Abstract
The exploratory immunogenicity objective of this analysis was to characterize the titer of vaccine human papillomavirus (HPV)-type immunoglobulins in both peripartum maternal blood and the cord blood of infants born to women who received blinded therapy. Data were derived from a randomized, placebo-controlled, double-blind safety, immunogenicity, and efficacy study (protocol 019; NCT00090220). This study enrolled 3,819 women between the ages of 24 and 45 years from 38 international study sites between 18 June 2004 and 30 April 2005. Data in the current analysis are from subjects enrolled in Philippines and Thailand. For each of HPV types 6, 11, 16, and 18, maternal anti-HPV was found in cord blood samples. Furthermore, HPV titers in cord blood samples were highly positively correlated with maternal HPV titers. Additionally, there were instances when anti-HPV antibodies were no longer detectable in maternal serum samples and yet were detected in matched cord blood samples. These results demonstrate that quadrivalent HPV (qHPV) vaccine-induced antibodies cross the placenta and could potentially provide some benefit against vaccine-type HPV infection and related diseases such as recurrent respiratory papillomatosis.
INTRODUCTION
Human papillomaviruses (HPVs) are double-stranded DNA viruses that infect the cutaneous and mucosal epithelium of humans. HPV infection can lead to benign genital warts or papillomas and low- or high-grade intraepithelial neoplasia, including cervical cancer. In addition, HPV type 6 (HPV-6) and HPV-11 can infect the squamous epithelium of the oral cavity, oropharynx, larynx, and hypopharynx and cause recurrent respiratory papillomatosis (RRP) (19, 31). RRP is a rare disease (roughly 4 cases per 100,000 children [10]) characterized by benign squamous papillomas, noncancerous tumors, or warts that grow in the larynx and within the respiratory tract.
The quadrivalent HPV (qHPV) (types 6/11/16/18) virus-like particle (VLP) vaccine was approved in 2006 for the prevention of genital warts caused by HPV types 6 and 11 as well as vaginal, vulvar, and cervical cancer caused by HPV types 16 and 18 (12, 13). The vaccine has since been studied in both adult women (2) and men (15).
Vaccination with qHPV has been shown to elicit a strong neutralizing antibody response and to engender immune memory (anamnestic response) upon reexposure to HPV vaccine (26). In addition, few data exist on the transfer of anti-HPV antibodies from mothers to newborns (18). To establish whether antibodies induced by natural infection or following vaccination with qHPV can cross the placenta, we evaluated whether a type 6, 11, 16, and 18 competitive Luminex immunoassay (cLIA) and a total IgG Luminex immunoassay (LIA) could measure IgG neutralizing antibodies in matched maternal serum and fetal cord blood samples.
MATERIALS AND METHODS
Objective and study data.
The exploratory immunogenicity objective of this analysis was to characterize the titer of vaccine HPV-type immunoglobulins both in peripartum maternal blood and in the cord blood of infants born to women who received blinded therapy. These data were derived from a randomized, placebo-controlled, double-blind safety, immunogenicity, and efficacy study (protocol 019; NCT00090220). This study enrolled 3,819 women between the ages of 24 and 45 years from 38 international study sites between 18 June 2004 and 30 April 2005. Subjects were enrolled from community health centers, academic health centers, and primary health care providers in Colombia, France, Germany, Philippines, Spain, Thailand, and the United States, although the data for the current analysis come from subjects enrolled from either Philippines or Thailand.
Subjects.
Women were eligible to participate in the vaccine study from which these data were taken if they were not pregnant and if they had not undergone hysterectomy. Subjects were asked to use effective contraception through month 7 of the study. Women with a history of genital warts or current/past cervical disease were not eligible for enrollment. Those with prior cervical definitive therapy and those having undergone a cervical biopsy within the past 5 years were also excluded. Additionally, those subjects infected with human immunodeficiency virus (HIV) and those who were otherwise immunocompromised were not eligible for enrollment. Further information on enrollment criteria and subject characteristics has been published previously (2, 25).
Vaccine.
Subjects were randomized and received either quadrivalent HPV (types 6, 11, 16, and 18) L1 VLP vaccine (Gardasil/Silgard; Merck & Co., Inc., Whitehouse Station, NJ) or visually indistinguishable adjuvant-containing placebo at day 1 and months 2 and 6. Details of the quadrivalent HPV vaccine have been published previously (26).
Serum samples and immunogenicity analyses.
Maternal serum samples (“mother anti-HPV result”) were obtained at the time of infant delivery from a study participant who became pregnant during the study and gave birth to an infant who contributed a cord blood sample (“infant anti-HPV result”). These samples were referred to as a “mother-infant pair”.
Mother-infant pair sample levels of vaccine-type epitope-specific neutralizing anti-HPV antibodies were analyzed with a competitive Luminex-based immunoassay (cLIA) developed by Merck Research Laboratories, as described previously (27). Samples were also analyzed with a total IgG assay in order to determine the total IgG antibody production specific for HPV types analyzed.
Total IgG Luminex immunoassay.
A nine-valent total IgG Luminex immunoassay was developed utilizing yeast-derived L1 VLPs of HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58 coupled to a set of nine distinct fluorescent Luminex microspheres (28). Samples were tested in duplicate in this qualified research assay, and titers were determined relative to a 12-point standard curve. The median fluorescent intensity (MFI) of the bound phycoerythrin (PE)-tagged mouse anti-human IgG monoclonal antibody (MAb) clone HP6043 (Biotrend, Destin, FL) was captured as the raw VLP-specific bound total IgG direct binding data. This MAb binds equally to all four human IgG isotypes, IgG1 to IgG4 (17). For the purpose of this study, only data generated for the qHPV types (6, 11, 16, and 18) were collected as the test subjects received the qHPV vaccine. The correlation of MFI units with an arbitrary IgG milli-Merck unit per milliliter (IgG mMU/ml) of VLP-specific IgG was made by serially diluting the reference serum and interpolating the MFI data through a 4-parameter curve-fitting algorithm.
The defined serostatus cutoffs were set at a level that delineated HPV-negative samples above VLP adsorption-depleted serum background. The serostatus cutoffs determined for HPV types 6, 11, 16, and 18 in the total IgG LIA were 3.4, 2.2, 8, and 5 IgG mMU/ml, respectively.
cLIA.
The competitive Luminex immunoassay (cLIA) simultaneously evaluates the presence of conformational, neutralizing antibodies to the four HPV types present in the quadrivalent vaccine: HPV-6, -11, -16, and -18 (11, 27). On each VLP, there are neutralizing epitopes that may or may not be HPV type specific, as well as epitopes that are nonneutralizing (1, 3–8, 14, 29). The cLIA is a multiplex assay that involves the displacement of a phycoerythrin (PE)-labeled HPV type-specific, VLP-conformation dependent, neutralizing monoclonal antibody (MAb): H6.M48, K11.B2, H16.V5, and H18.J4 for the HPV-6, -11, -16, and -18 assays, respectively (11, 27). Luminex microspheres are coated with HPV type 6, 11, 16, and 18 L1 VLPs and incubated with the PE-labeled MAbs. Displacement of the PE-labeled MAb is an indirect measure of human serum antibody binding to the monitored neutralizing epitopes relative to the reference standard. Human serum was diluted 1:4 and tested. Titers are reported in arbitrary cLIA milli-Merck units per milliliter (cLIA mMU/ml) determined by the MFI correlation with the reference standard interpolated through a four-parameter curve-fitting algorithm. As a unique reference standard curve is generated for each HPV type, and because each HPV type employs a type-specific MAb with a unique binding affinity, the recorded cLIA mMU/ml titers are not equivalent and cannot be directly compared between HPV types.
The defined serostatus cutoffs were set at the levels that clearly delineated HPV-positive and HPV-negative samples, above serum background. The cLIA serostatus cutoffs were 20, 16, 20, and 24 mMU/ml for HPV types 6, 11, 16, and 18, respectively.
Correlation analysis.
For each HPV type, two-dimensional scatter plots were generated to show graphically the mother (vertical axis) and infant (horizontal axis) pair of anti-HPV antibodies. The concordance between the mother and infant anti-HPV antibodies was measured using the Pearson correlation coefficient. The distribution of the ratios of the infant to mother anti-HPV antibodies (i.e., infant-anti-HPV/mother-anti-HPV) was also summarized.
RESULTS
There were a total of 44 subjects (qHPV, 24; placebo, 20) with available mother-infant pair serology data whose data were considered for the antibody analysis. In general, the serum samples obtained at the time of infant delivery from mothers given placebo were seronegative for vaccine-type anti-HPV. There were only 4 mothers who received placebo whose serum samples obtained at the time of infant delivery were seropositive for HPV-6.
Among the subjects whose mother-infant pairs were used in the correlation analysis, the median time after vaccine dose 3 when the maternal serum and cord blood samples were obtained was 28 months (range, 14 to 43 months; interquartile range, 19 to 35 months). Relative to day 1, the median time after vaccine dose 1 when the maternal serum and cord blood samples were obtained was 34 months (range, 19 to 48 months; interquartile range, 24 to 40 months).
As seen in Table 1, the majority of infants whose mothers received qHPV vaccine and were seropositive for a vaccine HPV type were also seropositive for that HPV type. Overall, 67%, 83%, 92%, and 39% of infants were seropositive via the cLIA assay to HPV types 6, 11, 16, and 18, respectively, when their mothers were also seropositive at the time of birth. When all HPV-specific IgG molecules are considered, 84%, 65%, 100%, and 62% of infants were seropositive for HPV-6, -11, -16, and -18, respectively, when their mothers were also seropositive at the time of birth.
Table 1.
Summary of serostatus of mother-infant pairs (all mother-infant pairs with serology data)a
| HPV type | cLIA |
IgG assay |
||||||
|---|---|---|---|---|---|---|---|---|
| n | Mother's serostatusb | Infant's serostatusb |
n | Mother's serostatusc | Infant's serostatusc |
|||
| Positive m (%d) | Negative m (%d) | Positive m (%d) | Negative m (%d) | |||||
| 6 | 24 | Positive | 16 (66.7) | 4 (16.7) | 13 | Positive | 11 (84.6) | 0 (0.0) |
| Negative | 1 (4.2) | 3 (12.5) | Negative | 2 (15.4) | 0 (0.0) | |||
| 11 | 24 | Positive | 20 (83.3) | 0 (0.0) | 14 | Positive | 9 (64.3) | 0 (0.0) |
| Negative | 1 (4.2) | 3 (12.5) | Negative | 3 (21.4) | 2 (14.3) | |||
| 16 | 24 | Positive | 22 (91.7) | 1 (4.2) | 14 | Positive | 14 (100) | 0 (0.0) |
| Negative | 0 (0.0) | 1 (4.2) | Negative | 0 (0.0) | 0 (0.0) | |||
| 18 | 23 | Positive | 9 (39.1) | 1 (4.3) | 13 | Positive | 8 (61.5) | 0 (0.0) |
| Negative | 0 (0.0) | 13 (56.5) | Negative | 3 (23.1) | 2 (15.4) | |||
Abbreviations: n, number of mother-infant pairs contributing to the analysis; m, number of mother-infant pairs with the indicated serostatus; cLIA, competitive Luminex immunoassay.
Serostatus positive (negative) for HPV-6, -11, -16, and -18 is defined as HPV cLIA values of ≥ (<) 20, 16, 20, and 24 mMU/ml, respectively.
Serostatus positive (negative) for HPV-6, -11, -16, and -18 is defined as HPV IgG values of ≥ (<) 15, 15, 9, and 14 mMU/ml, respectively.
Percent is calculated as 100 × (m/n).
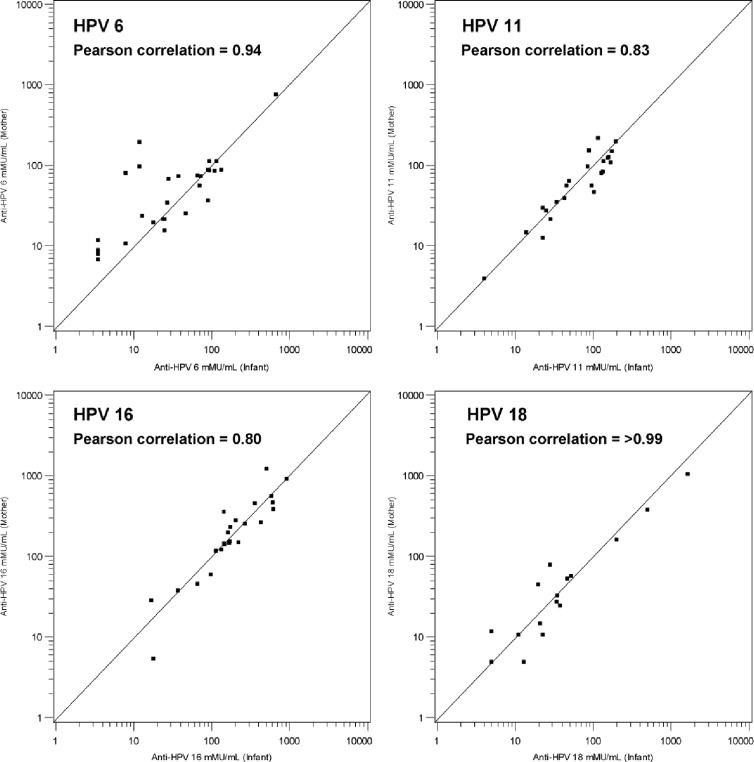
Figure 1 shows the scatter plot on the 2-dimensional xy plane of the HPV cLIA data points (x = cord blood anti-HPV result, y = maternal serum anti-HPV result) for each of HPV types 6, 11, 16, and 18. The data indicate that HPV titers in maternal serum samples are positively correlated with anti-HPV titers from infant cord blood samples. Pearson correlation coefficients of 0.94, 0.83, 0.80, and >0.99 were calculated for HPV-6, -11, -16, and -18, respectively.
Fig 1.
Scatter plots of mother-infant vaccine-type anti-HPV titers (all qHPV vaccinated with mother-infant serology result). The analysis of HPV-6 includes 4 mother-infant pairs such that the mother received placebo but had detectable anti-HPV-6.
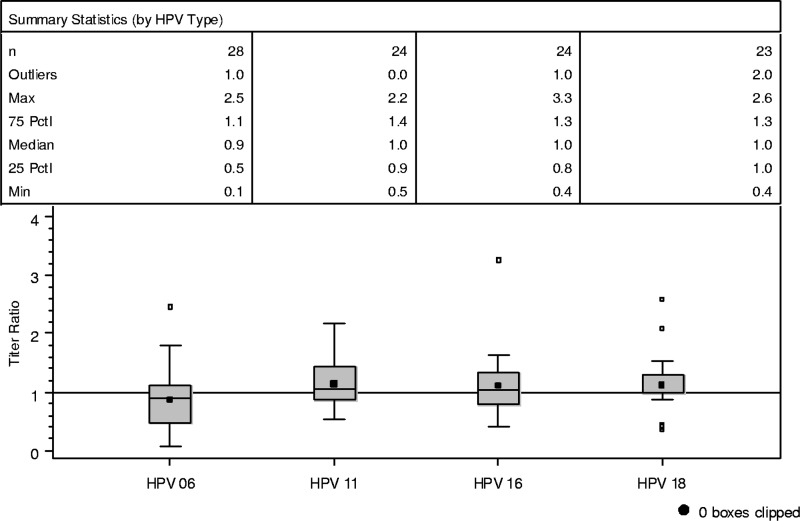
A plot of the distribution of the ratios of infant to mother cLIA anti-HPV results for each of the 4 vaccine HPV types is shown in Fig. 2. The distribution of the ratio of anti-HPV results centered on 1.0 further illustrates the highly concordant mother and infant anti-HPV antibody titers.
Fig 2.
Distribution of the ratios of infant to mother anti-HPV titers (all qHPV vaccinated with mother-infant serology result). The bottom and top edges of the box plot correspond to the 25th and 75th percentiles, respectively, of the distribution. The horizontal line inside the box represents the median of the distribution. The small solid square inside each box represents the arithmetic mean. The end of the bottom whisker corresponds to the minimum value of the distribution. The end of the top whisker represents the maximum value that is below the point equal to (75th percentile + 1.5× interquartile range). The small open squares above the top whisker represent extreme values that are greater than the point equal to (75th percentile + 1.5× interquartile range). The analysis of HPV-6 includes 4 mother-infant pairs such that the mother received placebo but had detectable anti-HPV-6. n, number of mother-infant pairs contributing to the analysis; Outliers, number of extreme anti-HPV values that are greater than the point equal to (75th percentile + 1.5× interquartile range); interquartile range, equal to 75th percentile − 25th percentile; Max, maximum anti-HPV value; Min, minimum anti-HPV value; Pctl, percentile.
DISCUSSION
For each of HPV types 6, 11, 16, and 18, maternal anti-HPV was found in infant cord blood samples. Furthermore, HPV titers in cord blood samples were highly positively correlated with maternal HPV titers. Moreover, there were instances when titers against vaccine HPV types that were no longer detectable in maternal serum samples were detected in cord blood samples, although the clinical relevance of this finding is unclear.
Newborn infants have immature immune systems, especially when the infant is born prematurely. This immature immune system is not fully capable of actively protecting against vaccine-preventable infections such as diphtheria, HPV infection, tetanus, and pertussis (9, 20, 30). Maternal immunoglobulins are transported across placental membranes during pregnancy by an active, receptor-mediated process. These antibodies are capable of protecting against infections until the infants' immune system has time to mature (24).
Transplacental transport of vaccine-induced antibodies has been shown to various degrees for a variety of vaccines, including vaccines against measles (16), varicella-zoster (23), rubella (22), and hepatitis A (21), among others. In the case of HPV, antibodies generated through qHPV vaccination and transported to the fetus across the placenta could potentially be capable of preventing infection.
ACKNOWLEDGMENTS
We thank all subjects taking part in this study, as well as all investigators and study coordinating personnel.
The study was sponsored by Merck, Sharp & Dohme Corp. S.M., O.B., S.V., and A.S. are employees of Merck, Sharp & Dohme Corp. and may hold stock and/or stock options in the company.
Footnotes
Published ahead of print 18 April 2012
REFERENCES
- 1. Carter JJ, et al. 2006. Identification of human papillomavirus type 16 L1 surface loops required for neutralization by human sera. J. Virol. 80: 4664–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Castellsague X, et al. 2011. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br. J. Cancer 105: 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Christensen ND, Kreider JW. 1990. Antibody-mediated neutralization in vivo of infectious papillomaviruses. J. Virol. 64: 3151–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Christensen ND, Kreider JW, Cladel NM, Galloway DA. 1990. Immunological cross-reactivity to laboratory-produced HPV-11 virions of polysera raised against bacterially derived fusion proteins and synthetic peptides of HPV-6b and HPV-16 capsid proteins. Virology 175: 1–9 [DOI] [PubMed] [Google Scholar]
- 5. Christensen ND, Kreider JW, Cladel NM, Patrick SD, Welsh PA. 1990. Monoclonal antibody-mediated neutralization of infectious human papillomavirus type 11. J. Virol. 64: 5678–5681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christensen ND, Kreider JW, Shah KV, Rando RF. 1992. Detection of human serum antibodies that neutralize infectious human papillomavirus type 11 virions. J. Gen. Virol. 73: 1261–1267 [DOI] [PubMed] [Google Scholar]
- 7. Christensen ND, Reed CA, Cladel NM, Hall K, Leiserowitz S. 1996. Monoclonal antibodies to HPV-6 L1 virus-like particles identify conformational and linear neutralizing epitopes on HPV-11 in addition to type-specific epitopes on HPV-6. Virology 224: 477–486 [DOI] [PubMed] [Google Scholar]
- 8. Culp TD, Spatz CM, Reed CA, Christensen ND. 2007. Binding and neutralization efficiencies of monoclonal antibodies, Fab fragments, and scFv specific for L1 epitopes on the capsid of infectious HPV particles. Virology 361: 435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'Angio CT. 2007. Active immunization of premature and low birth-weight infants: a review of immunogenicity, efficacy, and tolerability. Paediatr. Drugs 9: 17–32 [DOI] [PubMed] [Google Scholar]
- 10. Derkay CS. 1995. Task force on recurrent respiratory papillomas. A preliminary report. Arch. Otolaryngol. Head Neck Surg. 121: 1386–1391 [DOI] [PubMed] [Google Scholar]
- 11. Dias D, et al. 2005. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomavirus 6, 11, 16 and 18. Clin. Diagn. Lab. Immunol. 12: 959–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. FUTURE II Study Group 2007. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 356: 1915–1927 [DOI] [PubMed] [Google Scholar]
- 13. Garland SM, et al. 2007. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N. Engl. J. Med. 356: 1928–1943 [DOI] [PubMed] [Google Scholar]
- 14. Giroglou T, et al. 2001. Immunological analyses of human papillomavirus capsids. Vaccine 19: 1783–1793 [DOI] [PubMed] [Google Scholar]
- 15. Giuliano AR, et al. 2011. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N. Engl. J. Med. 364: 401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glick C, Feldman S, Norris MR, Butler J. 1998. Measles, mumps, and rubella serology in premature infants weighing less than 1,000 grams. South. Med. J. 91: 159–160 [DOI] [PubMed] [Google Scholar]
- 17. Hamilton RG. 1990. Engineered human antibodies as immunologic quality control reagents. Ann. Biol. Clin. (Paris) 48: 473–477 [PubMed] [Google Scholar]
- 18. Heim K, et al. 2007. Type-specific antiviral antibodies to genital human papillomavirus types in mothers and newborns. Reprod. Sci. 14: 806–814 [DOI] [PubMed] [Google Scholar]
- 19. Kashima HK, Mounts P, Shah K. 1996. Recurrent respiratory papillomatosis. Obstet. Gynecol. Clin. North Am. 23: 699–706 [PubMed] [Google Scholar]
- 20. Langkamp DL, Davis JP. 1996. Increased risk of reported pertussis and hospitalization associated with pertussis in low birth weight children. J. Pediatr. 128: 654–659 [DOI] [PubMed] [Google Scholar]
- 21. Linder N, et al. 1997. Placental transfer of hepatitis A antibodies in full term and preterm infants. Pediatr. Infect. Dis. J. 16: 245–247 [DOI] [PubMed] [Google Scholar]
- 22. Linder N, et al. 1999. Placental transfer of maternal rubella antibodies to full-term and preterm infants. Infection 27: 203–207 [DOI] [PubMed] [Google Scholar]
- 23. Linder N, et al. 2000. Placental transfer and decay of varicella-zoster virus antibodies in preterm infants. J. Pediatr. 137: 85–89 [DOI] [PubMed] [Google Scholar]
- 24. Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. 1996. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am. J. Reprod. Immunol. 36: 248–255 [DOI] [PubMed] [Google Scholar]
- 25. Munoz N, et al. 2009. Safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women between 24 and 45 years of age: a randomized, double-blind trial. Lancet 373: 1949–1957 [DOI] [PubMed] [Google Scholar]
- 26. Olsson S-E, et al. 2007. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like-particle vaccine. Vaccine 25: 4931–4939 [DOI] [PubMed] [Google Scholar]
- 27. Opalka D, et al. 2003. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16 and 18 by a multiplexed luminex assay. Clin. Diagn. Lab. Immunol. 10: 108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Opalka D, et al. 2010. Multiplexed serologic assay for nine anogenital human papillomavirus types. Clin. Vaccine Immunol. 17: 818–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ryding J, Dahlberg L, Wallen-Ohman M, Dillner J. 2007. Deletion of a major neutralizing epitope of human papillomavirus type 16 virus-like particles. J. Gen. Virol. 88: 792–802 [DOI] [PubMed] [Google Scholar]
- 30. Sadeghi K, et al. 2007. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J. Infect. Dis. 195: 296–302 [DOI] [PubMed] [Google Scholar]
- 31. Syrjanen S. 2005. Human papillomavirus (HPV) in head and neck cancer. J. Clin. Virol. 32: S59–S66 [DOI] [PubMed] [Google Scholar]