Abstract
Infections by Babesia bovis limit cattle production and cause important economic losses in tropical and subtropical areas around the world. Monitoring of calf sera can be used to detect unprotected cattle herds and to decide on strategic control measures, as well as for epidemiological studies. Merozoite surface antigen 2c (MSA-2c) is an immunodominant surface protein expressed in B. bovis merozoites and sporozoites and contains B-cell epitopes that are conserved among geographic isolates. A monoclonal antibody against recombinant MSA-2c (rMSA-2c) was previously shown to inhibit the binding of anti-B. bovis antibodies to a parasite B-cell epitope in a competitive enzyme-linked immunosorbent assay (cELISA) format. In the work at hand, the parameters of this cELISA were reevaluated and adjusted when necessary, and a cutoff value was determined by receiver operator characteristic (ROC) curve analysis of a total of 357 bovine sera of known reactivity, as assessed by indirect immunofluorescence assay (IFAT). The established rMSA-2c cELISA demonstrated a specificity of 98% and a sensitivity of 96.2%. An additional set of 303 field bovine sera from regions where ticks are endemic and tick-free regions of Argentina was tested by both rMSA-2c cELISA and IFAT, and the results were shown to be in very good agreement (kappa index, 0.8325). The performance shown by rMSA-2c cELISA in the detection of B. bovis-specific antibodies and its suitability for standardization and large-scale production, as well as the possibility of its application in most veterinary diagnostic laboratories, make the assay a powerful tool for the surveillance of herd immunity as a strategic measure for the control of bovine babesiosis.
INTRODUCTION
The hemoprotozooan Babesia bovis severely limits cattle breeding in vast tropical and subtropical areas of the world, where its tick vectors, belonging to the family Ixodidae, are endemic (5, 25). Economic losses caused by the parasite are due to a decrease in meat and milk production, treatment of clinical cases, abortions, and death, as well as losses of potential production and cattle trade restrictions (5, 30, 36). B. bovis-infected bovines that survive the infection before 10 months of age acquire long-lasting protective immunity, while previously unexposed adult animals often succumb to the infection (28). Accordingly, the presence of B. bovis-specific circulating antibodies identifies previously exposed immune animals that will not develop clinical disease upon reinfection. The risk of a babesiosis outbreak can thus be assessed by analyzing the immunological status of a herd (18, 28). In Argentina, monitoring of calves for the presence of anti-B. bovis antibodies is periodically performed in regions of enzootic instability to decide the application of control measures, such as vaccination with live attenuated vaccines (2, 24, 25).
Merozoite surface antigen 2c (MSA-2c) is one of the five variable merozoite surface antigens (VMSAs) that are encoded in the same genomic region (17, 34). Antibodies recognizing recombinant forms of all VMSA members (MSA-1, MSA-2a1, MSA-2a2, MSA-2b, and MSA-2c) have been demonstrated in calves infected with a homologous Mexican strain of B. bovis (17, 34). MSA-2c is a species-specific, immunodominant antigen and the most conserved member of this family, showing very high amino acid sequence identity among B. bovis strains from Argentina, the United States, Mexico, and Australia (12, 19, 38). These features encouraged the use of MSA-2c for the development of serological tests, like an indirect enzyme-linked immunosorbent assay (ELISA) and a rapid immunochromatographic diagnostic test (6, 26). A competitive ELISA (cELISA) is an adequate serological tool for the epidemiological surveillance of the spread of bovine babesiosis, as it can be easily standardized, is less laborious and less time-consuming than the traditionally used indirect immunofluorescence assay (IFAT) (immunofluorescence antibody test), and, in addition, has the potential to display higher specificity than an indirect ELISA. In a previous work, a monoclonal antibody (MAb) against recombinant MSA-2c (rMSA-2c) was generated which showed competitive binding for this antigen with antisera of B. bovis-infected bovines in a cELISA format (13). In this work, the conditions and parameters of this rMSA-2c cELISA were optimized, and its cutoff, sensitivity, and specificity were established. In addition, using field samples, its performance was compared with that of the currently accepted gold standard, IFAT. The results demonstrate the applicability of rMSA-2c cELISA in the field for the diagnosis of cattle naturally infected with B. bovis in Argentina (22, 32).
MATERIALS AND METHODS
Production and purification of recombinant antigen and monoclonal antibody.
Recombinant expression of MSA-2c with an N-terminal histidine tag and subsequent purification by affinity chromatography in Ni-agarose was carried out as described previously (13, 38). Validation and quality assessment of expression were analyzed by Western blotting. To this end, a sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-PAGE) was run, protein transfer was carried out, and the resulting blot was probed using either an anti-histidine antibody (GE Healthcare, Chalfont, United Kingdom) or the MAb H9P2C2 (20 μg/ml) as the primary antibody (see below). Anti-mouse alkaline phosphatase-conjugated IgG (KPL, Gaithersburg, MD; 1/1,500) was used as the secondary antibody, and immunodetection was carried out using nitroblue tetrazolium/5-bromo-4-chloro-3-indolylphosphate (NBT/BCIP) (Promega, Fitchburg, WI) as the substrate. Quantity assessment of rMSA-2c expression was carried out by comparison of band sizes with known amounts of bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO) after SDS-PAGE and Coomassie blue staining.
The H9P2C2 hybridoma cell line producing the anti-rMSA-2c MAb H9P2C2 was cultured (13). Subsequently, the culture supernatant was collected, and the MAb was purified by affinity chromatography using the Affi-Gel Protein A MAPS II Kit (Bio-Rad, Hercules, CA). After protein quantification with a BCA colorimetric kit (Pierce, Rockford, IL), the MAb was aliquoted and stored at −20°C until it was used.
Serum samples.
Bovine blood samples were aseptically collected without anticoagulants from different geographical regions of Argentina as indicated below. Serum was separated by centrifugation, aliquoted, and stored at −20°C until it was used. For calculation of the cutoff value by receiver operator characteristic (ROC) analysis, a set of known-positive and known-negative sera was used. The known-positive sera (n = 104) originated from (i) animals from regions of endemicity in the provinces of Salta and Chaco that tested positive by diagnostic nested PCR, as reported by Figueroa et al. (15) (n = 27), and (ii) experimentally B. bovis-infected bovines after inoculation with either the BboR1A or the BboS2P strain (n = 77). In each case, establishment of infection was verified by observation of B. bovis-infected erythrocytes in Giemsa-stained smears. Known-negative sera (n = 253) originated from (i) animals from tick-free regions (n = 200), (ii) animals that had been experimentally infected with Babesia bigemina (n = 28) after confirmation of their hemoparasite-free status by IFAT and nested PCR (18), and (iii) animals from tick-free regions that were naturally infected with Anaplasma marginale (n = 25). An additional set of field serum samples (n = 303) was evaluated in a blind test by IFAT and rMSA-2c cELISA to estimate Cohen's kappa value. These samples were obtained from bovines from areas where ticks are enzootic in the provinces of Salta (n = 91) and Santiago del Estero (n = 120) and tick-free areas in the province of Santa Fe (n = 92).
IFAT.
Diagnostic IFAT was carried out essentially as described by Rios et al. (32) with minor modifications. Briefly, smears were prepared using a suspension of in vitro-cultured B. bovis-parasitized erythrocytes (8% infection) diluted 1:3 in phosphate-buffered saline (PBS)-1% BSA. Sera were tested in a 1/60 dilution in PBS, and a 1/2,500 fluorescein isothiocyanate (FITC)-labeled rabbit anti-bovine IgG (Sigma-Aldrich, St. Louis, MO) was used. Microscopic detection of positive reactions was carried out using a Leitz microscope (500×) equipped for epifluorescence with a 50-W mercury vapor lamp after mounting cover slides with glycerol-PBS, pH 7.5 (1:2 [vol/vol]). Positive and negative reference sera were included in each slide. Recognition of B. bovis merozoites of the BboS2P (Argentina) and RAD (Mexico) strains by MAb H9P2C2 (20 μg/ml) was carried out as previously described (12).
cELISA conditions.
The format of the cELISA as reported by Dominguez et al. (13) was reevaluated in order to further optimize the conditions and test parameters (data not shown). Based on this thorough assessment, only minor modifications of the serum dilution (1:5 instead of 1:20) and the composition of the blocking solution (0.5% gelatin-0.1% Tween 20-PBS instead of 0.5% gelatin-0.051% Tween 20-PBS) as indicated were found to slightly increase its performance. In brief, the rMSA-2c cELISA protocol was as follows. Immulon 2HB plates (Thermo Fisher Scientific, Waltham, MA) were incubated overnight with rMSA-2c (8.5 ng/well) in a final volume of 50 μl in 50 mM carbonate/bicarbonate buffer, pH 9.6, at 8°C. After three washings with PBS-0.1% Tween 20 (PBS-T), the plates were blocked with 200 μl of PBS-0.05% gelatin-0.1% Tween 20 (blocking buffer) for 1 h at 37°C with shaking. Then, the wells were sequentially incubated with (i) a 1/5 dilution of either control or test sera, (ii) MAb H9P2C2 (25 ng/well), and (iii) a 1/2,000 dilution of horseradish peroxidase-conjugated goat anti-mouse IgG (Kirkegaard & Perry Laboratories, Gaithersburg, MD). In all cases, dilutions were made in blocking buffer; incubations were carried out in a final volume of 50 μl for 1 h at 37°C with shaking and were followed by extensive washing with PBS-T. Finally, 50 μl of the orthophenylenediamine/hydroperoxidase (OPD/H2O2) colorimetric substrate were added, which contains 0.4 mg/ml OPD (Sigma-Aldrich St. Louis, MO) in citrate-phosphate buffer (0.035 M citric acid–0.067 M disodic-phosphate, pH 5.0) and 0.024% (vol/vol) H2O2. After 10 min at room temperature, the reactions were stopped by the addition of 50 μl sulfuric acid (2 N H2SO4) in water. Absorbance at 490 nm (A490) was recorded in an ELISA plate reader (Multiskan EX; Thermo Fisher Scientific, Waltham, MA). All samples were tested in triplicate. Two known-positive and two known-negative control sera were included in each plate as reference controls. rMSA-2c cELISA results were expressed as inhibition percentages (PI) of the negative control and calculated with the following formula: PI = 100 − [(average A490 of test serum × 100)/average A490 of negative-control sera].
Calculations and statistical analysis.
A frequency distribution graph was plotted using the MedCalc 8.1.0.0 program, and the optimal cutoff was established by ROC analysis. This allowed an accurate estimation of the diagnostic specificity and sensitivity of the established cELISA. Concordance between IFAT and cELISA results was estimated by Cohen's kappa value using the same program (1, 16). The sensitivity (ss) and specificity (sp) of rMSA-2c cELISA applied to field samples, considering IFAT the gold standard, were calculated according to the method of Martin et al. (29) from a two-entry table of cELISA and IFAT positive and negative results, using the following formula: ss = (a/[a + c]) × 100 and sp = (d/[b + d]) × 100, where a and d are the number of positive and negative results, respectively, by both methods; c is the number of cELISA-positive and IFAT-negative results; and b is the number of cELISA-negative and IFAT-positive results.
RESULTS AND DISCUSSION
The availability of suitable, reliable, and specific diagnostic tools is imperative for efficient epidemiological surveillance of B. bovis infection and the rational use of control measures. Serological assays are currently most likely to meet these requirements, as B. bovis infection finally leads to an asymptomatic carrier animal state in which the parasite commonly escapes direct detection by PCR, reverse line blot hybridization (RLB), or Giemsa-stained blood smears (10, 23). A number of serological methods have been established for the detection of B. bovis-specific antibodies, of which ELISA is considered the most advantageous for epidemiological investigations, as it offers greater sensitivity and objectivity and can be easily adapted to test large numbers of serum samples (3, 4, 6, 7, 8, 11, 20, 21, 27, 31, 35, 37). Furthermore, this format is amenable to the use of recombinant antigens, which makes it independent of the necessity to produce large amounts of parasites in vivo or in vitro, facilitates standardization, and has the potential to overcome limitations caused by cross-reactivity (22, 32). The use of rMSA-2c as a diagnostic antigen in an indirect-ELISA format has been reported, and it has proved useful in the study of the development of antibody titers after experimental infection of bovines (6). However, the indirect-ELISA formats based on rMSA-2c suffer from low specificity and sensitivity and have not been recommended for field studies (6, 35).
A cELISA format based on an epitope located in the C-terminal region of the B. bovis rhoptry-associated protein 1 (CT-RAP-1) has been shown to have high specificity and sensitivity (20, 21). However, the first version of this test has been applied to only 130 field serum samples from Morocco, Bolivia, and Puerto Rico, while its validity for Argentina or any other geographic region with large cattle herds has not been assessed (20). In its second version, this cELISA features a different cutoff than the first version (21% versus 40%) and was exclusively applied to a fixed set of known-positive and -negative samples in different laboratories in order to validate the reproducibility of its results (21). It is noteworthy that the rMSA-2c and CT-RAP-1 antigens have both recently been directly compared in an indirect-ELISA format, and it was demonstrated that rMSA-2c clearly outperformed CT-RAP-1 with respect to specificity, sensitivity, and concordance with IFAT (35). This strongly suggests that a cELISA based on an MSA-2c epitope may have the potential to result in further improved test parameters.
In the present work, we evaluated the cELISA format using an MSA-2c epitope. The rMSA-2c cELISA format was applied to 357 bovine sera composed of a panel of known-positive (n = 104) and known-negative (n = 253) samples, as described in Materials and Methods, and PI values were calculated. By ROC analysis, the optimal cutoff was determined to be a PI of 29.5 (Fig. 1A). A scatter plot of the frequency distribution of the known-positive and -negative sera is shown in Fig. 1B. The area under the curve (AUC) was assessed to be 0.994, which corresponds to an excellent ability of the assay to discriminate truly infected from truly uninfected animals. At the established cutoff, the cELISA yields a salient specificity and sensitivity of 98% and 96.2%, respectively, resulting in only 5 false-positive and 4 false-negative test results. Importantly, all sera that originated from B. bigemina-infected (n = 28) or A. marginale-infected (n = 25) animals scored below the established cutoff value, and thus, the rMSA-2c cELISA did not show any cross-reactivity with antibodies against B. bigemina and A. marginale, the main B. bovis-coinfecting hemoparasites of cattle in Argentina.
Fig 1.
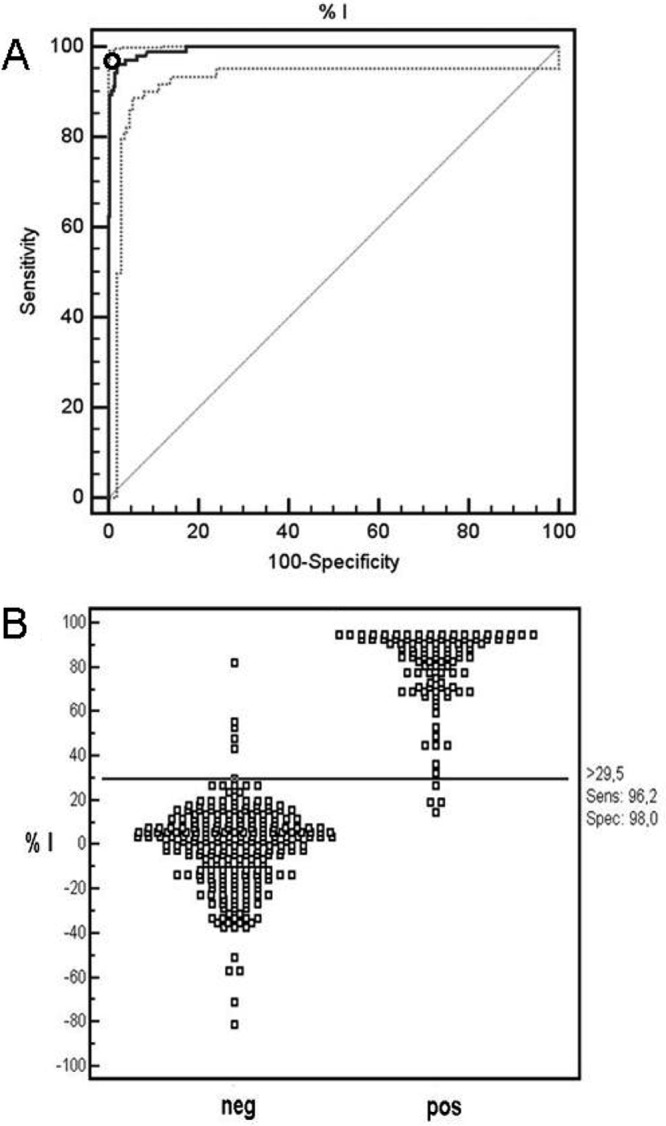
ROC analysis of rMSA2c cELISA. (A) Optimal cutoff point (o) providing the highest sensitivity and specificity. Solid lines indicate the fitted ROC curve (thick) and line of no discrimination (thin), and dotted lines indicate the 95% confidence interval of the fitted ROC curve. (B) Frequency distribution based on 253 known-negative (neg) and 104 known-positive (pos) B. bovis-infected bovine serum samples.
As 100 of the 104 known-positive sera reacted positively, the rMSA-2c cELISA results provide evidence that the target epitope is strongly conserved and immunodominant. This coincides with previously reported findings of the immunodominance and high sequence conservation of MSA-2c (>90%) (12, 19, 38). The expression of the epitope recognized by MAb H9C2P2 has been demonstrated by immunofluorescence assay of merozoites of the BoS2P (Argentine) and RAD (Mexican) strains, yielding a homogeneously stained parasite surface (data not shown). The presence of an immunodominant B-cell epitope in surface-exposed antigens, such as MSA-2c, agrees well with the “smoke screen” theory, in which elicitation of a humoral response to this kind of epitope may distract the host immune system, allowing other parasite epitopes to remain unnoticed and available to play important functional roles (33). Alternatively, the possibility that the target epitope of MAb H9C2P2 may be involved in erythrocyte invasion of the parasite, as has been reported for MSA-2c (38), cannot be excluded.
The predictive positive and negative values of the rMSA-2c cELISA at a supposed prevalence of 10% were estimated as 84% and 100%, respectively. In turn, a prevalence of 90% resulted in positive and negative predictive values of 100% and 74%, respectively. These results show that the rMSA-2c cELISA is a suitable tool in various epidemiological scenarios. The rMSA-2c cELISA identified specific antibodies in bovines experimentally infected with B. bovis for up to 3 (n = 10) and even 5 (n = 16) months, demonstrating that circulating epitope-specific antibodies are detected for a prolonged period. This result corresponds well to the detection of anti-MSA-2c antibodies up to at least month 4 postinfection (p.i.), as has been reported for an indirect-ELISA format based on the antigen (6).
In a blind trial, the concordance of cELISA with IFAT was thoroughly assessed using a panel of an additional set of 303 bovine sera, collected in the field from areas of endemicity and nonendemicity. Cross-tabulation of cELISA and IFAT results (Table 1) demonstrated a high level of agreement between the two tests, resulting in a kappa value of 0.833 (0.80 < κ ≤ 1.00; very good agreement) (1). A total of 278 of 303 sera (91.7%) tested either positive (n = 159) or negative (n = 119) by both methods, while 25 samples showed discrepant test results. In five of these samples (1.6%), the cELISA result was positive while that of IFAT was negative, and for 20 samples (6.7%), the cELISA result was negative while that of IFAT was positive. Using the formulas described by Martin et al. (29), the specificity and sensitivity of the rMSA-2c cELISA when applied to field samples were estimated at 96% and 89%, respectively, compared with IFAT (Table 1). Thus, the specificity was very similar to that based on known-infected and -uninfected sera (96% versus 98.5%). On the other hand, the sensitivity value obtained (89% versus 96.2%) was considerably lower when using samples of unknown serological status, which is consistent with the notion that IFAT produces a number of false-positive results.
Table 1.
Serological results of a blind test of IFAT and rMSA2c-cELISA on bovine field samples from Argentinaa
| IFAT result | No. with cELISA result: |
Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 159 | 20 | 179 |
| Negative | 5 | 119 | 124 |
| Total | 164 | 139 | 303 |
A total of 303 samples, 211 of which originated from areas where B. bovis is endemic and 92 from areas where it is not endemic.
Competitive ELISAs potentially have interesting advantages over indirect ELISAs. Since they are based on competition for a single B-cell epitope present in the antigen between host antibodies and a custom-designed monoclonal antibody, opposite to what happens in an indirect ELISA, the presence of E. coli antigens in the protein preparation does not lead to a decrease in assay specificity due to cross-reactivity reactions. In addition, since the target for detection is the MAb and not the antibodies present in the test serum samples, cELISAs can be directly applied to different host species. Accordingly, a preliminary version of the rMSA-2c cELISA presented proved useful for the detection of anti-B. bovis antibodies in water buffaloes, a type of cattle with increasing popularity in tick-infested regions of Argentina and Brazil (9, 14).
In summary, our results validate the developed rMSA-2c cELISA as a useful method for the detection of anti-B. bovis antibodies in Argentine cattle. This assay can contribute to future efforts to improve control of bovine babesiosis in the country. Studies to evaluate its applicability in other geographic regions are under way.
ACKNOWLEDGMENTS
This work was financed by grants from the Argentine Ministry of Science and Technology (PICT 2002-00054), the European Commission (MEDLABAB, INCO 003691; INTA, AESA-203961), and TCP INTA-Asoc. Coop. EEA Rafaela 426100. Salaries were provided by INTA (M.D., I.E., S.T.D.E., and O.Z.), CONICET (S.W., L.S., and M.F.-C.), and Universidad de Queretaro (J.J.M.).
Footnotes
Published ahead of print 4 April 2012
REFERENCES
- 1. Altman DG. 1991. Practical statistics for medical research, p 404 Chapman and Hall, London, England [Google Scholar]
- 2. Anziani OS, Guglielmone AA, Abdala AA, Aguirre DH, Mangold AJ. 1993. Proteccion conferida por Babesia bovis vacunal en novillos Holando Argentino. Rev. Med. Vet. 74: 47–49 [Google Scholar]
- 3. Araujo FR, et al. 1998. Comparison between enzyme-linked immunosorbent assay, indirect fluorescent antibody and rapid conglutination test in detecting antibodies against Babesia bovis. Vet. Parasitol. 74: 101–108 [DOI] [PubMed] [Google Scholar]
- 4. Barry DN, Rodweil BJ, Timms P, McGregor W. 1982. A microplate enzyme immunoassay for detectiong and measuring antibodies to Babesia bovis in cattle serum. Aust. Vet. J. 59: 136–140 [DOI] [PubMed] [Google Scholar]
- 5. Bock R, Jackson L, de Vos A, Jorgensen W. 2004. Babesiosis of cattle. Parasitology 129(Suppl): S247–S269 [DOI] [PubMed] [Google Scholar]
- 6. Bono MF, et al. 2008. Efficiency of a recombinant MSA-2c-based ELISA to establish the persistence of antibodies in cattle vaccinated with Babesia bovis. Vet. Parasitol. 157: 203–210 [DOI] [PubMed] [Google Scholar]
- 7. Böse R, Jacobson RH, Gale KR, Waltisbuhl DJ, Wright IG. 1990. An improved ELISA for the detection of antibodies against Babesia bovis using either a native or a recombinant B. bovis antigen. Parasitol. Res. 76: 648–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Böse R, Jorgensen RJ, Dalgliesh RJ, Friedhoff KT, de Vos AJ. 1995. Current state and future trends in diagnosis of babesiosis. Vet. Parasitol. 57: 61–74 [DOI] [PubMed] [Google Scholar]
- 9. Cadoppi A. 2010. Development for the demand for high quality water buffalo meat and elaborated meat products in Argentina and Germany. Rev. Vet. 21: 268 [Google Scholar]
- 10. Calder JAM, et al. 1996. Monitoring Babesia bovis infections in cattle by using PCR-based tests. J. Clin. Microbiol. 34: 2748–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Echaide ST, et al. 1995. Evaluation of an enzyme-linked immunosorbent assay kit to detect Babesia bovis antibodies in cattle. Prev. Vet. Med. 24: 277–283 [Google Scholar]
- 12. Dominguez M, et al. 2010. In silico predicted conserved B-cell epitopes in the Merozoite Surface Antigen-2 family of B. bovis are neutralization sensitive. Vet. Parasitol. 167: 216–226 [DOI] [PubMed] [Google Scholar]
- 13. Dominguez M, et al. 2004. Use of a monoclonal antibody against Babesia bovis Merozoite Surface Antigen-2c for the development of a competitive ELISA test. Ann. N. Y. Acad. Sci. 1026: 165–170 [DOI] [PubMed] [Google Scholar]
- 14. Ferreri L, et al. 2008. Water buffalos as carriers of Babesia bovis in Argentina. Ann. N. Y. Acad. Sci. 1149: 149–151 [DOI] [PubMed] [Google Scholar]
- 15. Figueroa JV, Chieves LP, Johnson GS, Buening GM. 1993. Multiplex polymerase chain reaction based assay for the detection of Babesia bigemina, Babesia bovis and Anaplasma marginale DNA in bovine blood. Vet. Parasitol. 50: 69–81 [DOI] [PubMed] [Google Scholar]
- 16. Fleiss JJ. 1981. Statistical methods for rates and proportions, 2nd ed Wiley, New York, NY [Google Scholar]
- 17. Florin-Christensen M, et al. 2002. The Babesia bovis merozoite surface antigen 2 locus contains four tandemly arranged and expressed genes encoding immunologically distinct proteins. Infect. Immun. 70: 3566–3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Food and Agriculture Organization of the United Nations 1984. Ticks and tick borne disease control: a practical field manual, vol 2. Tick borne disease control programme, p 300–321 Food and Agriculture Organization of the United Nations, Rome, Italy [Google Scholar]
- 19. Genis AD, et al. 2008. Phylogenetic analysis of Mexican Babesia bovis isolates using msa and ssrRNA gene sequences. Ann. N. Y. Acad. Sci. 1149: 121–125 [DOI] [PubMed] [Google Scholar]
- 20. Goff WL, et al. 2003. Competitive enzyme-linked immunosorbent assay based on a rhoptry-associated protein 1 epitope specifically identifies Babesia bovis-infected cattle. Clin. Diagn. Lab. Immunol. 10: 38–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goff WL, et al. 2008. Validation of a competitive enzyme-linked immunosorbent assay for detection of Babesia bigemina antibodies in cattle. Clin. Vaccine Immunol. 15: 1316–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goff WL, Wagner GG, Craig TM, Long RF. 1982. The bovine immune response to tick-derived Babesia bovis infection: serological studies of isolated immunoglobulins. Vet. Parasitol. 11: 109–120 [DOI] [PubMed] [Google Scholar]
- 23. Gubbels JM, et al. 1999. Simultaneous detection of bovine Theileria and Babesia species by reverse line blot hybridization. J. Clin. Microbiol. 37: 1782–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guglielmone AA. 1994. Epidemiología y control de los hemoparásitos (Babesia y Anaplasma) en la Argentina, p 461–479 In Nari AY, Fiel C. (ed), Enfermedades parasitarias de importancia económica en bovinos. Bases epidemiológicas para su prevención y control en Argentina y Uruguay. Hemisferio Sur, Montevideo, Uruguay [Google Scholar]
- 25. Guglielmone AA. 1995. Epidemiology of babesiosis and anaplasmosis in South and Central America. Vet. Parasitol. 57: 109–119 [DOI] [PubMed] [Google Scholar]
- 26. Kim C, et al. 2007. Development of two immunochromatographic tests for the serodiagnosis of bovine babesiosis. Vet. Parasitol. 148: 137–143 [DOI] [PubMed] [Google Scholar]
- 27. Machado RZ, et al. 1997. An enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against Babesia bovis in cattle. Vet. Parasitol. 71: 17–26 [DOI] [PubMed] [Google Scholar]
- 28. Mahoney DF, Ross DR. 1972. Epizootiological factors in the control of bovine babesiosis. Aust. Vet. J. 48: 292–298 [DOI] [PubMed] [Google Scholar]
- 29. Martin WS, Meek AH, Willerbert P. 1987. Measurement of disease frequency and production, p 48–76 In Martin WS, Meek AH, Willerberg P. (ed), Veterinary epidemiology: principles and methods. Iowa State Press, Iowa City, IA [Google Scholar]
- 30. McCosker PJ. 1981. The global importance of babesiosis, p 1–24 In Ristic M, Kreier JP. (ed), Babesiosis. Academic Press, New York, NY [Google Scholar]
- 31. Molloy JB, et al. 1998. Evaluation of an ELISA for detection of antibodies to Babesia bovis in cattle in Australia and Zimbabwe. Prev. Vet. Med. 33: 59–67 [DOI] [PubMed] [Google Scholar]
- 32. Rios LG, Aguirre DH, Gaido AB. 1988. Evaluación de la dinámica de la infección por Babesia bovis y Babesia bigemina en terneros, diagnostico por microscopia directa y prueba de inmunofluorescencia indirecta. Rev. Med. Vet. (Buenos Aires) 69: 255–260 [Google Scholar]
- 33. Sher A. 1988. Strategies for vaccination against parasites, p 169–172 In England PT, Sher A. (ed), The biology of parasitism. Alan R. Liss Inc., New York, NY [Google Scholar]
- 34. Suarez CE, et al. 2000. Characterization of allelic variation in the Babesia bovis merozoite surface antigen 1 (MSA-1) locus and identification of a cross-reactive inhibition-sensitive MSA-1 epitope. Infect. Immun. 68: 6865–6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Terkawi MA, et al. 2011. Spherical body protein 4 is a new serological antigen for global detection of Babesia bovis infection in cattle. Clin. Vaccine Immunol. 18: 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Uilenberg G. 1995. International collaborative research: significance of tick-borne hemoparasitic diseases to world animal health. Vet. Parasitol. 57: 19–41 [DOI] [PubMed] [Google Scholar]
- 37. Waltisbuhl DJ, Goodger BV, Wright IG, Commins MA, Mahoney DF. 1987. An enzyme linked immunosorbent assay to diagnose Babesia bovis infection in cattle. Parasitol. Res. 73: 126–131 [DOI] [PubMed] [Google Scholar]
- 38. Wilkowsky SE, et al. 2003. Babesia bovis merozoite surface protein-2c (MSA-2c) contains highly immunogenic, conserved B-cell epitopes that elicit neutralization-sensitive antibodies in cattle. Mol. Biochem. Parasitol. 127: 133–141 [DOI] [PubMed] [Google Scholar]


