Abstract
This study characterized the efficacy of the Brucella abortus strain RB51 vaccine in bison when delivered by single intramuscular vaccination (hand RB51), by single pneumatic dart delivery (dart RB51), or as two vaccinations approximately 13 months apart (booster RB51) in comparison to control bison. All bison were challenged intraconjunctivally in midgestation with 107 CFU of B. abortus strain 2308 (S2308). Bison were necropsied and sampled within 72 h of abortion or delivery of a live calf. Compared to nonvaccinated bison, bison in the booster RB51 treatment had a reduced (P < 0.05) incidence of abortion, uterine infection, or infection in maternal tissues other than the mammary gland at necropsy. Bison in single-vaccination treatment groups (hand RB51 and dart RB51) did not differ (P > 0.05) from the control group in the incidence of abortion or recovery of S2308 from uterine, mammary, fetal, or maternal tissues at necropsy. Compared to nonvaccinated animals, all RB51 vaccination groups had reduced (P < 0.05) mean colonization or incidence of infection in at least 2 of 4 target tissues, with the booster RB51 group having reduced (P < 0.05) colonization and incidence of infection in all target tissues. Our data suggest that booster vaccination of bison with RB51 enhances protective immunity against Brucella challenge compared to single vaccination with RB51 by hand or by pneumatic dart. Our study also suggests that an initial vaccination of calves followed by booster vaccination as yearlings should be an effective strategy for brucellosis control in bison.
INTRODUCTION
The zoonotic intracellular pathogens in the genus Brucella have plagued mankind for centuries. Osteoarticular lesions consistent with brucellosis infection have been described in individuals from the Middle Ages (7th century), Roman times (44 BC to AD 476), and the Bronze Age (3300 to 2200 BC) and from the skeletal remains of a 2.3- to 2.5-million-year-old hominid found in South Africa (8). Even today, brucellosis continues to be a significant worldwide, and it is reemerging in many parts of the world. Within the United States, billions of dollars of state and federal funds have been invested in regulatory programs since the 1930s to reduce the prevalence of brucellosis in domestic livestock. Addressing the disease in natural hosts of Brucella is the most cost-effective mechanism to prevent human infection (4, 18).
Currently, B. abortus in domestic cattle has essentially been eradicated from the United States. However, the prevalence of brucellosis in free-ranging bison and elk in the Greater Yellowstone Area (Yellowstone National Park and surrounding areas) has led to occasional transmission of infection to cattle herds. The bison within Yellowstone National Park, the crown jewel of the U.S. National Park Service, are historically important because they are direct descendants of the last free-ranging plains bison within the United States. In 1880, 8 years after its establishment, the park superintendent reported that three herds totaling approximately 600 wild bison remained (20). However, continued hunting and poaching reduced the herd such that only 25 bison were counted in the park in 1901 (13). After importation of 21 bison from captive herds in 1902 and more strenuous efforts by the park to protect the bison in the park, the bison population in Yellowstone increased to more than 1,000 by the 1930s (13). Herd reductions and other manipulations of the population continued from the 1920s until the 1960s (13). When the current policy of natural regulation was instituted in 1967, the population in Yellowstone was estimated at approximately 400 bison (13). From 1967 to the present day, the bison population in the park has increased. Over the last decade, bison populations within the park have ranged between 3,000 and 5,000 animals, with a brucellosis seroprevalence in adult bison of approximately 50%. Interestingly, historical data suggest that the brucellosis infections in bison most likely originated from domestic livestock (12).
Postinoculation immunologic responses of bison to vaccination with strain RB51 via single injections or pneumatic dart delivery or after booster vaccination were reported in a prior publication (18). Other studies have suggested that a single RB51 vaccination of bison induces protection against virulent B. abortus when evaluated in a standardized challenge model for cattle (15, 16). However, protection induced by RB51 vaccination against experimental challenge is reduced in bison compared to cattle evaluated under similar conditions (6). This may partly be explained by the observation that bison are more susceptible than cattle to abortion, uterine infection, and mammary infection when experimentally challenged using the standardized model (17).
In this study, we evaluated the efficacy of RB51 vaccine delivered by single injection, by pneumatic dart delivery, or with booster vaccination. Because correlates for protective immunity against brucellosis in domestic livestock are currently not adequately identified, characterization of vaccine-induced protection in ruminants requires evaluation using a standardized experimental challenge model.
MATERIALS AND METHODS
Brucella abortus cultures.
For experimental challenges, Brucella abortus strain 2308 (S2308) was isolated from infected bovine cotyledon tissue and grown on tryptose agar for 48 h at 37°C. After one passage, the bacteria were harvested from the agar by aspiration using saline. Suspensions of S2308 were adjusted by use of a spectrophotometer, and concentrations of viable bacteria were determined by standard plate counts.
Vaccination and experimental Brucella challenge.
Bison heifers were obtained from a brucellosis-free herd. Bison heifers in the single-inoculation (hand RB51) treatment group received 2.2 × 1010 CFU of a commercial B. abortus strain RB51 vaccine at between 8 and 10 months of age in the musculature of the cervical region by hand injection. Bison in the treatment group that received two inoculations of RB51 (booster RB51) were intramuscularly (i.m.) vaccinated by hand injection in the cervical region at 8 to 10 and 23 to 25 months of age with inoculums containing 1.1 × 1010 and 2.2 × 1010 CFU, respectively. Bison in the dart vaccination group (dart RB51) received 1.8 × 1010 CFU of RB51 at between 8 and 10 months of age, with delivery targeted to the cervical region. Bison heifers in the control treatment group received 2 ml of saline administered i.m. into the musculature of the cervical region by hand injection. Data on immunologic responses after vaccination have been previously reported (18).
Animals were raised to adulthood and pasture bred at approximately 30 months of age for hand RB51 and dart RB51 treatments and at 42 months of age for booster RB51 and control treatments. Breeding dates were determined by rectal palpation at between 40 and 90 days of gestation. At approximately 5 months of gestation, pregnant bison were transferred to a biosafety level 3 containment facility, where they were individually housed for the duration of the study. At between 170 and 180 days of gestation as determined by rectal palpation, bison were restrained in a squeeze chute and intraconjunctivally challenged with approximately 1 × 107 CFU of S2308 (50 μl of inoculum per eye). Concentrations of viable bacteria within each challenge inoculum were determined by serial dilution in saline and standard plate counts.
Serologic evaluation.
Blood samples were collected by jugular venipuncture prior to experimental challenge, at 4-week intervals, and at necropsy. Blood was allowed to clot for 12 h at 4°C and centrifuged. Serum was divided into 1-ml aliquots, frozen, and stored at −70°C. Serologic titers to Brucella after experimental challenge were determined by the standard tube agglutination test (STAT) (2).
Necropsy sampling.
Immediately following abortion or within 72 h of parturition, cows were euthanized with intravenous administration of sodium pentobarbitol. Maternal samples obtained at necropsy included blood, milk from all four quarters, lymph nodes (bronchial, hepatic, internal iliac, mandibular, mesenteric, parotid, prescapular, retropharyngeal, and supramammary), mammary gland tissue from all four quarters, placentome or caruncle, spleen, liver, lung, and vaginal swab. Fetal/calf samples obtained included spleen, lung, liver, blood, bronchial lymph node, gastric contents, and rectal swabs. Abortion was defined as the premature birth of a Brucella-infected, nonviable fetus after S2308 challenge. In addition to clinical assessment of viability of live calves, all calves considered viable had milk present within the abomasum at necropsy.
Bacterial culture.
For bacterial culture, tissues were triturated in 0.15 M NaCl (saline) using a tissue grinder and placed on tryptose agar plates containing 5% bovine serum as previously described (5, 14). Swabs were directly plated on tryptose agar plates containing 5% bovine serum. Following incubation at 37°C with 5% CO2 for at least 7 days, B. abortus was identified on the basis of colony morphology and growth characteristics (2) and confirmed by a PCR procedure using primers specific for identification of B. abortus omp2a (9).
Colonization (CFU/g) of B. abortus in the placentome and supramammary, parotid, and prescapular lymph nodes was determined by obtaining a cross-section of the tissue, weighing the sample (approximately 1 g), triturating the sample in a tissue grinder, preparing serial dilutions (1/10) in saline, and placing aliquots in duplicate on tryptose agar plates containing 5% bovine serum. Initial plates received 200 μl of the 2-ml total volume of the tissue homogenate, with dilutions extending to 10−9. The concentration in the tissue was determined by performing standard plate counts.
Dams and calves were considered to be infected if a single colony of B. abortus was recovered from any sample obtained at necropsy. Mammary infection was defined as the recovery of the 2308 challenge strain from supramammary lymph node, milk, or mammary gland tissue. Uterine infection was defined as the recovery of the 2308 challenge strain from the placentome or vaginal swab. Fetal infection was defined as recovery of S2308 from any fetal sample.
Statistical analysis.
Serologic and colonization data (CFU/g) were analyzed as the logarithm of the value. Serologic and tissue colonization data were compared by a general linear model procedure (SAS Institute Inc., Cary, NC). Means for individual treatments were separated by use of a least-significant-difference procedure (P < 0.05). Chi-square analysis was used to compare the incidences of abortion and S2308 infection in vaccinated and nonvaccinated animals following experimental challenge.
RESULTS
Challenge dosages and parturition results.
Standard plate counts indicated that the mean challenge dose was 1.5 × 107 ± 0.23 × 107 CFU of S2308.
In the control treatment, 5 of 6 animals aborted (Table 1), with a mean time between challenge and abortion of 37.8 ± 2.2 days and a mean crown-to-rump length of 82 cm. The control animal that did not abort delivered a live calf at 63 days after challenge, with a crown-to-rump length of 109 cm.
Table 1.
Efficacies of Brucella abortus strain RB51 vaccination strategies in protecting against experimental challenge at midgestation with 107 CFU of B. abortus strain 2308
| Vaccination strategy | Rate (%) of abortion or infection (no. aborted or infected/total)a |
||||
|---|---|---|---|---|---|
| Abortion | Infection |
||||
| Uterineb | Mammaryc | Fetald | Remaining maternal tissuese | ||
| Hand RB51f | 67 (2/6) | 66 (4/6) | 83 (5/6) | 100 (6/6) | 83 (5/6) |
| Dart RB51g | 57 (4/7) | 57 (4/7) | 100 (7/7) | 100 (7/7) | 94 (6/7) |
| Booster RB51h | 0 (0/5)* | 40 (2/5)* | 80 (4/5) | 100 (5/5) | 40 (2/5)* |
| Control | 83 (5/6) | 100 (6/6) | 100 (6/6) | 100 (6/6) | 100 (6/6) |
*, mean significantly different (P < 0.05) from that for the control treatment group.
Placentome, vaginal swab, and/or internal iliac lymph node.
Mammary tissues (4 quarters), milk, and/or supramammary lymph node.
Fetal lung, liver, spleen, gastric contents, bronchial lymph node, or rectal swab.
Any maternal tissue except mammary gland, placentome, milk, supramammary lymph node, or internal iliac lymph node.
The vaccination dose was 1.1 × 1010 CFU.
The vaccination dose was 2.2 × 1010 CFU.
The initial vaccination dose was 1.1 × 1010 CFU, and the booster vaccination dose was 2.2 × 1010 CFU.
In the RB51 single-vaccination group, 2 of 6 animals aborted, with the mean times between challenge and parturition for bison having viable calves or aborting being 86.8 ± 10.3 and 58 ± 5 days, respectively. Full-term, viable calves had mean crown-to-rump lengths of 96 cm, compared to 69 cm for the two calves that were aborted in this treatment.
In the dart vaccination group, 4 of 7 bison aborted, with mean times between challenge and parturition of 91.0 ± 11 and 51.5 ± 5.2 days for animals delivering viable calves or aborting, respectively. Mean crown-to-rump lengths were 106 cm and 78.5 cm, respectively, for full-term calves and calves that were aborted.
In the booster vaccination group, none of the 5 bison aborted. The mean time to parturition was 72 ± 14 days, with a mean crown-to-rump length of 100.6 cm.
There was no difference (P > 0.05) between treatments in mean crown-to-rump lengths or mean days between challenge and parturition for animals that delivered full-term, viable calves. In a similar comparison for bison that aborted, the mean crown-to-rump length and mean days between challenge and abortion did not differ (P > 0.05) between the control, hand RB51, and dart RB51 treatments.
Serologic responses.
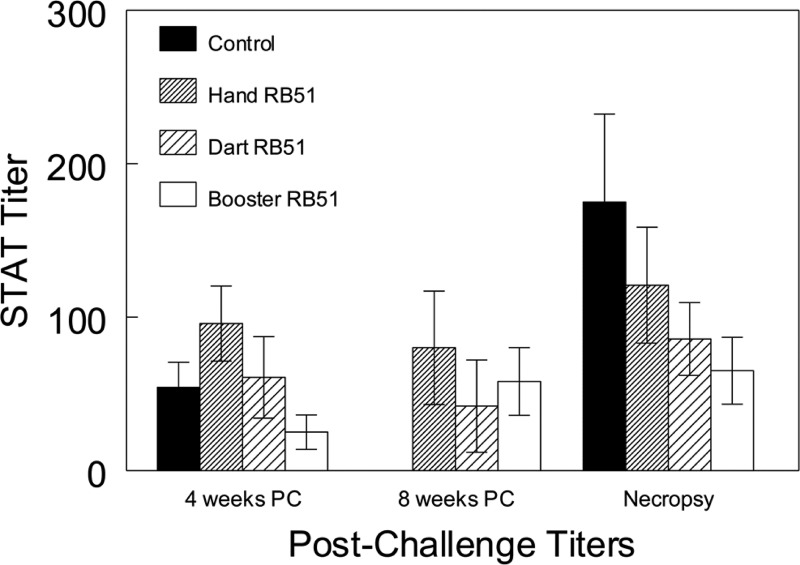
Prior to challenge, all animals were negative in the STAT. At 4 weeks after challenge 5 of 6, 6 of 6, 3 of 5, and 5 of 7 bison in the control, hand RB51, booster RB51, and dart RB51 groups, respectively, had seroconverted in the STAT. Mean STAT titers did not differ (P > 0.05) between treatments after challenge (Fig. 1).
Fig 1.
Standard tube agglutination responses of bison after experimental intraconjunctival challenge with 107 CFU of B. abortus strain 2308 in midgestation. Bison were vaccinated i.m. as calves with 1.1 × 1010 CFU (hand RB51) or 2.2 × 1010 CFU (dart RB51) RB51 or as calves and yearlings with 1.1 × 1010 and 2.2 × 1010 CFU (booster RB51). Data are reported as mean titer ± standard error of the mean (SEM). All bison in the control treatment aborted or had live calves prior to the 8-week postchallenge (PC) sampling.
Bacteriologic data.
Compared to nonvaccinated bison, bison that were booster vaccinated with RB51 had a reduced (P < 0.05) incidence of abortion, uterine infection, or infection in maternal tissues other than reproductive or mammary tissues after experimental challenge with S2308 (Table 1). Bison in the single-vaccination treatments (hand RB51 and dart RB51) did not differ (P > 0.05) from the control group in the incidence of abortion or recovery of S2308 from uterine, mammary, fetal, or remaining maternal tissues at necropsy.
Bison in all RB51 vaccination groups had reduced (P < 0.05) mean colonization or incidence of infection in at least two of four target tissues obtained at necropsy compared to nonvaccinated bison (Table 2). Bison in the booster RB51 treatment group had reduced (P < 0.05) mean colonization in all four tissues, including the supramammary lymph node and placentome, and a reduced (P < 0.05) incidence of infection (number culture positive/number in treatment group) compared to the control treatment group. Bison in the RB51 single-vaccination treatment groups (hand RB51 and dart RB51) had reduced (P < 0.05) mean colonization in the parotid and prescapular lymph nodes, but not (P > 0.05) in placentome or supramammary lymph node tissues, compared to those in the control treatment group. The incidence of S2308 infection in placentome, parotid lymph node, and prescapular lymph node tissues at necropsy was reduced (P < 0.05) in the hand RB51 treatment group compared to that in nonvaccinated bison. For bison in the dart RB51 treatment group, only the prescapular lymph node demonstrated a reduced (P < 0.05) incidence of recovery of S2308 compared to recovery from bison in the control treatment.
Table 2.
Colonization in target tissues obtained at necropsy after experimental challenge at midgestation with 107 CFU of B. abortus strain 2308
| Vaccination strategy | Log CFU/g (no. culture positive/total)a in: |
|||
|---|---|---|---|---|
| Parotid lymph node | Prescapular lymph node | Suprammamary lymph node | Placentome | |
| Hand RB51b | 0.8 ± 0.4 b (3/6)* | 0 ± 0 b (0/6)* | 0.7 ± 0.5 ab (2/6) | 4.0 ± 1.8 ab (3/6)* |
| Dart RB51c | 1.2 ± 0.5 b (4/7) | 0.3 ± 0.3 b (1/7)* | 0.9 ± 0.4 ab (4/7) | 4.5 ± 1.6 ab (4/7) |
| Booster RB51d | 0.8 ± 0.6 b (2/5)* | 0 ± 0 b (0/5)* | 0 ± 0 b (0/5)* | 1.7 ± 1.1 b (2/5)* |
| Control | 2.7 ± 0.3 a (6/6) | 1.7 ± 0.4 a (5/6) | 1.9 ± 0.5 a (5/6) | 7.6 ± 0.3 a (6/6) |
Data are means ± standard errors of the means. Colonization means with different letters are significantly different (P < 0.05). *, vaccination treatment group in which the incidence of infection in a tissue is significantly different (P < 0.05) from the incidence of infection in the control treatment group.
The vaccination dose was 1.1 × 1010 CFU.
The vaccination dose was 2.2 × 1010 CFU.
The initial vaccination dose was 1.1 × 1010 CFU, and the booster vaccination dose was 2.2 × 1010 CFU.
In a similar manner, the mean numbers of maternal or fetal tissues positive for recovery of the 2308 challenge strain at necropsy were reduced (P < 0.05) in bison in the hand RB51 and booster RB51 treatment groups compared to the control treatment group (Table 3). In comparison, bison in the dart RB51 treatment group did not differ (P > 0.05) from nonvaccinated bison in mean numbers of maternal or fetal tissues from which the challenge strain was recovered at necropsy.
Table 3.
Recovery of B. abortus strain 2308 from tissues obtained at necropsy after midgestational challenge from bison vaccinated with saline, Brucella abortus strain RB51 once by hand or pneumatic dart, or twice with RB51
| Vaccination strategy | No. of tissues (mean ± SEM)a |
|||
|---|---|---|---|---|
| Maternal |
Fetal |
|||
| Positive | Negative | Positive | Negative | |
| Hand RB51b | 9.2 ± 6.4 a | 11.8 ± 6.4 a | 4.0 ± 2.3 a | 3.0 ± 2.3 a |
| Dart RB51c | 11.0 ± 6.6 ab | 10.0 ± 6.6 ab | 3.4 ± 1.5 ab | 3.6 ± 1.5 ab |
| Booster RB51d | 5.8 ± 5.1 a | 15.2 ± 5.1 a | 3.4 ± 1.5a | 3.6 ± 1.5 a |
| Control | 17.4 ± 2.2 b | 3.6 ± 2.2 b | 6.6 ± 0.5b | 0.4 ± 0.5 b |
Means with different letters are significantly different (P < 0.05).
The vaccination dose was 1.1 × 1010 CFU.
The vaccination dose was 2.2 × 1010 CFU.
The initial vaccination dose was 1.1 × 1010 CFU, and the booster vaccination dose was 2.2 × 1010 CFU.
DISCUSSION
The results of this study indicate that booster vaccination of bison with RB51 enhances protective immunity against experimental Brucella challenge compared to single parenteral vaccination with RB51 by hand or by pneumatic dart. Although bison receiving booster vaccinations with RB51 did not abort, the ability to recover S2308 from mammary tissue and fetal tissues remained surprisingly high, at 80% and 100%, respectively. As there were no abortions and S2308 was recovered from uterine/placental samples at necropsy of only 40% of bison in the booster vaccination treatment group, we hypothesize that the recovery of the 2308 challenge strain from all calves in this group may reflect vertical infection through milk. Because all calves were determined to be viable and found to have milk in their abomasums at necropsy, the 48 to 72 h between parturition and necropsy in which they were allowed to nurse may have facilitated vertical transmission from S2308 localized within milk or maternal mammary tissues.
Our data affirm the preference of Brucella for localization in tissues associated with the mammary gland. As immunologic and challenge data indicate that bison in the booster vaccination treatment had developed adaptive immunity against brucellosis, the possibility that adaptive immunity may have played a role in the tissue distribution of Brucella in this group cannot be eliminated. It should be noted that mammary tissues, compared to other maternal tissues, had the highest recovery of Brucella across treatment groups in the current study. It is well known that Brucella localizes and causes inflammatory lesions in mammary tissues of natural hosts (7) and that unpasteurized dairy products have a high risk for transmitting brucellosis to humans. One study found that Brucella was isolated more frequently from milk than from mammary tissues of cattle (22), and work with B. abortus in goats found that milk stasis may cause the increased susceptibility of mammary gland to infection (10, 11). In a similar manner, shedding of Brucella within human breast milk (3, 23) and human mastitis caused by brucellosis (1) have been reported.
As implementation of a vaccination program for free-ranging wildlife will be expensive and require long-term commitment of human and financial resources for success, the failure of the vaccination strategies evaluated in the current study to provide sterile immunity may be of concern to some individuals. However, one model for brucellosis estimated a 24 to 66% reduction in seroprevalence in bison over a 30-year period, using an estimation of vaccine efficacy of 0.5 and an estimation of vaccination coverage of 1 to 29% of the population (21). As abortions are the most significant mechanism for horizontal transmission of B. abortus in ruminants, it should be noted that the current study found that booster vaccination reduced abortions by 83%, uterine infection by 60%, and colonization in uterine placentomes by 78% after experimental challenge in comparison to results for control bison. Using a similar comparison, a single hand vaccination with RB51 in our study was associated with a 33% reduction in abortions, a 34% reduction in uterine infection, and a 48% reduction in placentome colonization after experimental challenge compared to those in nonvaccinated animals. Cumulative data from previous efficacy studies in our laboratory, encompassing 67 vaccinated animals and 50 controls experimentally challenged in a manner similar to that in the current study, found that abortions were reduced by 55% and uterine infections were reduced by 45% in bison receiving a single RB51 vaccination at between 6 and 12 months of age (15, 16; S. C. Olsen, unpublished data) Recognizing that the numbers of experimental units in the current study were relatively small, additional studies are warranted to more accurately define the efficacy of booster vaccination of bison with RB51.
The relatively lower efficacy of pneumatic dart delivery of RB51 was unexpected. Standard plate counts indicated that the inoculum was within the 1 × 1010 to 3.4 × 1010 CFU RB51 dosage as recommended for calfhood vaccination of cattle. However, tissue trauma associated with pneumatic impact, failure to fully deliver the 2-ml volume of vaccine with the dart, leakage of inoculum from the site of injection, and other, unidentified factors are all possible explanations for the relative reduction in efficacy as observed in the current study. As pneumatic darts can administer vaccines at greater distances than ballistic systems, this delivery system may offer an advantage for use in wildlife. Increasing the RB51 dosage within the dart and administration of a booster vaccination via pneumatic dart are possibilities that could be explored for increasing the efficacy of brucellosis vaccines administered with this delivery system.
In summary, our study suggests that an initial vaccination of calves followed by booster vaccination as yearlings should be a more effective strategy for brucellosis control in bison than a single calfhood vaccination. Compared to single vaccination with RB51, booster vaccination appears to increase protection against abortion and infection in bison while reducing bacterial colonization within tissues. Reductions in abortion and tissue colonization should reduce the potential for brucellosis to be horizontally transmitted within an infected bison herd and assist in reducing disease prevalence. Although the most effective approach is to combine vaccination with test and removal programs, our data suggest that even in the absence of procedures to remove animals infected with field strains of B. abortus, vaccination will be an effective strategy for reducing the prevalence of brucellosis.
ACKNOWLEDGMENTS
We thank Deb Buffington, Katie Bunte, Aileen Bryant, Doug Ewing, John Kent, Tonia McNunn, Todd Pille, David Panthen, Darl Pringle, Jay Steffan, Dennis Weuve, and Robin Zeissness for technical assistance.
Product names are necessary to report factually on available data; however, the USDA neither guarantees nor warrants the standard of the product, and the use of a name by USDA implies no approval of the product to the exclusion of others that may also be suitable.
Footnotes
Published ahead of print 11 April 2012
REFERENCES
- 1. Akay H, Girgin S, Ozmen CA, Killic J, Sakarya H. 2007. An unusual bilateral mastitis in a postmenopausal woman caused by brucellosis. Acta Chir. Belq. 107:320–322 [DOI] [PubMed] [Google Scholar]
- 2. Alton GG, Jones LM, Angus RD, Verger JM. 1988. Techniques for the brucellosis laboratory, p 17–136. Institut National de la Recherche Agronomique, Paris, France [Google Scholar]
- 3. Barroso Espadero D, Arroyo Carrera I, Lõpez Rodriquez MJ, Lozano Rodríquez JA, Lõpez Lafuente A. 1998. The transmission of brucellosis via breast feeding. A report of 2 cases. An. Esp. Pediatr. 48:60–62 [PubMed] [Google Scholar]
- 4. Bernués A, Manrique E, Maza MT. 1997. Economic evaluation of bovine brucellosis and tuberculosis eradication programmes in a mountain area of Spain. Prev. Vet. Med. 30:137–149 [DOI] [PubMed] [Google Scholar]
- 5. Cheville NF, et al. 1992. Bacterial survival, lymph node changes and immunologic responses of cattle vaccinated with standard and mutant strains of Brucella abortus. Am. J. Vet. Res. 53:1881–1888 [PubMed] [Google Scholar]
- 6. Cheville NF, Stevens MG, Jensen AE, Tatum FM, Halling SM. 1993. Immune responses and protection against infection and abortion in cattle experimentally vaccinated with mutant strains of Brucella abortus. Am. J. Vet. Res. 54:1591–1597 [PubMed] [Google Scholar]
- 7. Corner LA, Alton GG, Iver H. 1987. Distribution of Brucella abortus in infected cattle. Aust. Vet. J. 64:241–244 [DOI] [PubMed] [Google Scholar]
- 8. D'Anastasio R, Staniscia T, Milia ML, Manzoli L, Capasso L. 2011. Origin, evolution and paleoepidemiology of brucellosis. Epidemikol. Infect. 139:149–156 [DOI] [PubMed] [Google Scholar]
- 9. Lee L-K, Olsen SC, Bolin CA. 2001. Effects of exogenous recombinant interleukin-12 on immune responses and protection against Brucella abortus in a murine model. Can. J. Vet. Res. 65:223–228 [PMC free article] [PubMed] [Google Scholar]
- 10. Meador VP, Deyoe BL. 1991. Effect of milk stasis on Brucella abortus infection of the mammary gland in goats. Am. J. Vet. Res. 52:886–890 [PubMed] [Google Scholar]
- 11. Meador VP, Deyoe BL, Cheville NF. 1989. Effect of nursing on Brucella abortus infection of mammary glands of goats. Vet. Pathol. 26:369–375 [DOI] [PubMed] [Google Scholar]
- 12. Meagher M, Meyer ME. 1994. On the origin of brucellosis in bison of Yellowstone National Park: a review. Conserv. Biol. 8:645–653 [Google Scholar]
- 13.National Park Service 2000. Bison Management for the state of Montana and Yellowstone National Park. Final environmental impact statement, p vii-viii, 282–283. National Park Service,U.S. Department of the Interior, Washington, DC [Google Scholar]
- 14. Olsen SC. 2000. Immune responses and efficacy after administration of a commercial Brucella abortus strain RB51 vaccine to cattle. Vet. Ther. 1:183–191 [PubMed] [Google Scholar]
- 15. Olsen SC, Boyle SM, Schurig GG, Sriranganathan NN. 2009. Immune responses and protection against experimental challenge after vaccination of bison with Brucella abortus strain RB51 or RB51 overexpressing superoxide dismutase and glycosyltransferase genes. Clin. Vaccine Immunol. 16:535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olsen SC, Jensen AE, Stoffregen WC, Palmer MV. 2003. Efficacy of calfhood vaccination with Brucella abortus strain RB51 in protecting bison against brucellosis. Res. Vet. Sci. 74:17–22 [DOI] [PubMed] [Google Scholar]
- 17. Olsen SC, Johnson C. 2011. Comparison of abortion and infection after experimental challenge of pregnant bison and cattle with Brucella abortus strain 2308. Clin. Vaccine Immunol. 18:2075–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olsen SC, Johnson C. 2012. Immune responses and safety after dart or booster vaccination of bison with Brucella abortus strain RB51. Clin. Vaccine Immunol. 19:642–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roth F, et al. 2003. Human health benefits from livestock vaccination for brucellosis: case study. Bull. World Health Organ. 81:867–876 [PMC free article] [PubMed] [Google Scholar]
- 20. Schullery P, Whittlesey L. 1991. The documentary record of wolves and related species in the Yellowstone National Park Area prior to 1882, p 1–173. In Varley JD, Brewster WG. (ed), Wolves in Yellowstone: a report to the United States Congress. National Park Service, Yellowstone National Park, WY [Google Scholar]
- 21. Treanor JJ, et al. 2010. Vaccination strategies for managing brucellosis in Yellowstone bison. Vaccine 28S:F64–F72 [DOI] [PubMed] [Google Scholar]
- 22. Xavier MN, Paixão TA, Poester FP, Lage AP, Santos RL. 2009. Pathological, immunohistochemical and bacteriological study of tissues and milk of cows and fetuses experimentally infected with Brucella abortus. J. Comp. Pathol. 140:149–157 [DOI] [PubMed] [Google Scholar]
- 23. Zuppa AA, et al. 2010. Breastfeeding and infectious diseases: state of the art. Minerva Pediatr. 62:397–409 [PubMed] [Google Scholar]