Abstract
Female rhesus macaques were immunized with HIV virus-like particles (HIV-VLPs) or HIV DNA administered as sequential combinations of mucosal (intranasal) and systemic (intramuscular) routes, according to homologous or heterologous prime-boost schedules. The results show that in rhesus macaques only the sequential intranasal and intramuscular administration of HIV-VLPs, and not the intranasal alone, is able to elicit humoral immune response at the systemic as well as the vaginal level.
TEXT
The vast majority of new HIV infections worldwide are acquired via the genital mucosa, and women account for close to 50% of them (39). The development of vaccination strategies able to elicit protective systemic and mucosal immune response represents a major goal in the HIV vaccine field, possibly providing a crucial method for halting the spread of HIV/AIDS.
Mucosal secretory immunoglobulin A (sIgA) specific for HIV-1 envelope glycoproteins is consistently detected in seropositive subjects (1, 14) and has been strongly associated with protection from HIV-1 infection in uninfected individuals having unprotected sexual intercourse with HIV-1-seropositive partners (13, 23, 25, 28). Furthermore, intravenous or intravaginal passive administration of the gp120-specific human neutralizing monoclonal antibody (Ab) b12 has been shown to be highly effective in protecting monkeys from a vaginal challenge (12, 30, 40).
Considering this epidemiological and experimental evidence, specific mucosal immunity is extremely relevant for controlling the primary HIV-1 infection. Intranasal (i.n.) immunization has been shown to be effective for protection against infectious respiratory diseases such as influenza (2, 22, 33, 35, 38, 43). However, the effectiveness of mucosal immunization often relies upon coadministration of appropriate adjuvants that can initiate and support the transition from innate to adaptive immunity (recently reviewed in reference 20). In addition to adjuvants, particulate antigens (e.g., virus-like particles [VLPs]) have been shown to be advantageous for intranasal immunization, given that they efficiently target antigen-presenting cells (APCs) and facilitate the induction of potent immune responses (7, 9, 10, 11, 16, 18, 31, 34, 41, 43, 44). However, several vaccine concepts have been evaluated in nonhuman primates (NHPs) by intranasal administration with inconsistent immunogenicity results, probably related to the different vaccination strategy (3, 17, 24, 26, 27, 29).
HIV-VLPs developed in our laboratory, and used in the present study, are based on HIV Gag protein and express the whole HIV gp120/140 envelope protein derived from an Ugandan clade A field isolate (4, 5, 6, 36, 37, 42). Elicitation of immune response at systemic as well as mucosal (vaginal and intestinal) levels has been previously evaluated in mice by intraperitoneal as well as intranasal administration (7, 8, 11). In particular, the mucosal immunogenicity of such HIV-VLPs has been evaluated by comparing a homologous (VLP + VLP) and a heterologous (DNA + VLP) prime-boost strategy by intranasal administration, in an adjuvant formulation (7).
In the present study, the immunogenicity of HIV-VLPs was evaluated in rhesus macaques immunized with HIV-VLPs administered via a sequential combination of mucosal (intranasal) and systemic (intramuscular [i.m.]) routes, according to homologous (VLP prime + VLP boost) or heterologous (DNA prime + VLP boost) prime-boost schedules.
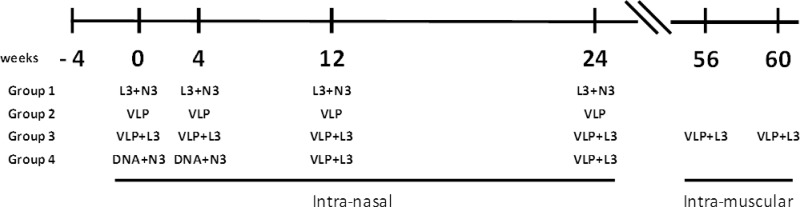
A total of 24 female rhesus macaques were equally divided into four experimental arms and immunized by the intranasal route as described in Fig. 1. Groups 2 and 3 were immunized using the homologous prime-boost protocol in the absence (group 2) or in the presence (group 3) of the Eurocine L3 nasal lipid adjuvant. Group 4 was immunized using the heterologous prime-boost protocol in the presence of Eurocine L3 and N3 adjuvants. Additionally, group 3 received two further boosting doses of VLPs by the intramuscular (i.m.) route, 22 weeks after the last intranasal (i.n.) administration. Group 1 was the control group administered adjuvants. VLPs were administered at 100 μg per immunization dose; DNA plasmids were administered at 200 μg per immunization dose. Antigens as well as adjuvants used in the study have all been previously described (5, 7, 8, 11, 15, 19, 21, 32).
Fig 1.
Immunization scheme in NHPs. Six animals per group were immunized as described at indicated weeks.
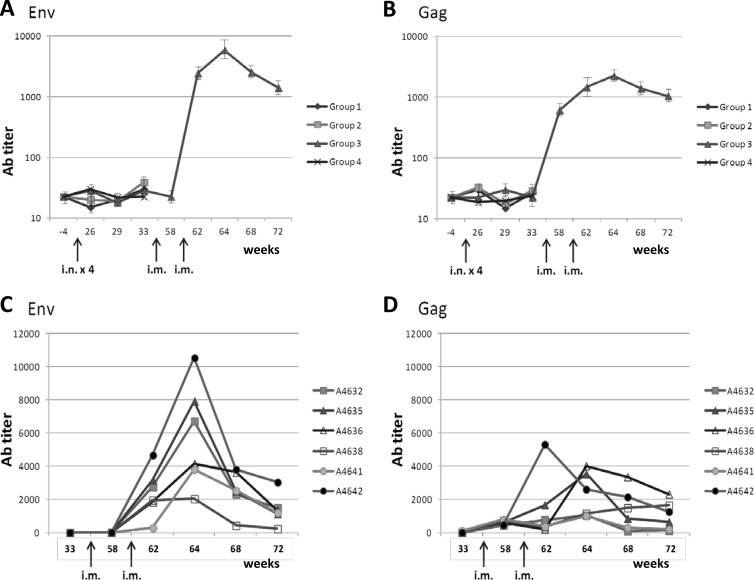
Sera were collected from 10 ml of whole blood 1 week before and 1 week after each antigen administration, and enzyme-linked immunosorbent assays (ELISAs) were performed in microwell plates coated with recombinant HIV gp120 or p24 of subtype B. The data show that intranasal administration of HIV-VLPs, in either the homologous or heterologous prime-boost protocol, does not elicit measurable serum anti-Env or anti-Gag Ab titers (Fig. 2A). Moreover, the i.n. administration protocol does not appear to efficiently prime the systemic immune system, since two subsequent i.m. injections were needed to observe significant serum Ab titers (>1:1,000) (Fig. 2B). In particular, evaluating the individual animals in such group, it is possible to identify the best responders for both Env and Gag (no. 4642 > 4635 > 4636) (Fig. 2C and D).
Fig 2.
Systemic immune response. Specific anti-env and anti-gag immune responses in serum of immunized animals were evaluated by ELISA. (A and B) Average Ab titer in each group. (C and D) Ab titer for each animal in group 3 after the two i.m. immunizations. Results are expressed as the reciprocal last dilution with a 3-fold optical density at 492 nm of the preimmune sera.
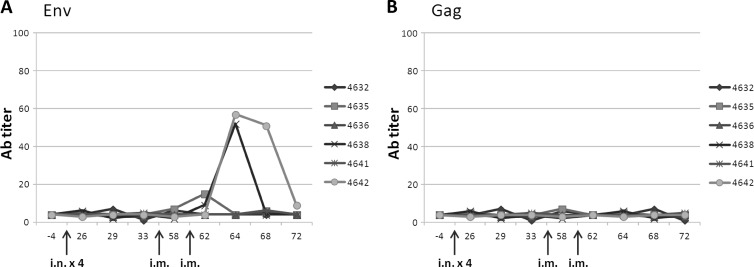
Vaginal washes were collected on the same days as the serum, and ELISAs were run in parallel. The data show that intranasal administration of HIV-VLPs, in either a homologous or a heterologous prime-boost protocol, does not elicit measurable mucosal titers (data not shown); however, it seems to prime the mucosal immune system which, 6 months after the last i.n. boost, is able to respond after the i.m. immunizations. The effect is evident in 2/6 animals in group 3 and appears to be selective for Env (Fig. 3). Furthermore, antibody titers elicited by the two i.m. administrations of VLPs do not show HIV neutralization or antibody-dependent cell-mediated cytotoxicity (ADCC) activity (data not shown).
Fig 3.
Vaginal immune response. Specific anti-env and anti-gag immune responses in vaginal washes of immunized animals were evaluated by ELISA. The Ab titer for each animal in group 3 is shown. Results are expressed as the reciprocal last dilution with a 3-fold optical density at 492 nm of the preimmune sera.
The results obtained in NHPs in the present study by i.n. administration of VLPs are in contrast to those obtained in mice (7); however, this could be due to either the administered dose (i.e., too low in NHPs) or lower permeability to antigens of the nasal epithelium in macaques. Indeed, our data are in agreement with results from others who have previously shown the limited or absent immune response in NHP by i.n. administration. Such results have been obtained using different vaccine delivery systems, which suggests that they are not vaccine related (3, 24, 26, 27).
In conclusion, the described NHP preclinical trial shows the elicitation of specific immune response by HIV-VLPs when administered by the i.m. route. On the other hand, at least in our experimental model, the i.n. administration is possibly only priming the humoral mucosal immunity for subsequent i.m. boosting doses in a few animals. However, such an observation needs further investigation and must be taken into consideration for future preclinical vaccine evaluations.
ACKNOWLEDGMENTS
The preclinical study in NHPs was fully supported by the Simian Vaccine Evaluation Unit (SVEU) of the Division of AIDS. M.T. and M.L.V. are supported by the European Community's Seventh Framework Programme NGIN (FP7/2007-2013) under grant agreement no. 201433.
We thank Nancy Miller and Yen Li for their continuous and invaluable support.
Footnotes
Published ahead of print 29 March 2012
REFERENCES
- 1. Alfsen A, Iniguez P, Bouguyon E, Bomsel M. 2001. Secretory IgA specific for a conserved epitope on gp41 envelope glycoprotein inhibits epithelial transcytosis of HIV-1. J. Immunol. 166: 6257– 6265 [DOI] [PubMed] [Google Scholar]
- 2. Asahi-Ozaki Y, et al. 2006. Intranasal administration of adjuvant-combined recombinant influenza virus HA vaccine protects mice from the lethal H5N1 virus infection. Microbes Infect. 8: 2706– 2714 [DOI] [PubMed] [Google Scholar]
- 3. Barnett SW, et al. 2008. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. AIDS 22: 339– 348 [DOI] [PubMed] [Google Scholar]
- 4. Buonaguro L, et al. 1998. A novel gp120 sequence from an HIV-1 isolate of the A clade identified in North Uganda. AIDS Res. Hum. Retroviruses 14: 1287– 1289 [DOI] [PubMed] [Google Scholar]
- 5. Buonaguro L, et al. 2001. High efficient production of Pr55gag virus-like particles expressing multiple HIV-1 epitopes, including a gp120 protein derived from an Ugandan HIV-1 isolate of subtype A. Antiviral Res. 49: 35– 47 [DOI] [PubMed] [Google Scholar]
- 6. Buonaguro L, et al. 1995. Heteroduplex mobility assay and phylogenetic analysis of V3 region sequences of human immunodeficiency virus type 1 isolates from Gulu, Northern Uganda. J. Virol. 69: 7971– 7981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buonaguro L, et al. 2007. DNA-VLP prime-boost intra-nasal immunization induces cellular and humoral anti-HIV-1 systemic and mucosal immunity with cross-clade neutralizing activity. Vaccine 25: 5968– 5977 [DOI] [PubMed] [Google Scholar]
- 8. Buonaguro L, et al. 2002. Induction of neutralizing antibodies and CTLs in Balb/c mice immunized with virus-like particles presenting a gp120 molecule from a HIV-1 isolate of clade A (HIV-VLPAs). Antiviral Res. 54: 189– 201 [DOI] [PubMed] [Google Scholar]
- 9. Buonaguro L, Tagliamonte M, Tornesello ML, Buonaguro FM. 2011. Developments in virus-like particle-based vaccines for infectious diseases and cancer. Expert Rev. Vaccines 10: 1569– 1583 [DOI] [PubMed] [Google Scholar]
- 10. Buonaguro L, Tornesello ML, Buonaguro FM. 2010. Virus-like particles as particulate vaccines. Curr. HIV Res. 8: 299– 399 [DOI] [PubMed] [Google Scholar]
- 11. Buonaguro L, et al. 2005. Induction of systemic and mucosal cross-clade neutralizing antibodies in BALB/c mice immunized with human immunodeficiency virus type 1 clade A virus-like particles administered by different routes of inoculation. J. Virol. 79: 7059– 7067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burton DR, et al. 2011. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc. Natl. Acad. Sci. U. S. A. 108: 11181– 11186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Devito C, et al. 2002. Cross-clade HIV-1-specific neutralizing IgA in mucosal and systemic compartments of HIV-1-exposed, persistently seronegative subjects. J. Acquir. Immune. Defic. Syndr. 30: 413– 420 [DOI] [PubMed] [Google Scholar]
- 14. Devito C, et al. 2000. Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS 14: 1917– 1920 [DOI] [PubMed] [Google Scholar]
- 15. Devito C, et al. 2004. Intranasal HIV-1-gp160-DNA/gp41 peptide prime-boost immunization regimen in mice results in long-term HIV-1 neutralizing humoral mucosal and systemic immunity. J. Immunol. 173: 7078– 7089 [DOI] [PubMed] [Google Scholar]
- 16. El-Kamary SS, et al. 2010. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J. Infect. Dis. 202: 1649– 1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Enose Y, et al. 2002. Protection by intranasal immunization of a nef-deleted, nonpathogenic SHIV against intravaginal challenge with a heterologous pathogenic SHIV. Virology 298: 306– 316 [DOI] [PubMed] [Google Scholar]
- 18. Guerrero RA, et al. 2001. Recombinant Norwalk virus-like particles administered intranasally to mice induce systemic and mucosa (fecal and vaginal) immune responses. J. Virol. 75: 9713– 9722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haile M, et al. 2004. Immunization with heat-killed Mycobacterium bovis bacille Calmette-Guerin (BCG) in Eurocine L3 adjuvant protects against tuberculosis. Vaccine 22: 1498– 1508 [DOI] [PubMed] [Google Scholar]
- 20. Harandi AM, Medaglini D. 2010. Mucosal adjuvants. Curr. HIV Res. 8: 330– 335 [DOI] [PubMed] [Google Scholar]
- 21. Hinkula J, et al. 2006. A novel DNA adjuvant, N3, enhances mucosal and systemic immune responses induced by HIV-1 DNA and peptide immunizations. Vaccine 24: 4494– 4497 [DOI] [PubMed] [Google Scholar]
- 22. Ichinohe T, et al. 2010. Intranasal administration of adjuvant-combined vaccine protects monkeys from challenge with the highly pathogenic influenza A H5N1 virus. J. Med. Virol. 82: 1754– 1761 [DOI] [PubMed] [Google Scholar]
- 23. Kaul R, et al. 1999. HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS 13: 23– 29 [DOI] [PubMed] [Google Scholar]
- 24. Koopman G, et al. 2007. Comparison of intranasal with targeted lymph node immunization using PR8-Flu ISCOM adjuvanted HIV antigens in macaques. J. Med. Virol. 79: 474– 482 [DOI] [PubMed] [Google Scholar]
- 25. Lo Caputo S, et al. 2003. Mucosal and systemic HIV-1-specific immunity in HIV-1-exposed but uninfected heterosexual men. AIDS 17: 531– 539 [DOI] [PubMed] [Google Scholar]
- 26. Manrique M, et al. 2011. Long-term control of simian immunodeficiency virus mac251 viremia to undetectable levels in half of infected female rhesus macaques nasally vaccinated with simian immunodeficiency virus DNA/recombinant modified vaccinia virus Ankara. J. Immunol. 186: 3581– 3593 [DOI] [PubMed] [Google Scholar]
- 27. Manrique M, et al. 2009. Nasal DNA-MVA SIV vaccination provides more significant protection from progression to AIDS than a similar intramuscular vaccination. Mucosal Immunol. 2: 536– 550 [DOI] [PubMed] [Google Scholar]
- 28. Mazzoli S, et al. 1997. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat. Med. 3: 1250– 1257 [DOI] [PubMed] [Google Scholar]
- 29. Miyake A, et al. 2004. Induction of HIV-specific antibody response and protection against vaginal SHIV transmission by intranasal immunization with inactivated SHIV-capturing nanospheres in macaques. J. Med. Virol. 73: 368– 377 [DOI] [PubMed] [Google Scholar]
- 30. Parren PW, et al. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75: 8340– 8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perrone LA, et al. 2009. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J. Virol. 83: 5726– 5734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petersson P, et al. 2010. The Eurocine L3 adjuvants with subunit influenza antigens induce protective immunity in mice after intranasal vaccination. Vaccine 28: 6491– 6497 [DOI] [PubMed] [Google Scholar]
- 33. Prabakaran M, et al. 2008. Protective immunity against influenza H5N1 virus challenge in mice by intranasal co-administration of baculovirus surface-displayed HA and recombinant CTB as an adjuvant. Virology 380: 412– 420 [DOI] [PubMed] [Google Scholar]
- 34. Sedlik C, et al. 1999. Intranasal delivery of recombinant parvovirus-like particles elicits cytotoxic T-cell and neutralizing antibody responses. J. Virol. 73: 2739– 2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stephenson I, et al. 2006. Phase I evaluation of intranasal trivalent inactivated influenza vaccine with nontoxigenic Escherichia coli enterotoxin and novel biovector as mucosal adjuvants, using adult volunteers. J. Virol. 80: 4962– 4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tagliamonte M, et al. 2010. Constitutive expression of HIV-VLPs in stably transfected insect cell line for efficient delivery system. Vaccine 28: 6417– 6424 [DOI] [PubMed] [Google Scholar]
- 37. Tagliamonte M, et al. 2011. HIV-Gag VLPs presenting trimeric HIV-1 gp140 spikes constitutively expressed in stable double transfected insect cell line. Vaccine 29: 4913– 4922 [DOI] [PubMed] [Google Scholar]
- 38. Tumpey TM, Renshaw M, Clements JD, Katz JM. 2001. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J. Virol. 75: 5141– 5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. UNAIDS 2010. UNAIDS report on the global AIDS epidemic 2010. UNAIDS, Geneva, Switzerland: http://www.unaids.org/globalreport/Global_report.htm [Google Scholar]
- 40. Veazey RS, et al. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9: 343– 346 [DOI] [PubMed] [Google Scholar]
- 41. Velasquez LS, et al. 2011. Intranasal delivery of Norwalk virus-like particles formulated in an in situ gelling, dry powder vaccine. Vaccine 29: 5221– 5231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Visciano ML, et al. 2011. Generation of HIV-1 virus-like particles expressing different HIV-1 glycoproteins. Vaccine 29: 4903– 4912 [DOI] [PubMed] [Google Scholar]
- 43. Wang BZ, et al. 2010. Intranasal immunization with influenza VLPs incorporating membrane-anchored flagellin induces strong heterosubtypic protection. PLoS One 5: e13972 doi:10.1371/journal.pone.0013972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yao Q, Vuong V, Li M, Compans RW. 2002. Intranasal immunization with SIV virus-like particles (VLPs) elicits systemic and mucosal immunity. Vaccine 20: 2537– 2545 [DOI] [PubMed] [Google Scholar]