Abstract
Opsonophagocytic killing assays (OPAs) are important in vitro surrogate markers of protection in vaccine studies of Streptococcus pneumoniae. We have previously reported the development of a 4-fold multiplexed OPA (MOPA) for the 13 serotypes in Prevnar 13. Because new conjugate vaccines with increased valence are being developed, we developed 4-fold MOPAs for an additional 13 serotypes: serotypes 6C and 6D, plus the 11 serotypes contained in Pneumovax but not in Prevnar 13. A high level of nonspecific killing (NSK) was observed for three serotypes (10A, 15B, and 33F) in multiple batches of baby rabbit complement. The NSK could be reduced by preadsorbing the complement with encapsulated, as well as unencapsulated, pneumococcal strains. The MOPA results compared well with the results of single-serotype OPA for all serotypes except for serotype 3. For serotype 3, the results obtained from the MOPA format were ∼40% higher than those of the single-serotype format. Interassay precision of MOPA was determined with 5 serum samples, and the coefficient of variation was generally <30% for all serotypes. MOPA was also specific for all serotypes except for serotype 20; i.e., free homologous polysaccharide (PS), but not unrelated PS, could completely and efficiently inhibit opsonization. However, serotype 20 PS from ATCC could efficiently inhibit opsonization of one serotype 20 target strain but not three other type 20 target strains even at a high (>80 mg/liter) PS concentration. This suggests the presence of serologic heterogeneity among serotype 20 strains.
INTRODUCTION
Streptococcus pneumoniae is a Gram-positive bacterium capable of causing pneumonia and otitis media as well as severe invasive diseases such as sepsis and meningitis, primarily in young children and elderly adults (6). To control pneumococcal infections, a 23-valent polysaccharide (PS) pneumococcal vaccine (PPV23) is currently available for the elderly (≥65 years of age) and persons (age 2 to 65 years) that are at increased risk for invasive pneumococcal disease. Since the PS vaccine is not effective in young children, various conjugate vaccines have been introduced. A 7-valent conjugate vaccine was licensed in the United States in 2000, and 10- and 13-valent conjugate vaccines were introduced in 2010 and 2011, respectively (4, 5). A 15-valent conjugate vaccine is now under development (18).
During vaccine development and evaluation, pneumococcal vaccine immunogenicity has been primarily determined by measuring anticapsular PS antibody levels by enzyme-linked immunosorbent assay (ELISA) (7). While ELISA was successful in correlating protection against homologous serotypes in children, ELISA results may not correlate with cross-protection (8). Also, adults are susceptible to pneumococcal infections despite having generally high levels of pneumococcal antibodies (13, 14, 16, 17), so ELISA results may not correlate with protection in adults. Further, with increasing serotypes included in the conjugate vaccines, these vaccines are also being evaluated for use in adults; for example, Prevnar 13 is now approved for use in adults >50 years of age (www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm285431.htm). Therefore, there is a need to directly measure the protective capacity of anticapsular antibodies, which function by opsonizing pneumococci for phagocytes (14), in adults.
The in vitro opsonophagocytic killing assay (OPA) has become a practical tool with the development of a 4-fold multiplexed OPA (MOPA) for 13 serotypes (2) by significantly increasing assay throughput and reducing the serum volume necessary for testing. However, PPV23 and the newest conjugate vaccine formulation (18) contain additional serotypes. Also, new serotypes (6C [12] and 6D [1]) have been discovered, and the protective capacities against these new serotypes need to be measured. We have therefore developed and characterized 13 additional target strains, enabling one to perform MOPA for the 23 serotypes contained in PPV23, along with serotype 6A and the newly found serotypes 6C and 6D. Unexpectedly, our studies also show evidence of subtypes among serotype 20 isolates.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in this study are listed in Table 1 along with their respective wild-type parental strain names. Strains were made resistant to one of four antibiotics (optochin, spectinomycin, streptomycin, or trimethoprim; all purchased from Sigma, St. Louis, MO) by natural selection with increasing antibiotic concentrations. Previously described were OREP3, OREP7F, SPEC6B, STREP14, and TREP19A (2); DBL2 (19); BGO-2197 (11); and MNZ920 (9). R36A, strain 6320 (S. pneumoniae serotype 20), and strain 6538 (Staphylococcus aureus) were obtained from ATCC (Manassas, VA). The remaining wild-type strains were obtained from the Centers for Disease Control and Prevention (CDC) in Atlanta, GA.
Table 1.
Bacteria strain composition and antibiotic resistance of opsonization assay groups
| Strain | Assay groupa |
Antibiotic resistance and concn. in overlay (mg/liter) | |||
|---|---|---|---|---|---|
| D | E | F | G | ||
| 1 | OREP3 (Wu2) | OREP10A (DS3032-06) | OREP17F (DS3022-06) | Empty | Optochin, 2 |
| 2 | SPEC6C (BGO-2197) | SPEC6D (MNZ920) | SPEC9N (DS1398-00) | SPEC20B (DS3014-06) | Spectinomycin, 300 |
| 3 | STREP33F (DS3052-06) | STREP8 (DS5675-06) | STREP2 (DBL2) | Empty | Streptomycin, 300 |
| 4 | TREP22F (DS3433-06) | TREP12F (DS4031-06) | TREP11A (DS3160-06) | TREP15B (DS0556-97) | Trimethoprim, 25 |
Serotype is included in the name (e.g., OREP10A is a strain expressing type 10A capsule PS). Parental strains are in parentheses. OREP3 has been described previously (2). Currently, group G contains only two strains. However, when running group G, we recommend that an unrelated optochin-resistant strain (such as OREP3) and an unrelated streptomycin-resistant strain (such as STREP2) be included to maintain the effector/target ratio.
To create assay working stocks, bacterial master stocks were streaked onto blood agar plates and cultured overnight at 37°C and 5% CO2. Multiple colonies were inoculated into filter-sterilized Todd-Hewitt broth (Becton Dickinson, Sparks, MD) with 0.5% yeast extract (THY) and were cultured in a 37°C water bath until the optical density at 600 nm (OD600) was ∼0.8. At that time, multiple working assay stocks were cryopreserved with ∼15% glycerol and stored at −80°C until needed.
Serum samples.
Pool 32 (P1) was prepared by combining equal volumes of serum from 100 anonymous adults (>65 years of age) who had been vaccinated with Pneumovax 1 month prior. Pool 34 (P2) was prepared by combining equal volumes of serum from 52 anonymous adults (>65 years of age) who had been vaccinated with Pneumovax 2 months prior. Forty single-donor serum samples collected from anonymous adults (>65 years of age) were also used in this study (designated S1, S2, etc.). Thirty of these samples were collected 1 month after vaccination with Pneumovax, and 10 were collected prevaccination. All serum samples were deemed free of antibiotics based on their inability to inhibit growth of R36A bacteria and were incubated at 56°C for 30 min to inactivate endogenous complement activity prior to use in the assays.
MOPA.
The MOPA procedure has been described previously (2), and a detailed protocol can be found at www.vaccine.uab.edu. Briefly, serum samples were serially diluted (3-fold) in opsonization assay buffer B (OBB; Hanks' balanced salt solution [with magnesium and calcium] with 0.1% gelatin and 5% defined fetal bovine serum [FBS; HyClone, Logan, UT]) with 20 μl of serum tested in duplicate in 96-well round-bottom plates. Frozen working stocks of each of the four target strains were thawed and washed once with OBB by centrifugation (13,000 rpm for 2 min), and a bacterial mixture was prepared in OBB that contained ∼5 × 104 CFU/ml of each of the four strains. Ten microliters of the bacteria mixture was added to each well, and plates were incubated at room temperature for 30 min with shaking. After incubation, 10 μl of baby rabbit complement (BRC; Pel-Freez Biological, Rogers, AR) collected from 3- to 4-week-old rabbits was added to all wells except control A wells, which received 10 μl of heat-inactivated BRC (heated at 56°C for 30 min). Forty microliters of differentiated HL60 cells (containing 4 × 105 cells) was added to all wells, and the plates were incubated for 45 min in a 37°C/5% CO2 incubator with shaking. Afterwards, a 10-microliter aliquot of the final reaction product from each well was spotted onto THY agar (1.5%) plates. Overlay agar (THY with 0.75% agar) containing one of the four antibiotics and 2,3,5-triphenyltetrazolium chloride (TTC; Sigma) was added, and plates were incubated overnight (37°C/5% CO2). The number of surviving colonies was enumerated, and the opsonic indices (OIs) were calculated using linear interpolation. OI is defined as the reciprocal of the interpolated dilution of serum that kills 50% of bacteria.
For experiments involving the single-serotype format, the same protocol was followed except after being washed, the bacteria were diluted to ∼1 × 105 CFU/ml. After the assay, five microliters of the final reaction product from each well was spotted onto THY agar (1.5%) plates.
Adsorption of BRC.
For the indicated experiments, BRC was preadsorbed with bacteria. The indicated bacterial strain was grown in THY broth to an OD600 of ∼0.5. Eleven-milliliter aliquots of the culture were prepared, glycerol was added to ∼15%, and aliquots were stored at −80°C. For adsorption, aliquots of bacteria were thawed and washed two times with OBB and centrifugation. The bacterial pellet (∼20 μl) was suspended in 10 ml of BRC, and tubes were incubated at 4°C for 30 min with rotation. After incubation, the bacteria were pelleted by centrifugation and the BRC was filtered to remove any remaining bacteria. Adsorbed BRC was either used immediately or was aliquoted and stored at −80°C.
Specificity determination.
For experiments involving specificity, serum samples were prediluted with OBB, and capsular polysaccharides were diluted to twice the indicated concentration with OBB. Equal volumes of diluted serum and diluted PS were mixed and incubated at room temperature for 15 min. After incubation, serial dilutions of sera were performed with OBB. The rest of the assay was the same as described above. All capsular PSs were purchased from ATCC (Manassas, VA) except where noted.
CH50 assay.
The CH50 assay was adapted from Rose (15), and a detailed procedure can be found at www.vaccine.uab.edu (the CH50 measures the total hemolytic activity of a test sample and is the reciprocal of the dilution of serum complement needed to lyse 50% of a standardized suspension of sheep erythrocytes coated with antierythrocyte antibody). Briefly, lyophilized rabbit antiserum against sheep red blood cell (Sigma) was reconstituted with 2 ml of deionized water, heat inactivated at 56°C for 30 min, diluted 100-fold with 0.9% NaCl, and stored at −80°C until needed. After being washed with assay buffer (140 mM NaCl, 5 mM sodium barbital, 0.1% gelatin, 0.004% NaN3, 0.06 mM CaCl2, 0.4 mM MgCl2, pH 7.35) and centrifugation (1,300 × g for 10 min at 4°C), sheep red blood cells (sRBC; Colorado Serum Company, Denver, CO) were opsonized by coincubation with the diluted rabbit antiserum at 37°C for 30 min with gentle agitation. After being washed by centrifugation, the opsonized sRBC were suspended in assay buffer and stored at 4°C until needed (for up to 1 month).
For the assay, 50 μl of test sera (serially diluted 2-fold in assay buffer) was added, in duplicate, to wells of 96-well round-bottom plates. Opsonized sRBC were washed by centrifugation (1,300 × g for 10 min at 4°C) and diluted to ∼2 × 108 cells/ml in assay buffer, and 50 μl was added to each well. Control wells consisted of opsonized sRBC in assay buffer without test serum (representing 0% lysis) and opsonized sRBC in water without test serum (representing 100% lysis). Plates were incubated for 60 min at 37°C with shaking. After incubation, 150 μl of cold assay buffer was added to each well, except the 100% lysis wells that received 150 μl of water. Plates were centrifuged at ∼ 1,300 × g for 10 min at 4°C. Supernatant (150 μl) from each well was transferred to a 96-well flat-bottomed plate and the OD405 was determined. CH50 values are defined as the reciprocal of the interpolated dilution of serum that lyses 50% of the sRBC.
RESULTS
Development of target bacteria.
The 13 new target strains derived for this study are presented in Table 1, along with OREP3, which has been described previously (2). The capsule type of the target strain was confirmed by our own assay (20) as well as by conventional serotyping methods performed by B. Beall of the U.S. CDC in Atlanta, GA. Each strain is resistant to the indicated antibiotic at a concentration 2-fold higher than that used in the assay and sensitive to the other three antibiotics at concentrations one-half of those used in the assay. All strains except STREP2 were sensitive to erythromycin, tetracycline, cefotaxime, moxifloxacin, oxacillin, vancomycin, and clindamycin as shown by the large zones of inhibition (>18 mm) in response to disks containing the appropriate antibiotic (Sensi-Disc; Becton Dickinson, Sparks, MD). STREP2 was resistant to both erythromycin and clindamycin but sensitive to the remaining antibiotics. OREP17F and TREP12F consistently displayed a mixture of transparent and opaque colonies, whereas all other strains were >95% opaque.
Nonspecific killing.
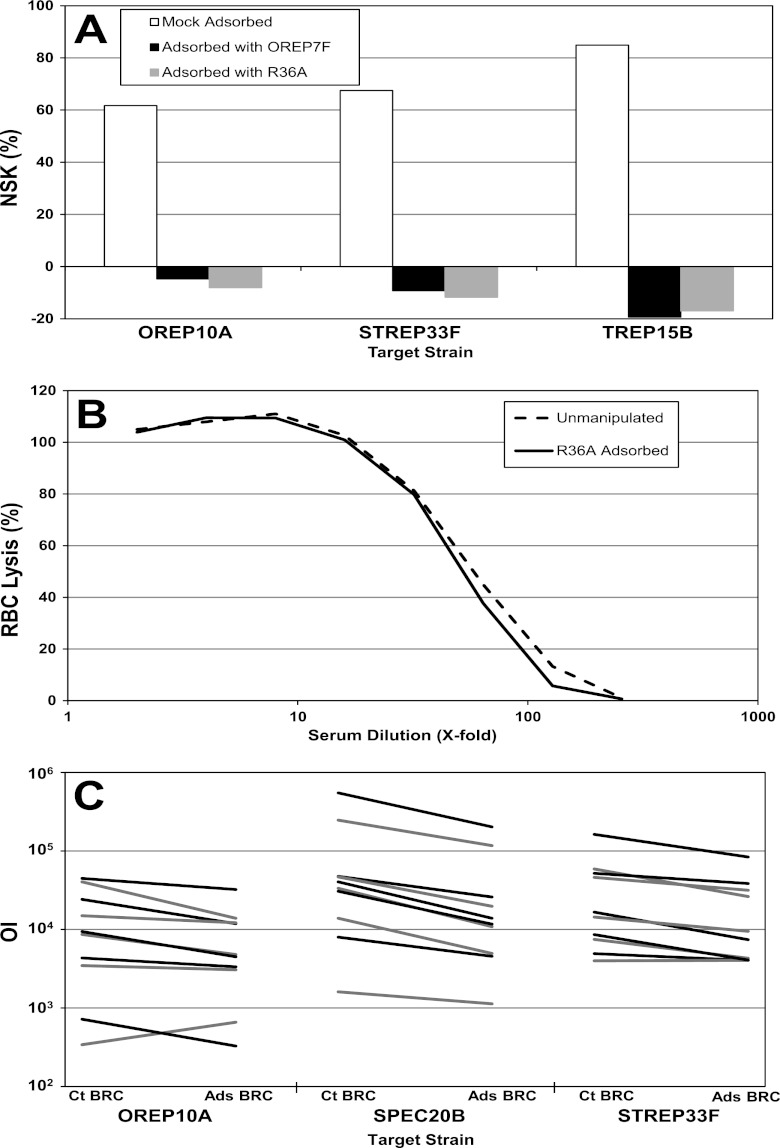
Low nonspecific killing (NSK), defined as killing of bacteria in the absence of test serum, by BRC is important in making the assay robust. The percentage of NSK is calculated by the formula %NSK = [1 − (CFU in control B/CFU in control A)] × 100%, where “control A” contains bacteria, heat-inactivated BRC, and phagocytes and “control B” contains bacteria, active BRC, and phagocytes. Although most serotypes in this study had low NSK (less than 30%), three target strains (TREP15B, OREP10A, and STREP33F) typically had high NSK (>50%; Fig. 1A). In most instances, the NSK of TREP15B was >70% and often exceeded 90%. As this high NSK was observed with multiple pneumococcal isolates for each serotype and multiple lots of BRC (data not shown), we hypothesized that BRC has factor(s) that are capable of opsonizing pneumococci. Therefore, BRC was preadsorbed with an unencapsulated strain (R36A), as well as an encapsulated pneumococcal strain (OREP7F), before evaluation for NSK (Fig. 1A). Adsorption with either strain reduced nonspecific killing to <10%. Similar results were obtained when heat-killed R36A (heated at 60°C for 2 h), other encapsulated pneumococcal strains (OREP4, SPEC6B, TREP15B, STREP14, TREP19A), or a staphylococcal strain (strain 6538) were used for adsorption (data not shown).
Fig 1.
Effects of preadsorption of BRC with bacteria. (A) NSK of BRC after mock adsorption (white bars), adsorption with OREP7F (black bars), or adsorption with R36A (gray bars). OREP10A, STREP33F, and TREP15B are target strains. (B) Comparison of the lysis of sheep red bloods by unmanipulated BRC (dashed line) and R36A-adsorbed BRC (solid line) in the CH50 assay. The y axis indicates the percent lysis of sensitized sheep red blood cells at the indicated dilution of BRC (x axis). (C) Opsonization index (y axis) obtained with 10 different sera using the unmanipulated (Ct BRC) or R36A-adsorbed BRC (Ads BRC) in the MOPA of 3 serotypes. Each line connects the OI using unmanipulated BRC and the OI using R36A-adsorbed BRC, one line for each of the 10 sera. Lines are alternately colored black and gray for visualization purposes.
To ensure that the preadsorption does not deplete complement, the CH50 was determined. The titration curves of the unmanipulated and adsorbed BRC were nearly identical (Fig. 1B), indicating that the adsorption did not reduce overall complement activity. The R36A-adsorbed BRC was also compared with unmanipulated BRC in the MOPA by measuring the OIs of 10 single-donor postvaccination sera for serotypes 10A, 20 (analyzed as a control), and 33F (Fig. 1C). For these serotypes, the average ratios of OI using unmanipulated BRC to OI using adsorbed BRC for the 10 sera were 1.6-, 2.4-, and 1.7-fold for OREP10A, SPEC20B, and STREP33F, respectively. A similar trend was seen when serotypes 6B, 18C, 19F, and 22F were tested with adsorbed and unmanipulated BRC as well (data not shown). The general shape of the dose-response curves was not affected (data not shown). R36A-adsorbed BRC was used for precision, specificity, and accuracy experiments involving assay group G (which contains serotype 15B).
Assay precision (reproducibility).
The interassay precision of the new target strains was ascertained by testing a panel of sera (2 serum pools and 3 single-donor sera) five times over a 3-week period. Sera were selected based solely on the criterion that they had detectable OIs for all serotypes in the bacteria group. For each sample, the mean OI, the standard deviation, and the %CV (standard deviation/mean × 100) of the five runs were calculated. The %CVs are presented in Table 2. For serotype 6C, S2 and S3 had irregular dose-response curves that contributed to the high CVs. For serotype 9N, 3 of the 5 sera had a CV of ≤30%, with the remaining two sera having CVs of 32% and 35%. For all other serotypes, at least 4 of the 5 sera had a CV of ≤30%.
Table 2.
Interassay precision (%CV) of MOPA with the new target strains
| Group designation | Group member | %CV of serum samplesa |
||||
|---|---|---|---|---|---|---|
| P1 | P2 | S1 | S2 | S3 | ||
| D | OREP3 | 11 | 10 | 16 | 10 | 8 |
| SPEC6C | 41 | 18 | 24 | 70 | 56 | |
| STREP33F | 25 | 17 | 41 | 14 | 14 | |
| TREP22F | 16 | 10 | 28 | 11 | 36 | |
| E | OREP10A | 30 | 11 | 18 | 8 | 43 |
| SPEC6D | 27 | 18 | 16 | 7 | 22 | |
| STREP8 | 8 | 13 | 4 | 11 | 4 | |
| TREP12F | 12 | 10 | 19 | 3 | 8 | |
| F | OREP17F | 33 | 13 | 16 | 22 | 30 |
| SPEC9N | 14 | 35 | 13 | 32 | 14 | |
| STREP2 | 14 | 10 | 19 | 33 | 4 | |
| TREP11A | 3 | 27 | 9 | 25 | 20 | |
| G | SPEC20B | 26 | 13 | 21 | 29 | 19 |
| TREP15B | 12 | 28 | 3 | 21 | 28 | |
P1 and P2 are serum pools 32 and 34, respectively. S1, S2, and S3 are anonymous single-donor sera and are different for each target bacterial group.
Assay sensitivity and accuracy (assay comparability).
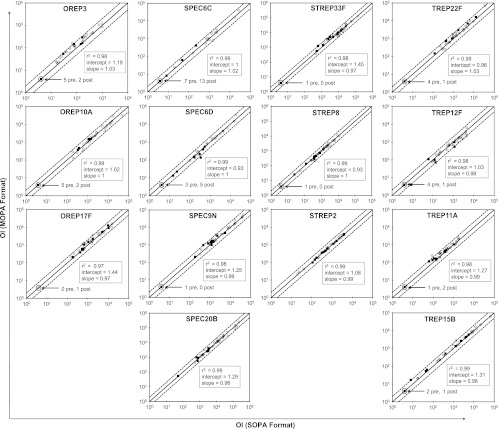
Since there is no standard or reference serum with assigned OPA values, there is no way to measure assay sensitivity and accuracy. Therefore, to ensure that the multiplexed OPA produces results comparable to those obtained by the reference method, the single-serotype OPA (SOPA), 40 sera (30 postvaccination and 10 prevaccination) were tested in both assay formats, and the resultant OIs were compared. Since bacteria group G has only two strains (TREP15B and SPEC20B), OREP3 and STREP2 bacteria were included for the MOPA to maintain the effector-to-target ratio. Figure 2 shows the results of these comparisons with the SOPA OIs on the x axis and the MOPA OIs on the y axis. Each graph also has the line of identity (solid line) as well as 2-fold deviations from identity (dashed lines). Although some minor bias may be seen for some serotypes (e.g., types 11A and 15B in Fig. 2), the results obtained with both assays agreed well, with 14 of 560 comparisons (14 serotypes × 40 sera) differing by more than 2-fold.
Fig 2.
Comparison of MOPA results with single-serotype OPA (SOPA) results. Ten prevaccination (black symbols) and 30 postvaccination (open symbols) sera were tested in the SOPA format (x axis) and the MOPA format (y axis). Each panel shows the target strain, the line of identity (solid line), and 2-fold deviations from identity (dashed lines). The number of serum samples with undetectable OIs (assigned an OI of 4) are indicated. Also, the r2 value, the slope, and y intercept of the line of best fit (all calculated from the log-transformed OIs) are provided.
After testing the initial 40 samples, there appeared to be a slight bias toward the MOPA for OREP3. The average ratio of MOPA OI to SOPA OI for samples with detectable OIs (i.e., ≥8) was 1.43 (n = 33). This apparent bias was seen over a wide range of OIs and was seen in both pre- and postvaccination sera (Fig. 2). To confirm this finding, we tested an additional 20 single-donor sera (6 pre- and 14 postvaccination). A similar trend was seen with the new samples (data not shown), and the average ratio (MOPA OI/SOPA OI) for the 60 samples was 1.39.
Assay specificity.
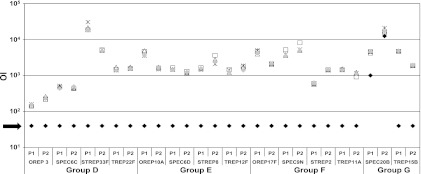
The specificity of the MOPA was determined by preabsorbing two immune serum pools with homologous capsular polysaccharide (purchased from ATCC). The serum pools were also tested after preabsorption with the 3 heterologous PSs included in that assay group, with each heterologous PS tested individually. As shown in Fig. 3, the OIs against all strains, except SPEC20B, were undetectable in the presence of homologous capsular PS (20 mg/liter). For SPEC20B, absorption with ATCC serotype 20 PS reduced the OIs by 80% and 25% for P1 and P2, respectively. Conversely, for all strains (including SPEC20B), there was little or no change in the OIs when the test sera were preincubated with any of the three heterologous capsular PSs in that assay group (each also 20 mg/liter).
Fig 3.
Specificity of the MOPA. To determine the specificity of the MOPA, two serum pools, P1 and P2, were preincubated with homologous PS (20 mg/liter, black diamond), one of three heterologous PS (star, open triangle, open square, each 20 mg/liter), or OBB as a control (gray diamond). The black arrow indicates the sensitivity limit in the assays.
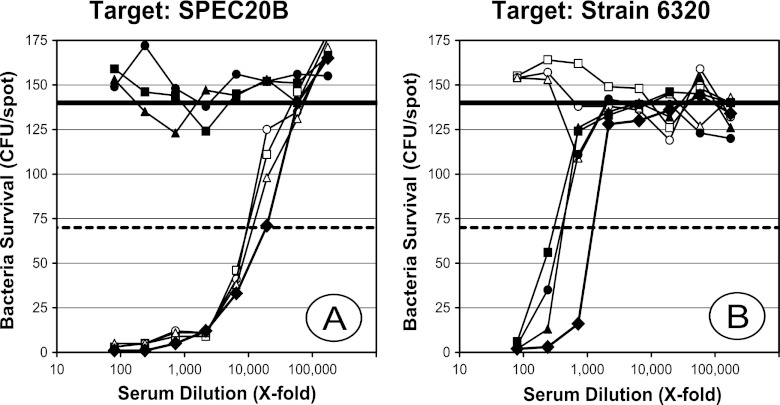
Because of the low level of inhibition of SPEC20B killing with homologous PS from ATCC, serum P2 was chosen for further exploration using multiple ATCC PS concentrations for preabsorption. In addition, we purified capsular PS from another serotype 20 strain from CDC (5931-06) and tested its ability to inhibit killing. As shown in Fig. 4A, preincubation of P2 with ATCC PS decreased the OI against SPEC20B by only about 40 to 50% compared to that of the buffer control. However, preincubation with as little as 5 mg/liter of PS from 5931-06 completely abrogated killing of SPEC20B (Fig. 4A). Conversely, when strain 6320 from ATCC was used as a target, its killing was completely inhibited with 5 mg/liter of ATCC PS but was again only partially (∼75%) inhibited with PS (Fig. 4B) from 5931-06 even at 80 mg/liter. For each target strain, there was no significant inhibition of killing with heterologous PSs (data not shown). When we tested four additional serotype 20 isolates (strains 3014-06, 4579-06, and 5931-06 from CDC and SSISP20/2 from Staten Serum Institute in Denmark) as target strains, we found them to behave like SPEC20B (data not shown). These data indicated that strain 6320 and SPEC20B are serologically distinct, and we therefore provisionally assigned them to serotype 20A and serotype 20B, respectively.
Fig 4.
Inhibition of type 20 killing. The number of surviving CFU (y axis) at various dilutions of serum P2 (x axis) obtained with SPEC20B (A) or strain 6320 (B) as target bacteria. Serum P2 was preabsorbed with 5, 20, or 80 mg/liter of type 20 PS from ATCC (white triangles, white circles, and white squares, respectively), type 20 PS purified from strain 5931-06 (black triangles, black circles, and black squares, respectively), or with buffer alone (black diamonds) as a control. The solid and dashed horizontal lines represent 0% and 50% killing, respectively.
DISCUSSION
We have developed 13 additional target serotypes for the MOPA, in order to complete the serotype coverage of MOPA to PPV23, serotype 6A, and the new serotypes 6C and 6D. Since there is no OPA reference material, it is impossible to establish the accuracy of MOPA. Instead, MOPA results were compared to those obtained by SOPA, the reference method. The results obtained with both formats were quite comparable for all serotypes except for serotype 3. For serotype 3, the MOPA format produced OIs ∼40% higher than the SOPA format. While it is difficult to explain this difference, type 3 bacteria produce a copious amount of capsular PS that is not covalently anchored to bacterial cell wall and is released to the surroundings. Since the absolute concentration of type 3 bacteria is 2-fold higher in the SOPA than the MOPA, the extra bacteria in SOPA may release additional free PS that can behave as an opsonization inhibitor.
MOPA for the new serotypes had adequate intermediate precision. CVs were generally less than 30% for most test samples, but high CVs were associated both with certain test samples and with certain serotypes. High CVs (>50%) were found for serotype 6C with two samples that produced irregular (not monotonically increasing) dose-response curves. The irregular curves that were associated with low precision may arise because the sera contain antibodies weakly cross-reactive with 6C. The serum donors were vaccinated with 6B PS (not 6C PS), and vaccination with 6B has been shown to elicit antibodies with poor cross-reactivity to 6C (10).
During our studies, some serotypes, particularly serotype 15B, had high NSK, which can significantly reduce the robustness of the MOPA. In our experience, high NSK is generally associated with specific serotypes. For instance, we found moderately high NSK (30% to 70%) for serotypes 6A and 6B in the past (2) and high NSK for serotypes 10A, 15B, and 33F in the current study. This high NSK was associated with multiple target strains of the same serotype (data not shown). By combining serotypes 10A, 15B, and 33F into one bacterial group, we were able to efficiently screen BRC lots for NSK, and the six lots of BRC tested in this study all had high NSK for these three target strains (data not shown). However, we found empirically that preadsorbing the BRC with an unencapsulated pneumococcal strain (R36A) dramatically decreased the NSK. Although this adsorption did not reduce the activity of the classical complement pathway (as measured by CH50 assay), it may have removed innate opsonins reacting with the three serotypes, and the adsorbed BRC produced generally lower (∼2-fold) OIs than those obtained with unadsorbed BRC. The lower OIs may not reflect innate opsonins and may be more accurate OIs, but this is difficult to conclude definitively due to lack of an OPA standard. More experience with R36A-adsorbed BRC is required.
Analysis of assay specificity showed that MOPAs involving all new serotypes, except serotype 20, were deemed specific, as absorption of test sera with homologous capsular PS resulted in undetectable OIs, and absorption with heterologous capsular PSs resulted in no significant change in OIs (Fig. 3). For serotype 20, two distinct PS inhibition patterns were observed. As we have demonstrated with other serotypes, opsonization of strain 6320 could be completely blocked with as little as 5 mg/liter of free 20A PS from ATCC but was only partially inhibited by 20B PS. In contrast, opsonization of SPEC20B could be inhibited only partially with 20A PS concentrations up to 80 mg/liter but was completely inhibited with 5 mg/liter of 20B PS. Also, postvaccination sera have much higher (about 10-fold) OIs against SPEC20B than strain 6320, as can be seen in Fig. 4 by comparing the serum dilution required to kill 50% for each of the two target bacteria with no inhibitor PS. At the moment, it is unclear as to which serotype 20 strains are appropriate targets for OPA.
Although all the isolates were reproducibly typed as serotype 20 by the currently available serologic methods, the most likely explanation is that subtypes exist among serotype 20 isolates. We have provisionally assigned ATCC 6320 to serotype 20A and the remaining type 20 strains tested in this study to serotype 20B. Finding subtypes among the serotypes defined by conventional typing methods is not surprising since new serotypes 6C and 11E were found among isolates that were typed as 6A and 11A, respectively, by the conventional methods (3, 12). Preliminary data suggest strains 6320 and SPEC20B produce PSs with different monosaccharide compositions (unpublished data), and more biochemical and genetic studies are being performed. Elucidation of potential subtypes should permit us to identify the subtype included in the PPV23 and perform epidemiologic surveys of the subtypes. The information should then help us select target bacteria for OPA. This situation also underscores the need for more specific serotyping assays.
In summary, we have developed 13 new target strains for MOPA. These additional serotypes may be useful when evaluating future formulations of PCVs, as well as new target populations for the current vaccines, in vaccine efficacy trials. Also, target strains expressing serotypes 6C and 6D will be useful to determine the level of cross-protection afforded by immunizing with 6A and/or 6B PS. To facilitate OPA standardization, these new target strains will be deposited at BEI Resources (ATCC) for wide distribution.
ACKNOWLEDGMENTS
The work was supported by a National Institutes of Health contract AI-30021 from the National Institute of Allergies and Infectious Disease, Division of Microbiology and Infectious Disease.
We thank Bernard Beall and Dee Jackson of the CDC in Atlanta, GA, for providing us with the parental bacteria strains and for confirming the serotypes of the target strains.
The University of Alabama at Birmingham has intellectual property rights on the target strains, and R.L.B. and M.H.N. are employees of the University of Alabama at Birmingham. M.H.N. is a consultant to Merck for pneumonia diagnosis.
Footnotes
Published ahead of print 18 April 2012
REFERENCES
- 1. Bratcher PE, Kim KH, Kang JH, Hong JY, Nahm MH. 2010. Identification of natural pneumococcal isolates expressing serotype 6D by genetic, biochemical, and serological characterization. Microbiology 156: 555–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burton RL, Nahm MH. 2006. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin. Vaccine Immunol. 13: 1004–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calix JJ, Nahm MH. 2010. A new pneumococcal serotype, 11E, has variably inactivated wcjE gene. J. Infect. Dis. 202: 29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chevallier B, et al. 2009. Safety and reactogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with routine childhood vaccines. Pediatr. Infect. Dis. J. 28: S109–S118 [DOI] [PubMed] [Google Scholar]
- 5. Esposito S, et al. 2010. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine compared to those of a 7-valent pneumococcal conjugate vaccine given as a three-dose series with routine vaccines in healthy infants and toddlers. Clin. Vaccine Immunol. 17: 1017–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fedson DS, Musher DM. 1994. Pneumococcal vaccine, p 517–564 In Plotkin SA, Mortimer EA. (ed), Vaccines, 2nd ed W.B. Saunders Co., Philadelphia, PA [Google Scholar]
- 7. Jodar L, et al. 2003. Serological criteria for evaluation and licensure of pneumococcal conjugate vaccine formultions for use in infants. Vaccine 21: 3265–3272 [DOI] [PubMed] [Google Scholar]
- 8. Lee H, Nahm MH, Burton R, Kim KH. 2009. Immune response in infants to the heptavalent pneumococcal conjugate vaccine against vaccine-related serotypes 6A and 19A. Clin. Vaccine Immunol. 16: 376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nahm MH, et al. 2011. A report of Streptococcus pneumoniae serotype 6D in Europe. J. Med. Microbiol. 60: 46–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park IH, et al. 2008. Differential effects of pneumococcal vaccines against serotypes 6A and 6C. J. Infect. Dis. 198: 1818–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park IH, Park S, Hollingshead SK, Nahm MH. 2007. Genetic basis for the new pneumococcal serotype, 6C. Infect. Immun. 75: 4482–4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park IH, et al. 2007. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 45: 1225–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park S, Nahm MH. 2011. Older adults have a low capacity to opsonize pneumococci due to low IgM antibody response to pneumococcal vaccinations. Infect. Immun. 79: 314–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Romero-Steiner S, et al. 2006. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin. Vaccine Immunol. 13: 165–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rose NR. 1997. Manual of clinical immunology. ASM, Washington, DC [Google Scholar]
- 16. Rubins JB, Alter M, Loch J, Janoff EN. 1999. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect. Immun. 67: 5979–5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schenkein JG, Park S, Nahm MH. 2008. Pneumococcal vaccination in older adults induces antibodies with low opsonic capacity and reduced antibody potency. Vaccine 26: 5521–5526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skinner JM, et al. 2011. Pre-clinical evaluation of a 15-valent pneumococcal conjugate vaccine (PCV15-CRM197) in an infant-rhesus monkey immunogenicity model. Vaccine 29: 8870–8876 [DOI] [PubMed] [Google Scholar]
- 19. Strand TA, et al. 2001. Pneumococcal pulmonary infection, septicaemia and survival in young zinc-depleted mice. Br. J. Nutr. 86: 301–306 [DOI] [PubMed] [Google Scholar]
- 20. Yu J, Lin J, Kim KH, Benjamin WH, Jr, Nahm MH. 2011. Development of a multiplexed and automated serotyping assay for Streptococcus pneumoniae. Clin. Vaccine Immunol. 18: 1900–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]