Abstract
Pneumococcal conjugate vaccines (PCVs) are recommended for the prevention of invasive pneumococcal disease (IPD) in young children. Since the introduction of the heptavalent pneumococcal vaccine (PCV7) in 2000, IPD caused by serotypes in the vaccine has almost been eliminated, and previously uncommon capsular serotypes now cause most cases of pediatric IPD in the United States. One way to protect against these strains would be to add cross-reactive protein antigens to new vaccines. One such protein is pneumococcal surface protein A (PspA). Prior to 2000, PspA families 1 and 2 were expressed by 94% of isolates. Because PCV7 vaccine pressure has resulted in IPD caused by capsular serotypes that were previously uncommon and unstudied for PspA expression, it was possible that many of the new strains expressed different PspA antigens or even lacked PspA. Of 157 pediatric invasive pneumococcal isolates collected at a large pediatric hospital in Alabama between 2002 and 2010, only 60.5% had capsular serotypes included in PCV13, which came into general use in Alabama after our strains were collected. These isolates included 17 serotypes that were not covered by PCV13. Nonetheless, pneumococcal capsular serotype replacement was not associated with changes in PspA expression; 96% of strains in this collection expressed PspA family 1 or 2. Continued surveillance will be critical to vaccine strategies to further reduce IPD.
INTRODUCTION
Streptococcus pneumoniae is a major cause of morbidity and mortality worldwide due to pneumonia, bacteremia, and meningitis. Pneumococcal infections are estimated to cause 826,000 deaths in children less than 5 years of age globally (16). The introduction of the heptavalent pneumococcal conjugate vaccine (PCV7) led to almost complete elimination of invasive pneumococcal disease (IPD) caused by the seven PCV capsular types (4, 6B, 9V, 14, 18C, 19F, and 23F) causing IPD prior to the introduction of that vaccine. Subsequently, an increase in the incidence of IPD caused by non-PCV7 capsular types has been observed (11, 21). In 2010, a new 13-valent vaccine was introduced to provide protection against the original PCV7 serotypes plus an additional 6 capsular serotypes (1, 3, 5, 6A, 7F, and 19A) known to cause IPD. In the European Union, serotypes 3 and 19A cause 2.5% and approximately 15% of IPD cases, respectively. A recent report indicates that PCV13 is expected to cover no more than 68% of IPD isolates and that the non-PCV13 isolates appear to be as virulent as those covered by the vaccine (18). Since there are >90 known capsular serotypes (5), continuing to increase the number of serotypes in conjugate vaccines may not remain a practical means of closing the gap in PCV coverage and countering future serotype replacement.
A potential strategy to reduce serotype replacement could be the inclusion of protein vaccine immunogens that could provide protection that is not dependent on antibody responses to capsular polysaccharides. One candidate protein antigen is the cross-protective protein antigen pneumococcal surface protein A (PspA). Prior to the use of PCV7, this cell surface-associated protein virulence factor (13) was found on virtually all clinically relevant strains of pneumococci (6), and almost all strains express one of 2 major serologic/sequence families. Prior to the licensure and extensive use of PCV7, PspA was expressed on more than 94% of strains reported from studies of 3 collections comprising more than 2,200 strains from around the world. It had been judged that a PspA-containing vaccine should include representatives of each of these two major families (1, 12, 23). Strains of the most common seven capsular types before the year 2000 almost exclusively expressed either PspA family 1 or family 2. For many of the other capsular types, the numbers of strains examined were so few that little information could be gained about whether they were also going to be primarily PspA family 1 or 2. Thus, although the use of PCV7 would not be expected to put selection pressure on PspA per se, it was possible that some of the pneumococcal clones that expanded in frequency following the use of PCV7 might already have expressed PspAs that were not within families 1 or 2.
In this study, we present data on the PspA family and capsular serotype distribution of 157 isolates from IPD collected between 2002 and 2010 at a children's hospital. Our study examined the proportion of IPD isolates with serotypes not included in PCV13 and the distribution of two major PspA families. Forty percent of strains in this collection were not covered by PCV13. We found, however, that PspA families 1 and 2 were expressed by 96% of IPD isolates, overall. These findings indicate that a vaccine containing PspA molecules from these two families would still have the potential to cover IPD isolates following the introduction of PCV7 and, probably, PCV13.
(This work was presented in part at the 48th Annual Meeting of the Infectious Disease Society of America, Vancouver, British Columbia, Canada, October 2010.)
MATERIALS AND METHODS
Data and patient selection.
All viable pneumococci from sequential routine clinical specimens submitted to the Clinical Microbiology Laboratory at Children's of Alabama in Birmingham, AL, between July 2002 and June 2010 were collected prospectively. The site of isolation, clinical disease diagnosis, date of culture, antimicrobial susceptibilities, and patient demographic data associated with each strain were retrieved from the electronic medical record under an approved protocol of the Institutional Review Board of the University of Alabama at Birmingham with waiver of informed consent. IPD for this study was defined as infection with Streptococcus pneumoniae isolated from a normally sterile site, including blood, cerebrospinal fluid (CSF), pleural fluid, sputum, peritoneal fluid, and bone or joint aspirates. Seven clinical disease categories were considered: bacteremia, bacteremic pneumonia (bacteremia in association with a chest X ray interpreted by a pediatric imaging specialist as consistent with bacterial pneumonia), complicated pneumonia (chest X ray with pneumonia with effusion or empyema and pneumococci isolated from pleural fluid), pneumonia (chest X ray with pneumonia and pneumococci isolated from sputum or bronchoalveolar lavage fluid), mastoiditis (pneumococci obtained at surgery), meningitis (cerebrospinal fluid indices compatible with bacterial meningitis and pneumococci isolated from CSF and/or blood), and other IPD, including endocarditis (vegetations on echocardiogram and pneumococci in blood cultures) and bone or joint infection (compatible clinical diagnosis with pneumococci isolated from bone or joint aspirate). Two hundred thirty pneumococcal isolates were obtained from individual patients with IPD; 73 strains were excluded due to receiving multiple copies of an isolate, loss of a viable sample, multiple samples from the same clinical illness, or patient age (>18 years old). The analyses included 157 IPD isolates.
Multiplex assays for serotype detection.
Strains were typed serologically and/or by PCR for all 93 known pneumococcal capsular types. Capsular serotyping was performed using a multiplex immunoassay with monoclonal antibodies specific for each of the following serotypes as described by Yu et al. (25, 26): 4, 6B, 9V, 14, 18C, 19F, 23F, 1, 3, 5, 6A, 7F/A, 19A, 2, 6C, 6D, 8, 9N, 10A/39, 11A/D/F, 11E, 12F/B, 15B/C, 17F/A, 20, 22F/A, 25, and 33F/A. Isolates not typeable in the multiplex immunoassay were further studied using a multiplex PCR assay for the remainder of the 93 known serotypes that also included detection of autolysin and cpsA. 7F/A strains were furthered typed by agglutination with factor sera (Statens Serum Institut, Denmark). Strains were then grouped according to whether their capsular serotype was included or not included as an antigen in PCVs, including (i) the original heptavalent PCV (PCV7), (ii) the capsule antigens for the 7 serotypes in PCV7 plus six additional serotypes (PCV13), (iii) capsule types not included in PCV13 but including isolates with typeable capsules (nonvaccine type [NVT]), and (iv) nontypeable capsules (NT). All strains NT for capsule were also observed to have cpsA (24), which is known to be present in the capsule loci of strains of almost all capsular types (24).
Confirming nontypeable isolates as S. pneumoniae.
NT strains were confirmed to be S. pneumoniae through testing for optochin sensitivity, bile solubility, and the presence of the pneumolysin gene (ply). Strains that failed all three tests or were bile insoluble and not positive for ply were removed from the analyses. PCR for ply was performed using colony DNA preparation by inoculation with 100 μl deionized water, boiling for 15 min, and cooling on ice for 15 min. The DNA in the resultant supernatant was examined by PCR. The following primers were used to amplify a 348-bp region in Ply: ply F′, 5′-ATTTCTGTAACAGCTACCAACGA-3′, and ply R′, 5′-GAATTCCCTGTCTTTTCAAAGTC-3′.
Pneumococcal surface protein A family typing.
Determination of the PspA family type (1, 2, and 3, respectively) was initially performed by PCR as previously described by Hollingshead et al. (13). For isolates not typeable by PCR, a dot blot assay was performed (23), and this result was considered to define the PspA family.
Statistical analysis.
Statistical analyses between groups were performed using the χ2 test, the χ2 test for trend, or Fisher's exact test. A P value of <0.05 was considered to be significant. All tests were performed in GraphPad InStat, version 5.0 (GraphPad, La Jolla, CA).
RESULTS
Patient demographics and serotype distribution of isolates from 2002 to 2010.
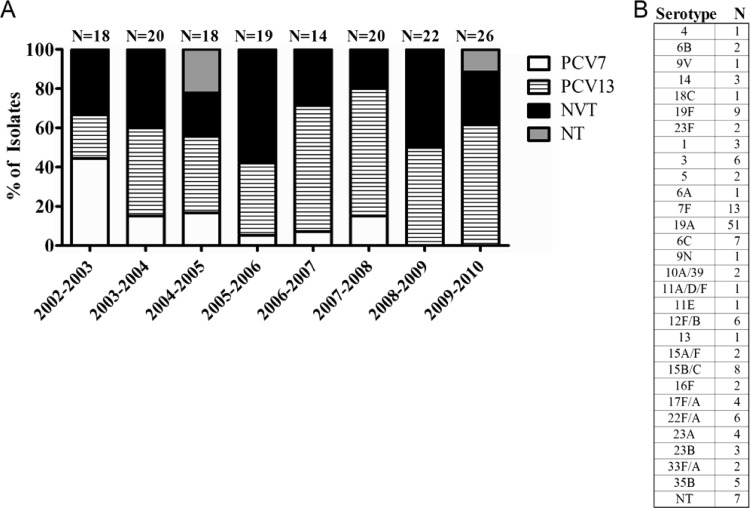
The population was 62.4% male, 50.3% Caucasian, and 26.8% African American. Of 157 IPD isolates collected, 64/157 (40.8%) were obtained from children less than 24 months of age, 43 (27.4%) were from children aged 24 to 60 months, and 50 (31.8%) were from children older than 60 months (Table 1). There was no statistically significant difference between age and capsular serotype distribution (P = 0.0664). Among the three age groups, patients >60 months of age had the greatest diversity in capsular serotype distribution (the highest number of prevalent serotypes and change over time within age group); 50% of strains from this group were non-PCV13 capsular types. Although the highest percentage (41%) of serotype 19A isolates was found among children <24 months old, there was no significant difference in the occurrence of serotype 19A between any of the age groups (P = 0.0845). PCV13 isolates were found in the highest proportions in Caucasians, but this difference was not statistically significant. In order to capture the seasonality of pneumococcal illness, the data were grouped by year from July 1 through June 30. Between 2002 and 2010, the PCV7 capsular types causing IPD virtually disappeared. Although the proportion of non-PCV capsular types differed from year to year, overall, 39.5% of the 157 IPD isolates failed to express PCV13 capsular types. A total of 17 different non-PCV13 capsular types were observed (Fig. 1). Two serotypes (19A and 7F) accounted for 55.7% of the PCV13 strains from 2002 to 2008 (39/70; 19A, 74%, and 7F, 26%) and for 92.0% (23/25; 19A, 87%, and 7F, 13%) of strains from 2008 to 2010 (data not shown). This increase of serotype 19A strains largely accounted for the increase of PCV13 strains.
Table 1.
Demographics of patients with IPD at Children's of Alabama
| Characteristic | Overall no. (%) | No. (%) of isolates of a serotype ina: |
|
|---|---|---|---|
| PCV13 | NVT | ||
| Age | 157 (100) | 95 (60.5) | 62 (39.5) |
| <24 months | 64 (40.8) | 43 (67.2) | 21 (32.8) |
| 24–60 months | 43 (27.4) | 27 (62.8) | 16 (37.2) |
| >60 months | 50 (31.8) | 25 (50.0) | 25 (50.0) |
| Ethnicity/race | |||
| Caucasian | 78 (50.3) | 51 (64.6) | 28 (35.4) |
| Black/African American | 42 (26.8) | 21 (50.0) | 21 (50.0) |
| Other | 10 (6.4) | 5 (50.0) | 5 (50.0) |
| Unknown | 26 (16.6) | 18 (69.2) | 8 (30.8) |
| Gender | |||
| Male | 98 (62.4) | 60 (61.2) | 38 (38.8) |
| Female | 59 (37.6) | 35 (59.3) | 24 (40.7) |
PCV13, all serotypes included in the 13-valent vaccine; NVT, encapsulated and nonencapsulated nonvaccine types.
Fig 1.
Serotype distribution of IPD isolates by period of isolation. (A) Serotype distributions for IPD isolates are shown for the years 2002 through 2010. Each seasonal period spans from July 1 through June 30 of the second year. N, total isolates from the indicated period; PCV7, capsular serotypes in the heptavalent pneumococcal conjugate vaccine; PCV13, capsular serotypes in the 13-valent PCV that are not included in PCV7; NVT, typeable serotypes not included in PCV13 (nonvaccine types); NT, nontypeable isolates. Overall, 60.5% of IPD cases were caused by serotypes included in Prevnar 13 (PCV13). (B) Raw serotype data of all IPD isolates. N, total number of isolates for indicated serotype. Serotype 19A accounted for 32.5% of all IPD isolates.
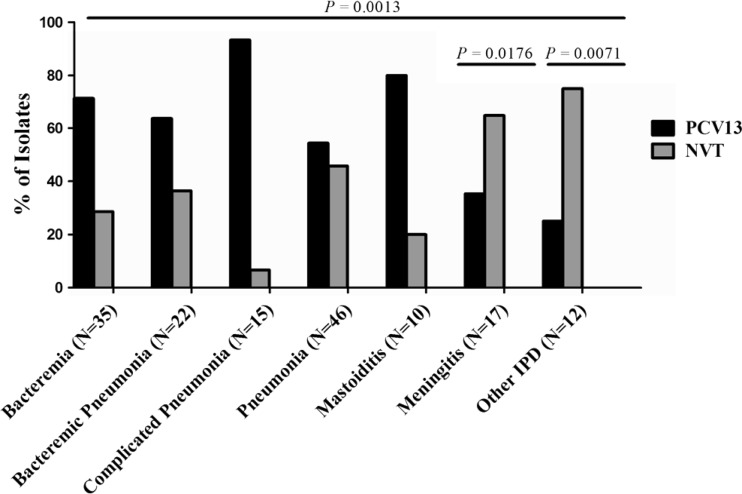
Serotype distribution also varied by clinical disease (P = 0.0013) (Fig. 2). Meningitis and “other IPD” (i.e., endocarditis, septic hip, and bone/joint isolates) were statistically more strongly associated than bacteremia with nonvaccine serotypes (P = 0.0176 and P = 0.0071, respectively). The remaining clinical disease categories (bacteremia, bacteremic pneumonia, complicated pneumonia, pneumonia, and mastoiditis) trended toward being more likely to be caused by PCV13 serotypes.
Fig 2.
Serotype distribution of pneumococci causing clinical disease. N, number of isolates; PCV13, serotypes included in PCV13; NVT, all pneumococci not expressing the serotypes included in the 13-valent vaccine (nonvaccine types), including both typeable isolates and those with nontypeable capsules. Serotype distribution versus disease manifestation overall was statistically significant (P = 0.0013, χ2 test for trend; all groups were compared as PCV13 versus NVT). Individual P values were obtained by setting bacteremia as the reference group and comparing with the disease of interest using Fisher's exact test. Bacteremic pneumonia was defined as a patient with a chest X ray (interpreted by a pediatric imaging specialist) for bacterial pneumonia and pneumococci isolated from a blood culture, complicated pneumonia as a chest X ray with pneumonia with effusion and pneumococci isolated from pleural fluid or pleural space, and pneumonia as a chest X ray positive for pneumonia and culture positive for pneumococci. “Other IPD” included endocarditis and bone or joint infection.
Characterization of IPD isolates by PspA family.
PspA typing by dot blotting and PCR using existing antisera and pairs of primers for families 1, 2, and 3 (13, 23) were able to ascertain the PspA family type for 97% of the 157 isolates. Among the 157 isolates, 64 (40.8%) were PspA family 1, 87 (55.4%) were PspA family 2, and 2 (1.3%) were PspA family 3, while 4 (2.5%) had nontypeable PspA (Table 2). A shift from majority PspA family 1 to family 2 occurred (P = 0.0052) during the study period; however, there were no differences between the other variables that were studied. We also observed no statistical difference in PspA families 1 and 2 among the strains with PCV13 serotypes versus those with NVT serotypes overall (P = 0.1777) (Table 3). When we looked at individual serotypes versus the overall population distribution, serotypes 7F, 6C, 22F/A, and 23A were found to be significantly different from the population as a whole in terms of PspA family type (P = 0.01, 0.002, 0.005, and 0.03, respectively). Eleven of 13 7F strains expressed PspA family 2. For capsular types 6C, 22F/A, and 23A, 100% of the 4 to 7 strains in each group expressed PspA family 1. Among the PCV13 and NVT strains, the percentages of strains expressing either family 1 or family 2 PspA were virtually identical (96.8% and 95.2%, respectively). Lastly, of the two PspA family 3 strains, both had NT capsules. Of the four strains that expressed NT PspA, one also had a nontypeable capsule.
Table 2.
Frequencies of PspA families by characteristics of S. pneumoniae isolates
| No. (%) of isolates expressinga: |
|||||
|---|---|---|---|---|---|
| Patient characteristic | PspA family: |
NT PspA | Total no. (%) (n = 157) | ||
| 1 | 2 | 3 | |||
| Age | |||||
| <24 months | 25 (39.1) | 36 (56.2) | 1 (1.6) | 2 (3.1) | 64 (40.8) |
| 24–60 months | 18 (41.9) | 24 (55.8) | 1 (2.3) | 43 (27.4) | |
| >60 months | 21 (42.0) | 27 (54.0) | 2 (4.0) | 50 (31.8) | |
| Clinical disease | |||||
| Bacteremia | 15 (42.9) | 20 (57.1) | 35 (22.3) | ||
| Bacteremic pneumonia | 6 (40.0) | 9 (60.0) | 15 (9.6) | ||
| Complicated pneumonia | 13 (59.1) | 9 (40.9) | 22 (14.0) | ||
| Pneumonia | 15 (32.6) | 26 (56.5) | 2 (4.3) | 3 (6.5) | 46 (29.3) |
| Mastoiditis | 3 (30.0) | 7 (70.0) | 10 (6.4) | ||
| Meningitis | 5 (29.4) | 12 (70.6) | 17 (10.8) | ||
| Other IPDb | 7 (58.3) | 4 (33.3) | 1 (8.3) | 12 (7.6) | |
| Period of isolationc | |||||
| 2002–2005 | 29 (51.8) | 26 (46.4) | 1 (1.8) | 56 (35.7) | |
| 2005–2008 | 24 (45.3) | 27 (50.9) | 2 (3.8) | 53 (33.8) | |
| 2008–2010 | 11 (22.9) | 34 (70.8) | 2 (4.2) | 1 (2.1) | 48 (30.6) |
PspA, pneumococcal surface protein A; NT, PspA nontypeable.
Other IPD was defined as endocarditis and bone or joint isolates.
P = 0.0052, χ2 test for trend, period of isolation versus PspA family 1 or 2.
Table 3.
Capsular serotype and PspA family type for all isolates
| Serotypea | No. (%) of isolates expressingb: |
Total no. (%) | |||
|---|---|---|---|---|---|
| PspA family: |
NT PspA | ||||
| 1 | 2 | 3 | |||
| 4 | 1 (100) | 1 | |||
| 6B | 2 (100) | 2 | |||
| 9V | 1 (100) | 1 | |||
| 14 | 1 (33.3) | 2 (66.7) | 3 | ||
| 18C | 1 (100) | 1 | |||
| 19F | 1 (11.1) | 8 (88.9) | 9 | ||
| 23F | 2 (100) | 2 | |||
| 1 | 3 (100) | 3 | |||
| 3 | 3 (50.0) | 3 (50.0) | 6 | ||
| 5 | 2 (100) | 2 | |||
| 6A | 1 (100) | 1 | |||
| 7Fc | 1 (7.7) | 11 (84.6) | 1 (7.7) | 13 | |
| 19A | 18 (35.3) | 31 (60.8) | 2 (3.9) | 51 | |
| 6Cc | 7 (100) | 7 | |||
| 9N | 1 (100) | 1 | |||
| 10A/39 | 2 (100) | 2 | |||
| 11A/D/F | 1 (100) | 1 | |||
| 11E | 1 (100) | 1 | |||
| 12F/B | 1 (16.7) | 5 (83.3) | 6 | ||
| 13 | 1 (100) | 1 | |||
| 15A/F | 2 (100) | 2 | |||
| 15B/C | 3 (37.5) | 5 (62.5) | 8 | ||
| 16F | 1 (50.0) | 1 (50.0) | 2 | ||
| 17F/A | 4 (100) | 4 | |||
| 22F/Ac | 6 (100) | 6 | |||
| 23Ac | 4 (100) | 4 | |||
| 23B | 2 (66.7) | 1 (33.3) | 3 | ||
| 33F/A | 2 (100) | 2 | |||
| 35B | 5 (100) | 5 | |||
| NT Capsule | 4 (57.1) | 2 (28.6) | 1 (14.3) | 7 | |
| Total | 64 | 87 | 2 | 4 | 157 |
The first 7 serotypes are in PCV7, the next 6 are the additional serotypes in PCV13, and the last 17 serotypes are in neither PCV7 nor PCV13.
PspA, pneumococcal surface protein A; NT, nontypeable.
P < 0.05, Fisher's exact test, individual serotype versus overall distribution of PspA family 1 or 2.
DISCUSSION
When PCV7 was licensed in 2000, 81% of cases of serious pneumococcal disease in the United States were caused by pneumococci with one of the vaccine capsule types. PCV7 was highly effective at reducing IPD caused by vaccine serotypes and reduced the incidence in children <5 years of age by about 75% by 2002 (11, 21). The incidence of disease by PCV7 isolates continued to decrease through 2007; however, the overall incidence of IPD failed to decrease significantly below the 2002 level due to an increase in IPD caused by non-PCV7 capsule type strains (18). PCV13 was anticipated to have lower coverage for pneumococcal capsular serotypes in childhood IPD (67.8% in children <5 years of age) prior to its introduction than did PCV7 (18), and our data support this expectation. Although the relative capsular serotype distribution of our IPD isolates from Alabama children varied considerably from year to year, IPD coverage by the recently licensed PCV13 among these strains collected from 2002 to 2010 was only 60.5% overall, even prior to the introduction of the vaccine in 2010. This relatively high proportion of non-PCV13 serotypes in this Alabama pneumococcal collection at the time of vaccine introduction probably reflects the unmasking of previously carried strains or new serotypes entering the niche originally occupied by the PCV7 strains. It should be noted that the serotype coverage by PCV13 in our strain collection had not yet been affected by PCV13 use since it was not in general clinical use until after the collection was closed.
The multiplex serotyping assay used in this study was capable of typing pneumococci of all 93 currently known serotypes (5). Nonetheless, 4.5% (7/157) of the IPD isolates collected remained nontypeable, even though these isolates each had the pneumococcal capsular gene cpsA, whose absence is most commonly associated with nonencapsulated noninvasive isolates (10). The lack of a detectable or identifiable capsule may be the result of capsular types not yet described or the result of dysfunction in the capsule locus (10).
A capsular polysaccharide vaccine containing capsule antigens for the 40% of IPD in Alabama not covered by PCV13 would have required the inclusion of an additional 17 or more (total of 30) capsular types. Based on our data, these 17 capsular types not covered by PCV13 would provide coverage of 1.6% to 13% of the non-PCV13 capsular types. Since the present collection of isolates is limited to 157 isolates from a relatively small geographic region of the southeastern United States, it is possible that a larger sample size from a larger geographic area or more diverse region would have identified more non-PCV13 IPD capsular types or a different distribution of serotypes (18).
A cost-effective way to expand serotype coverage of the present pneumococcal conjugate vaccines may be to include highly cross-reactive protection-eliciting pneumococcal protein antigens (22) or to develop a protein-only vaccine consisting of several protection-eliciting protein antigens. A vaccine containing proteins from both PspA families 1 and 2 would cover 96% of IPD isolates in this study, which is in accordance with previous studies of PspA family distribution from global isolates of pneumococci collected prior to the effect of PCV7 on capsular type distribution (12, 23). Our present results indicate that among the non-PCV7 strains which have expanded to fill the niche following mass immunization with PCV7, only 4% failed to express PspA family 1 or 2. To see whether this result can be generalized for strains around the world following the introduction of PCVs, it will be necessary to study additional isolates.
Immunization of healthy adults with a single PspA family 2 molecule resulted in the production of antibody that was able to passively protect mice infected with pneumococcal strains expressing either family 1 or family 2 PspAs (3). However, it was previously shown that PspAs exhibited the strongest cross-reactivity with PspAs of the same family. Thus, an effective PspA vaccine was expected to require epitopes from a single family 1 PspA and one or two family 2 PspAs to cover all strains (3, 15, 20). This could be achieved by mixing PspAs together (3, 20), making fusion proteins between two or more PspA alpha-helical regions (8), or using PspA as a carrier for one or more capsular polysaccharides. It has also been reported that careful selection of PspA molecules may make it possible to select individual molecules with wide cross-reactivity with other PspAs (14). Finally, recent work has shown that the relatively conserved proline-rich domain, which lacks any alpha-helical epitopes and is common to all families of PspA and PspC proteins, can protect against pneumococcal infection in mice (7). The proline-rich domain of PspA alone has not yet been studied in clinical trials (3).
Several experiments have shown that mixtures of different pneumococcal proteins can, in some cases, elicit much more protection than the individual proteins in the mixtures (2, 4, 17). Other promising proteins that could be used in combination with PspA for a protein vaccine to protect against strains not covered by conjugate vaccines include pneumolysoid, PspC (CbpA), PcpA, and PhtD (9, 17, 19, 22). Regardless of how new vaccines are constructed, it is likely that continued surveillance of pneumococcal serotypes associated with invasive disease will be critical to determine the effectiveness of PCV13 in reducing IPD and to help identify the best vaccine strategy for further improvement in the control of pneumococcal disease.
ACKNOWLEDGMENTS
We thank the Clinical Microbiology Laboratory at Children's of Alabama for all of their help. Without them, there would be no study. We also thank Janice King and Evida Dennis for help with maintenance of the isolate collection and Jigui Yu for technical assistance and support with multiplex serotyping.
This work was supported by grants AI-021458 and AI-30021 from the National Institute of Allergy and Infectious Diseases, grant P30 DK072482 from the National Institute of Diabetes and Digestive and Kidney Diseases, grant 5TL1 RR025775-04 from the National Center for Research Resources, and the Howard Hughes Medical Institute through the Med into Grad Initiative to University of Alabama at Birmingham.
The contents of this paper are solely the responsibility of the authors and do not represent the views of HHMI, NIAID, NIDDK, NCRR, or NIH.
Potential conflicts of interest include the fact that David E. Briles is a consultant for Sanofi Pasteur and the PATH Foundation. The University of Alabama at Birmingham (UAB) holds intellectual property rights related to protein vaccine antigens, including PspA, and David E. Briles is an inventor on the relevant patents. Moon H. Nahm is a consultant to Merck. UAB also holds intellectual property rights for monoclonal antibodies used in this study. C.M.C., M.H.N., D.E.B., and M.J.C. are employees of UAB.
Footnotes
Published ahead of print 25 April 2012
REFERENCES
- 1. Brandileone MC, et al. 2004. Typing of pneumococcal surface protein A (PspA) in Streptococcus pneumoniae isolated during epidemiological surveillance in Brazil: towards novel pneumococcal protein vaccines. Vaccine 22:3890–3896 [DOI] [PubMed] [Google Scholar]
- 2. Briles DE, et al. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Briles DE, et al. 2000. Immunization of humans with rPspA elicits antibodies, which passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 182:1694–1701 [DOI] [PubMed] [Google Scholar]
- 4. Briles DE, et al. 2003. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J. Infect. Dis. 188:339–348 [DOI] [PubMed] [Google Scholar]
- 5. Calix JJ, Nahm MH. 2010. A new pneumococcal serotype, 11E, has a variably inactivated wcjE gene. J. Infect. Dis. 202:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crain MJ, et al. 1990. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect. Immun. 58:3293–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daniels CC, et al. 2010. The proline-rich region of pneumococcal surface proteins A and C contains surface-accessible epitopes common to all pneumococci and elicits antibody-mediated protection against sepsis. Infect. Immun. 78:2163–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Darrieux M, et al. 2007. Fusion proteins containing family 1 and family 2 PspAs elicit protection against Streptococcus pneumoniae that correlates with antibody-mediated enhancement of complement deposition. Infect. Immun. 75:5930–5938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glover DT, Hollingshead SK, Briles DE. 2008. Streptococcus pneumoniae surface protein PcpA elicits protection against lung infection and fatal sepsis. Infect. Immun. 76:2767–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hathaway LJ, Stutzmann Meier P, Battig P, Aebi S, Muhlemann K. 2004. A homologue of aliB is found in the capsule region of nonencapsulated Streptococcus pneumoniae. J. Bacteriol. 186:3721–3729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hicks LA, et al. 2007. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J. Infect. Dis. 196:1346–1354 [DOI] [PubMed] [Google Scholar]
- 12. Hollingshead SK, et al. 2006. Pneumococcal surface protein A (PspA) family distribution among clinical isolates from adults over 50 years of age collected in seven countries. J. Med. Microbiol. 55:215–221 [DOI] [PubMed] [Google Scholar]
- 13. Hollingshead SK, Becker R, Briles DE. 2000. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 68:5889–5900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moreno AT, et al. 2010. Immunization of mice with single PspA fragments induces antibodies capable of mediating complement deposition on different pneumococcal strains and cross-protection. Clin. Vaccine Immunol. 17:439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nabors GS, et al. 2000. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 18:1743–1754 [DOI] [PubMed] [Google Scholar]
- 16. O'Brien KL, et al. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902 [DOI] [PubMed] [Google Scholar]
- 17. Ogunniyi AD, Grabowicz M, Briles DE, Cook J, Paton JC. 2007. Development of a vaccine against invasive pneumococcal disease based on combinations of virulence proteins of Streptococcus pneumoniae. Infect. Immun. 75:350–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pilishvili T, et al. 2010. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J. Infect. Dis. 201:32–41 [DOI] [PubMed] [Google Scholar]
- 19. Posfay-Barbe KM, et al. 2011. Immunity to pneumococcal surface proteins in children with community-acquired pneumonia: a distinct pattern of responses to pneumococcal choline-binding protein A. Clin. Microbiol. Infect. 17:1232–1238 [DOI] [PubMed] [Google Scholar]
- 20. Roche H, Ren B, McDaniel LS, Hakansson A, Briles DE. 2003. Relative roles of genetic background and variation in PspA in the ability of antibodies to PspA to protect against capsular type 3 and 4 strains of Streptococcus pneumoniae. Infect. Immun. 71:4498–4505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singleton RJ, et al. 2007. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 297:1784–1792 [DOI] [PubMed] [Google Scholar]
- 22. Tai SS. 2006. Streptococcus pneumoniae protein vaccine candidates: properties, activities and animal studies. Crit. Rev. Microbiol. 32:139–153 [DOI] [PubMed] [Google Scholar]
- 23. Vela Coral MC, et al. 2001. Pneumococcal surface protein A of invasive Streptococcus pneumoniae isolates from Colombian children. Emerg. Infect. Dis. 7:832–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yother J. 2011. Capsules of Streptococcus pneumoniae and other bacteria: paradigms for polysaccharide biosynthesis and regulation. Annu. Rev. Microbiol. 65:563–581 [DOI] [PubMed] [Google Scholar]
- 25. Yu J, et al. 2005. Rapid multiplex assay for serotyping pneumococci with monoclonal and polyclonal antibodies. J. Clin. Microbiol. 43:156–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu J, Lin J, Kim KH, Benjamin WH, Jr, Nahm MH. 2011. Development of a multiplexed and automated serotyping assay for Streptococcus pneumoniae. Clin. Vaccine Immunol. 18:1900–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]