baseline was associated with protection against V. cholerae in subjects with low baseline vibriocidal antibody titers (P = 0.081; ninfected = 32; nuninfected = 45).
DISCUSSION
The continued burden of disease caused by V. cholerae O1, already high in countries where it is endemic and also notable in recent epidemics in Zimbabwe and Haiti (10, 18), underscores the need for cholera vaccines. Although currently licensed vaccines offer substantial short-term protection, an optimal cholera vaccine would confer protection for several years, much like natural infection. One obstacle to this is the lack of knowledge regarding which components of the immune system confer long-term protection following infection. The objective of our study was to determine whether antigen-specific memory B cells present on exposure were correlated with protection from subsequent infection in Bangladesh, where cholera is endemic. We chose to study household contacts of patients with cholera because of the high rate of infection in this population (8, 19, 27, 29).
Several studies have found a strong relationship between vibriocidal antibody titers and protection from V. cholerae infection. In areas of endemicity, such as Bangladesh, up to 60% of the population has elevated vibriocidal titers at baseline, and the percentage increases with age, which is likely explained by previous exposure to V. cholerae (8). The present study confirms the known association between protection from infection and both serum vibriocidal titer and age. Contacts that remained uninfected were significantly older than infected contacts and had significantly higher vibriocidal antibody levels at day 2 than both patients and the contacts who subsequently became infected.
In previous univariate analyses, increased baseline serum CtxB- and LPS-specific IgA antibodies in contacts were significantly associated with a decreased risk of subsequent infection, although only CtxB-specific IgA antibodies remained associated with protection following multivariate analysis (11). Plasma IgA antibodies may reflect secretion of IgA at the mucosal surface, where protection is likely achieved. In a previous volunteer study of individuals who had been infected once with V. cholerae O1 Ogawa, none of the individuals developed symptomatic disease on rechallenge, and roughly half mounted a strong anamnestic secretory IgA response in jejunal fluid, indicating that anamnestic immune responses at the mucosal surface could mediate long-term protective immunity in the absence of preformed antibody (17). When mucosal antibody responses were measured in duodenal biopsy samples taken from cholera patients, levels declined to baseline earlier than plasma levels did, indicating that constitutive secretion of antibody at the mucosal surface is not the predominant mechanism of protection from cholera (28).
In the present study, we did not observe an association between plasma IgA antibodies specific for CtxB or LPS and protection from infection seen previously (11), likely due to the sample size limitation in our current study. We did observe that baseline IgG antibodies to CtxB and LPS and IgA antibodies to LPS were lower in patients than in all household contacts, which suggests that lower levels of these antibodies may have been associated with risk for more-severe disease in patients. The higher levels of antigen-specific antibodies seen in contacts may also be due to recent infection with either V. cholerae or, for those contacts with high CtxB antibodies, enterotoxigenic Escherichia coli (ETEC), which produces a heat-labile toxin (LT) which is antigenically similar to cholera toxin.
It has previously been shown that memory B cells develop in the circulation after V. cholerae infection (9). In the present study, we found that while baseline IgG memory B cells specific for CtxB and IgA memory B cells specific for CtxB and LPS were not associated with protection against V. cholerae O1 infection, baseline LPS IgG memory B cells were significantly more numerous for contacts protected from subsequent infection than for those who became infected. We found a surprisingly high baseline LPS IgA memory B cell response in both groups of contacts, as well as increased variability in levels of IgA MBC among contacts. These factors suggest that a larger sample size may have been needed to determine whether these are also associated with protection. While the presence of any detectable IgG or IgA memory B cells against CtxB or IgA memory B cells against LPS was not protective, the presence of detectable LPS-specific IgG memory B cells on study enrollment conferred 68% protection against subsequent infection with V. cholerae. This association appears robust and is discernible even after exclusion of those contacts with high vibriocidal antibody levels on enrollment, suggesting that the presence of LPS-specific IgG memory B cells could mediate protection even in persons whose vibriocidal antibody titers have declined to lower levels.
In a previous study (9), LPS-specific IgG memory B cells in the circulation returned to baseline levels in most patients by day 360 of follow-up, although these memory B cell responses achieved the highest peak levels following infection compared to the other antigen-specific MBC responses studied. Thus, it is possible that the increase in LPS-specific IgG memory B cells seen in the circulation of uninfected contacts in this study reflect more-recent infection and that these circulating LPS-specific IgG memory B cells may not directly mediate the long-term protection seen after cholera. It is also possible that the LPS-specific IgG memory B cells seen in the circulation may correlate with LPS-specific memory B cells in gut mucosa and that the latter may be associated with actual protection.
While CtxB has been shown to be more immunogenic than LPS, possibly due in part to the T-cell-dependent immune response it produces (9), there is a growing body of evidence that LPS may play a larger role in mediating immunity than previously thought despite being a T-cell-independent antigen. It has been shown that the vibriocidal antibody response is directed primarily against components of LPS (21). Our finding that LPS-specific IgG memory B cells in the circulation are correlated with protection, as well as the previous finding that LPS-specific, but not CtxB-specific, IgA ASC remain in the gut mucosa for several months following infection, suggest that immune responses against LPS may be the most protective against subsequent cholera. This hypothesis is strengthened by the observed lack of cross-protection between infection due to V. cholerae serogroups O1 and O139, which have different LPS compositions but are otherwise virtually identical and produce identical cholera toxins (20).
Given the lower efficacy of vaccination seen for children than for adults (3), as well as the significant difference in age between infected and uninfected contacts seen in this cohort, we also examined the effect of age on antigen-specific memory B cell response. We found no significant correlation between age and antigen-specific memory B cell response in this cohort, which is consistent with a separate recently published analysis (16), which also found no significant difference in antigen-specific memory B cell response between young children, older children, and adults. The finding that uninfected contacts were significantly older than infected contacts is consistent with results of prior studies that have found that exposure to cholera (as indicated by an elevated baseline vibriocidal titer) is correlated with increasing age, and it is unlikely that age acted as a confounding factor in this analysis.
There were some limitations to this study. Due to the small sample size, we did not assess the impact of factors that have previously been shown to impact the immune response and susceptibility to disease in contacts, such as helminth coinfection and retinol levels (11, 12). It was also not possible to adequately evaluate the association between immunologic parameters and the risk of symptomatic versus asymptomatic infection, in which CtxB neutralizing responses may play a greater role. Another limitation is that some contacts were likely to be infected prior to the onset of symptoms in the index case. We controlled for this by excluding from immunologic analysis those contacts who reported diarrhea in the 7 days prior to enrollment. The predominance of the Ogawa serotype of V. cholerae O1 in this study, which reflects trends seen in a recent surveillance study of areas in Dhaka of high endemicity (5), was also a limitation because differing immune responses have been noted in response to infection with the Ogawa serotype versus the Inaba serotype in a similar study population, and the preponderance of Ogawa strains may have masked any trends in immune response specific to the Inaba serotype (14).
In summary, it has been previously shown that V. cholerae antigen-specific memory B cells develop following cholera infection and persist longer than other previously identified immunologic markers of V. cholerae infection. Our finding that circulating LPS-specific IgG memory B cells are associated with protection against V. cholerae infection highlights the importance of LPS-specific responses in protection against cholera and suggests that memory B cell responses to this antigen may be a useful surrogate marker of protection and a tool for the development and assessment of improved cholera vaccines.
ACKNOWLEDGMENTS
We thank the patients and their household members for their participation in this study, as well as the field and laboratory workers of the Protective Immunity to Cholera Study at the International Centre for Diarrhoeal Disease Research, Bangladesh.
This work was supported by the icddr,b and grants from the National Institutes of Health, including the National Institute of Allergy and Infectious Diseases (U01 AI058935 [to S.B.C. and E.T.R.], R03 AI063079 [to F.Q.], and U01 AI077883 [to E.T.R.]), a Training Grant in Vaccine Development from the Fogarty International Center (TW005572 [to M.M.A. and F.Q.]), an American Recovery and Reinvestment Act (ARRA) FIC Post-doctoral Fellowship in Global Infectious Diseases (TW05572 [to D.T.L.]), Career Development Awards (K01) from the Fogarty International Center (TW007409 [to J.B.H.] and TW07144 [to R.C.L.]), the Harvard Initiative for Global Health Post-doctoral Fellowship in Global Infectious Diseases (to D.T.L), and a Physician Scientist Early Career Award from the Howard Hughes Medical Institute (to R.C.L.). S.M.P. is the recipient of a Fogarty International Clinical Research Scholars award from the Fogarty International Center and Vanderbilt University (R24 TW007988) and the American Relief and Recovery Act.
Footnotes
Published ahead of print 18 April 2012
REFERENCES
- 1. Alam MM, et al. 2011. Antigen-specific memory B-cell responses in Bangladeshi adults after one or two dose oral killed cholera vaccination, and comparison with responses following natural cholera. Clin. Vaccine Immunol. 18:844–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ali M, Emch M, Park JK, Yunus M, Clemens J. 2011. Natural cholera infection-derived immunity in an endemic setting. J. Infect. Dis. 204:912–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anonymous. 2010. Cholera vaccines: WHO position paper. Wkly. Epidemiol. Rec. 85:117–128 [PubMed] [Google Scholar]
- 4. Cassel D, Selinger Z. 1977. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc. Natl. Acad. Sci. U. S. A. 74:3307–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chowdhury F, et al. 2011. Impact of rapid urbanization on the rates of infection by Vibrio cholerae O1 and enterotoxigenic Escherichia coli in Dhaka, Bangladesh. PLoS Negl. Trop. Dis. 5:e999 doi: 10.1371/journal.pntd.0000999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clemens JD, et al. 1990. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet 335:270–273 [DOI] [PubMed] [Google Scholar]
- 7. Crotty S, Aubert RD, Glidewell J, Ahmed R. 2004. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J. Immunol. Methods. 286:111–122 [DOI] [PubMed] [Google Scholar]
- 8. Glass RI, et al. 1985. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J. Infect. Dis. 151:236–242 [DOI] [PubMed] [Google Scholar]
- 9. Harris AM, et al. 2009. Antigen-specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect. Immun. 77:3850–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris JB, et al. 2010. Cholera's western front. Lancet. 376:1961–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris JB, et al. 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl Trop. Dis. 2:e221 doi: 10.1371/journal.pntd.0000221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harris JB, et al. 2009. Immunologic responses to Vibrio cholerae in patients co-infected with intestinal parasites in Bangladesh. PLoS Negl. Trop. Dis. 3:e403 doi: 10.1371/journal.pntd.0000403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kelly DF, Pollard AJ, Moxon ER. 2005. Immunological memory: the role of B cells in long-term protection against invasive bacterial pathogens. JAMA 294:3019–3023 [DOI] [PubMed] [Google Scholar]
- 14. Khan AI, et al. 2010. Comparison of clinical features and immunological parameters of patients with dehydrating diarrhoea infected with Inaba or Ogawa serotypes of Vibrio cholerae O1. Scand. J. Infect. Dis. 42:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koelle K, Rodo X, Pascual M, Yunus M, Mostafa G. 2005. Refractory periods and climate forcing in cholera dynamics. Nature 436:696–700 [DOI] [PubMed] [Google Scholar]
- 16. Leung DT, et al. 2011. A comparison of memory B cell, antibody secreting cell, and plasma antibody responses in young children, older children, and adults with infection caused by Vibrio cholerae O1 El Tor Ogawa in Bangladesh. Clin. Vaccine Immunol. 18:1317–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levine MM, et al. 1981. Duration of infection-derived immunity to cholera. J. Infect. Dis. 143:818–820 [DOI] [PubMed] [Google Scholar]
- 18. Mintz ED, Guerrant RL. 2009. A lion in our village—the unconscionable tragedy of cholera in Africa. N. Engl. J. Med. 360:1060–1063 [DOI] [PubMed] [Google Scholar]
- 19. Mosley WH, Ahmad S, Benenson AS, Ahmed A. 1968. The relationship of vibriocidal antibody titre to susceptibility to cholera in family contacts of cholera patients. Bull. World Health Organ. 38:777–785 [PMC free article] [PubMed] [Google Scholar]
- 20. Nelson EJ, Harris JB, Morris JG, Jr, Calderwood SB, Camilli A. 2009. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat. Rev. Microbiol. 7:693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neoh SH, Rowley D. 1970. The antigens of Vibrio cholerae involved in the vibriocidal action of antibody and complement. J. Infect. Dis. 121:505–513 [DOI] [PubMed] [Google Scholar]
- 22. Qadri F, et al. 1999. Lipopolysaccharide- and cholera toxin-specific subclass distribution of B-cell responses in cholera. Clin. Diagn. Lab. Immunol. 6:812–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qadri F, et al. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect. Immun. 65:3571–3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ryan ET, et al. 2000. Mortality, morbidity, and microbiology of endemic cholera among hospitalized patients in Dhaka, Bangladesh. Am. J. Trop. Med. Hyg. 63:12–20 [DOI] [PubMed] [Google Scholar]
- 25. Saha D, et al. 2004. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J. Infect. Dis. 189:2318–2322 [DOI] [PubMed] [Google Scholar]
- 26. Sharp GW, Hynie S. 1971. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature 229:266–269 [DOI] [PubMed] [Google Scholar]
- 27. Tamayo JF, et al. 1965. Studies of cholera El Tor in the Philippines. 3. Transmission of infection among household contacts of cholera patients. Bull. World Health Organ. 33:645–649 [PMC free article] [PubMed] [Google Scholar]
- 28. Uddin T, et al. 2011. Mucosal immunologic responses in cholera patients in Bangladesh. Clin. Vaccine Immunol. 18:506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weil AA, et al. 2009. Clinical outcomes in household contacts of patients with cholera in Bangladesh. Clin. Infect. Dis. 49:1473–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. WHO 2003. Cholera unveiled. WHO, Geneva, Switzerland: http://whqlibdoc.who.int/hq/2003/WHO_CDS_CPE_ZFK_2003.3.pdf [Google Scholar]
- 31. Zuckerman JN, Rombo L, Fisch A. 2007. The true burden and risk of cholera: implications for prevention and control. Lancet Infect. Dis. 7:521–530 [DOI] [PubMed] [Google Scholar]