Abstract
Effective adjuvants capable of inducing strong cytotoxic T cell responses in humans are lacking. In this study, we tested 4-1BBL as an adjuvant for activation of human memory antiviral CD8 T cell responses ex vivo. A recombinant replication-defective 4-1BBL adenovirus was used to convert autologous monocytes into efficient antigen-presenting cells after overnight incubation, bypassing the need to generate dendritic cells. Together with viral peptides, 4-1BBL led to robust memory responses of human Epstein–Barr virus- and influenza virus-specific cytotoxic T cells, with expansion of peptide-specific CD8 effector cells; up-regulation of Bcl-xL, granzyme A, and perforin; enhanced cytotoxic activity; and increased cytokine production. The response was significant even at a 100-fold lower peptide dose, compared with responses obtained with control adenovirus. Adenovirus-delivered B7.1 also expanded and activated virus-specific CD8 T cells, but 4-1BBL was more effective in driving the T cells toward a more fully differentiated CD27– effector state. Thus, 4-1BBL is a promising adjuvant for human memory CD8 T cells and will likely be most effective in the boost phase of a prime-boost strategy.
Efficient activation of the cellular arm of the immune system requires a specific T cell antigen receptor signal delivered upon recognition of peptide/MHC together with costimulatory signals. It has now been well established that dendritic cells are potent antigen-presenting cells (APC) for initiation of immune responses (1). Upon maturation, these cells up-regulate costimulatory molecules required for T cell activation. As a result, there is now considerable interest in the use of dendritic cells loaded with antigens as adjuvants for therapeutic vaccines (2, 3). A limitation of this approach is the need to derive the syngeneic dendritic cells in culture, a process that takes 7 days. In this report we describe the conversion of monocytes into efficient APC for activation of T cell memory responses by overnight incubation with recombinant adenoviruses expressing costimulatory molecules.
Although the best characterized costimulatory molecule is CD28, recently other costimulatory molecules have been characterized (4–6). The emerging picture is that CD28 is important for the initial activation of an immune response and that other costimulatory ligand-receptor pairs act later to help sustain and diversify the response (4–6).
4-1BB is an inducible costimulatory member of the tumor necrosis factor receptor family expressed on activated CD4 and CD8 T cells. Its ligand, 4-1BBL, is expressed on activated APC (6, 7). 4-1BB can enhance both the proliferation and the survival of murine CD4 and CD8 T cells (8–14). Recent evidence in mouse models suggests that 4-1BB/4-1BBL interaction plays an important role in the memory CD8 T cell response to viruses (15–18).
In humans, several studies have looked at the role of 4-1BBL or anti-4-1BB in polyclonal activation of T cells, but the specific role of 4-1BBL in activation of antigen-specific memory T cells in humans has not been addressed (19–23). In view of the evidence in mice that 4-1BBL is important in CD8 T cell memory (18), we set out to directly test the ability of 4-1BBL to stimulate antiviral cytotoxic memory T cells from humans. The results show that 4-1BBL-mediated costimulation is highly effective in expanding and activating T cell memory responses to influenza virus and Epstein–Barr virus (EBV) and does so with faster kinetics than B7.1.
Materials and Methods
Tetramers, Antibodies, and Peptides. Class I peptide tetramers were produced as described (24). Peptides (purified to >90%) were obtained from the Alberta Peptide Institute (Edmonton, Alberta, Canada). The peptides used were influenza-M1 (GILG-FVFTL) and EBV-BMLF1 (GLCTLVAML). Class I HLA-peptide monomers were biotinylated with BirA enzyme (Avidity, Denver) and purified by gel filtration on an FPLC. Biotinylated monomers were then mixed with extravidin-phycoerythrin (Sigma-Aldrich) at a 4:1 molar ratio to form the tetramers.
Antibodies for CD3, CD4, CD28, HLA-A2, and HLA-DR were purified and labeled with FITC or biotin. Antibodies for CD8, CD14, CD27, CD28, CD45RA, and B7.2 were purchased from eBioscience (San Diego), and antibodies for 4-1BBL, B7.1, CCR7, CXCR3, IFN-γ, tumor necrosis factor α, IL-2, perforin, granzyme A, and Bcl-2 were purchased from BD Pharmingen. Bcl-xL-specific antibody was purchased from Southern Biotechnology Associates. CCR5- and CXCR4-specific purified antibodies were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (Bethesda), and labeled with FITC or biotin.
Generation of Recombinant Adenoviruses. Recombinant replication-defective adenovirus 5 (Adv) expressing full-length human 4-1BB ligand (4-1BBL-Adv) was generated by two-plasmid rescue method (25). Human full-length 4-1BBL cDNA was isolated (20) and cloned into EcoRI site of shuttle plasmid pDC104, and its sequence was confirmed. The purified shuttle plasmid was combined with rescue plasmid pBHGlox-delE1/E3.cre2 and cotransfected into 293 cells to rescue the adenovirus (25). 4-1BBL transgene expression was confirmed by fluorescence-activated cell sorting analysis of 4-1BBL-Adv-infected A549 cells. 293N3S cells were infected for large-scale virus production, viruses were purified from cell lysates by cesium chloride gradient ultracentrifugation, and viral titers were measured by plaque assay. A similar protocol was used for generation of human B7.1-Adv. Endotoxin levels in the adenovirus stocks were ≤0.06 units/ml (Limulus amebocyte lysate assay, BioWhittaker).
Donors, T Cell Purification, and APC Preparation. Eighty milliliters of venous blood was obtained from healthy volunteers, and peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Paque Plus gradient centrifugation (Amersham Biosciences). All donors gave informed consent, as approved by the University of Toronto human subjects review board. PBMC were used fresh or after freezing in 10% DMSO in a 50/50 FCS/medium mixture and storage in liquid nitrogen at –150°C. No significant differences between fresh and frozen samples were observed. Donors were screened for HLA-A2 by staining with the BB7.2 antibody. Donors were confirmed to be HLA-A*0201+ by using sequence-based HLA typing (provided by the Canadian Vaccines and Immunotherapeutics Network, Toronto). In most donors studied, a tetramer+ population was detectable in unstimulated PBMC, ranging from 0.05% to 0.3% of CD8+ T cells.
For costimulation cultures, fresh or freshly thawed PBMC were plated at 3.5 million per 48 wells for 1 h to allow the adherent fraction to attach to the plastic wells. The nonadherent fraction was removed and kept overnight at 37°C. After washing, control Adv, 4-1BBL-Adv or B7.1-Adv were added at a multiplicity of infection of 50 and the plate was centrifuged at 37°C at 1,643 × g for 1 h. Influenza matrix protein peptide (GILG-FVFTL) or the EBV-BMLF1 peptide (GLCTLVAML) dissolved in DMSO and diluted in medium were then added at the concentrations indicated. Irrelevant melanoma gp100 peptide (IMDQVPFSV) or DMSO in suspension medium were used as negative controls (indicated as no-antigen), with identical results. After overnight incubation with adenovirus and peptides, APC were washed twice with prewarmed medium. T cells were purified with a Pan T cell negative selection kit from Miltenyi Biotec (magnetic cell sorting). T cell purity was routinely >99.5%, as determined by flow cytometry. Purified T cells were added to adherent cell cultures at a concentration of 1 million per well of a 48-well dish. The cultures were stimulated for 7–9 days. In initial experiments, we compared cultures with and without exogenous IL-2 (added at 3 units/ml) and found that IL-2 did not improve T cell expansion and resulted in increased background, so remaining experiments were done without any exogenous cytokines.
Flow Cytometry. Samples were stained with tetramers at 37°C for 15 min, followed by a wash with cold buffer. All subsequent antibody stains were done on ice. For intracellular cytokine staining, samples were restimulated with 5 μM peptide for 5 h in the presence of GolgiPlug (BD Biosciences). Cells were stained for surface markers, followed by intracellular staining with anti-cytokine antibodies. The CytoFix/CytoPerm kit (BD Biosciences) was used to fix and permeabilize the cells for intracellular staining. FlowJo software was used for data analysis.
Cytotoxic T Lymphocyte (CTL) Assays. The human HLA-A2.1+ T2 cell line was used for target cells. Targets were pulsed with 3 μM influenza or EBV peptides and DMSO in suspension medium or irrelevant melanoma control peptides overnight. The next day, targets were labeled with 200 μCi of Na251 CrO4 and incubated with effector T cells for 4 h. Supernatant was analyzed for the release of radioactive chromium.
Results
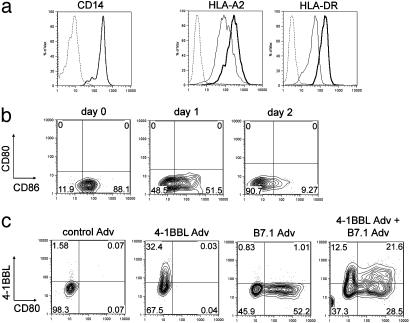
Delivery of Costimulatory Molecules to Donor APC by Means of Recombinant Adenovirus. Replication-defective recombinant adenoviruses were used to deliver costimulatory molecules to the adherent cell fraction of healthy donor PBMC. The adherent cell fraction was composed almost exclusively of CD14 monocytes (Fig. 1a), with no detectable B7.1 and negligible 4-1BBL (<2%) expressed on control adenovirus-treated cells (Fig. 1c). Some B7.2 (CD86) was detected on monocytes of most donors (Fig. 1b, day 0). Expression of CD86 decreased significantly after 2 days in culture on control or 4-1BBL-adenovirus-infected monocytes (Fig. 1b, days 1–2). Adherent monocytes expressed class I (HLA-A2) and class II (HLA-DR), both of which were up-regulated after infection with adenoviruses (Fig. 1a). The delivery of both costimulatory molecules through recombinant adenoviruses resulted in 32–55% of monocytes expressing 4-1BBL or B7.1 (Fig. 1c). The marker for human dendritic cells, CD83, was not detected on the adenovirus-infected adherent cells, as measured up to day 4 postinfection (data not shown).
Fig. 1.
Characterization of adherent cells and gene transfer efficiency with recombinant adenoviruses. (a) Adherent cells were stained for expression of CD14 (monocytes), HLA-A2, and HLA-DR before (thin lines) and 24 h after (thick lines) infection with adenovirus. Similar results were obtained by using control-Adv or 4-1BBL-Adv. The dashed lines indicate staining with isotype control antibody. (b) Monocytes were stained for B7.1 (CD80) and B7.2 (CD86) before (day 0) and 1 and 2 days after infection with 4-1BBL adenovirus. (c) Expression of B7.1 and 4-1BBL 24 h after infection with control or recombinant adenoviruses, as indicated. In b and c, the percentage of cells in each quadrant is indicated. Results are representative of three different donors.
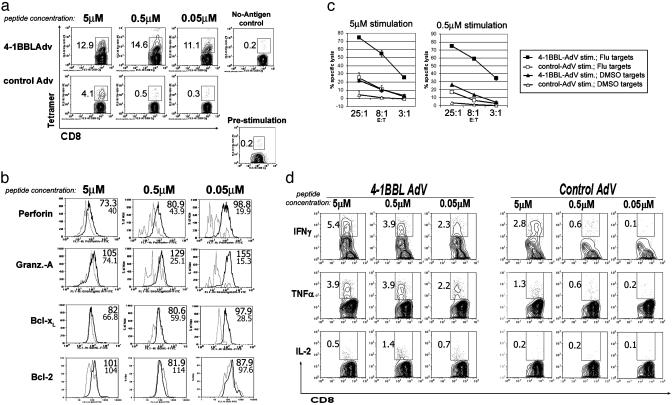
4-1BBL Acts as a Potent Adjuvant for Influenza- and EBV-Specific Memory CD8 T Cell Responses. To test the effect of 4-1BBL on memory responses to viruses, T cells from healthy donors were incubated with adherent monocytes that had been preactivated with 4-1BBL-Adv or control Adv plus influenza matrix peptides. 4-1BBL costimulation was tested over a 100-fold range of peptide concentrations and significantly enhanced the expansion of influenza-specific tetramer+ T cells over control adenovirus-treated cultures at all peptide doses. At the lowest peptide dose, a 35-fold enhancement of T cell expansion by 4-1BBL-Adv over control Adv-treated cultures was observed (Fig. 2a). Intracellular levels of perforin and granzyme A were consistently higher in 4-1BBL stimulated cultures, especially at lower peptide doses (Fig. 2b). The ability of 4-1BBL-stimulated T cells to kill influenza peptide-pulsed targets was consistently better than that observed with control adenovirus plus peptide-treated cultures, correlating with the increased expansion of tetramer+ cells and increases in effector molecule expression (Fig. 2c).
Fig. 2.
4-1BBL costimulates expansion and activation of influenza-specific memory CD8 T cells. T cells were cultured with adherent monocytes preincubated with 4-1BBL-Adv or control-Adv and influenza matrix peptide at the concentrations indicated. Seven days later, cells were analyzed for CD8 and HLA-A*0201/influenza matrix peptide tetramer staining (a). Before stimulation, total purified T cells contained memory CD8 T cells specific for the influenza matrix protein epitope (range 0.05–0.27% of CD8 T cells in seven donors). “No-Antigen control” indicates cultures incubated with irrelevant peptide or peptide suspension medium only. Data shown are gated on CD8+ cells. (b) Intracellular levels of perforin, granzyme A, Bcl-xL, and Bcl-2 were measured by intracellular flow cytometry. 4-1BBL-Adv-costimulated cultures (thick line) and control-Adv (thin line) are shown. All plots are gated on CD8+ influenza-tetramer+ T cells, as shown in a. Mean fluorescence intensities of CD8 tetramer+ population for 4-1BBL (top number) and control adenovirus cultures (bottom number) are indicated beside the histograms. (c) Killing of influenza peptide-coated T2 target cells by effector T cells from a, with 7-day stimulation conditions as indicated. (d) Cytokine production by 4-1BBL- or control-stimulated T cells after a 5-h restimulation in the presence of monensin and 5 μM influenza peptide, measured by intracellular flow cytometry. Concentrations above each plot indicates the concentration of peptide during the 8-day stimulation. Percent of cells in the indicated population are included in the plots for a and c. Similar results were obtained with six donors, with one to four experiments per donor. EBV-specific T cells showed similar results in three donors.
Expression of the survival protein Bcl-xL was found to be higher in 8 of 10 experiments in the 4-1BBL-costimulated as compared with control Adv cultures, mainly at lower doses of peptide (Fig. 2b). Bcl-2 levels, in contrast, were relatively constant. Cultures with 4-1BBL costimulation showed efficient production of cytokines after a 6-h restimulation with the peptide (Fig. 2d), whereas control adenovirus cultures showed moderate production of cytokines only in cultures that received a high dose of peptide during the 7-day culture period. In some donors, peptide restimulation for cytokine analysis was performed at 0.15 μM. Even this low dose of antigen resulted in nearly similar production of IFN-γ in the 4-1BBL-costimulated cultures, as compared to restimulation with 5 μM peptide (data not shown). Control adenovirus cultures showed no significant response at this low restimulation dose, suggesting 4-1BBL-costimulated T cells have an increased sensitivity and respond well at lower concentrations of peptide. The T cells produced mainly IFN-γ and tumor necrosis factor α and relatively small amounts of IL-2 (a phenotype associated with memory responses). Because IL-2 was not added to the cultures (see Materials and Methods), this small amount of IL-2 may be important in the observed CD8 T cell expansion.
Similar to the results observed with influenza-specific responses, 4-1BBL costimulation dramatically enhanced T cell responses to the EBV BMLF1 (GLCTLVAML) epitope. Strong 4-1BBL-dependent proliferation, up-regulation of survival markers and effector molecules, and enhancement in cytotoxic capability were observed (data not shown). Taken together, these results indicate that 4-1BBL is an efficient adjuvant for enhancing memory cytotoxic T cell responses to two different viruses. 4-1BBL was particularly effective at enhancing the response to low doses of peptide.
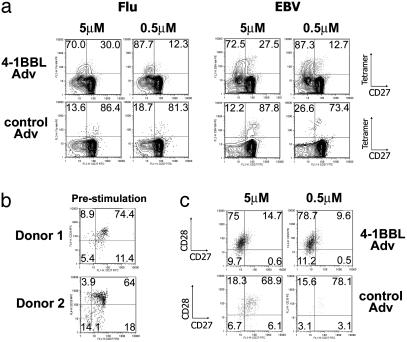
4-1BBL Costimulation Induces Mature CD27– Effector T Cells. The costimulatory molecules CD27 and CD28 have been used as markers of different subsets of memory T cells, differing in proliferative potential and cytotoxicity (26–28). The loss of CD27 is thought to herald a more committed effector cell, with lower proliferative potential and greater cytotoxicity (29). Most virus-specific CD8 T cells in 4-1BBL-costimulated cultures were CD27– (70–88%) (Fig. 3a). In contrast, only ≈10–25% of tetramer+ T cells were CD27– in control Adv cultures (Fig. 3a). Both influenza- and EBV-specific-responses exhibited a similar trend. Before stimulation, the majority of tetramer+ T cells (64–86%) were CD27+ CD28+ (Fig. 3b). In some donors CD27+ CD28– and CD27– CD28– populations were also found (Fig. 3b Lower). In most donors influenza-specific CD8 T cells remained CD28+ for the first 7 days after activation (Fig. 3c), consistent with a differentiation pathway that first results in the down-regulation of CD27, followed by CD28, at least for recently activated memory T cells. Thus, 4-1BBL costimulation drives the differentiation of memory T cells into more mature effector cells as measured by loss of CD27 and gain of effector function.
Fig. 3.
4-1BBL costimulation induces mature CD27– CD8 effector T cells. (a) T cells were cultured with adherent monocytes preincubated with 4-1BBL-Adv or control-Adv and EBV or influenza peptides. Seven days later expression of CD27 on tetramer+ CD8 T cells in influenza or EBV cultures was analyzed, with or without 4-1BBL costimulation. The percentage of tetramer+ cells that are CD27+ or CD27– are indicated. Data shown were gated on CD8+ cells. (b) Expression of CD27 and CD28 on influenza tetramer+ CD8 T cells in freshly isolated, unstimulated T cells in two donors. Most cells are double positive (range, 64–86%). EBV tetramer+ T cells contained more CD28– T cells. (c) Expression of CD27 and CD28 on tetramer+ T cells after a 7-day stimulation with 4-1BBL (Upper) or control Adv (Lower) plus peptide. Data shown were gated on CD8+ tetramer+ T cells. The percent of cells in each quadrant is indicated.
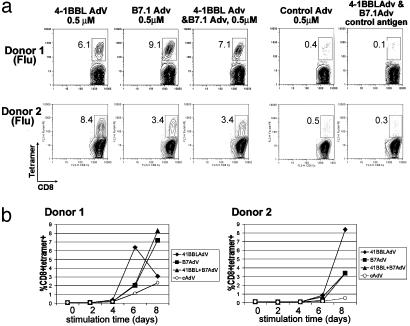
Comparison of 4-1BBL- Versus B7.1-Mediated Costimulation of Antiviral CD8 T Cell Responses. The preceding data suggest that 4-1BBL is an effective costimulatory molecule for memory cytotoxic T cells. To determine the effectiveness of 4-1BBL compared with a more conventional costimulation regimen, we set up parallel cultures in which 4-1BBL, B7.1, or both were delivered to the APC. In all five donors tested, 4-1BBL and B7.1 could each enhance T cell expansion when tested in isolation, albeit with some variability in efficacy among donors (Fig. 4a). Further kinetic analysis in additional experiments in four of the same donors showed that the weaker effect of 4-1BBL compared with B7.1 on day 8 in some experiments was due to the response developing more rapidly and exhausting the 4-1BBL cultures (Fig. 4b). Thus, taking into account the kinetic analysis, 4-1BBL is able to expand memory antiviral CD8 T cells with faster kinetics than B7.1 in all donors examined. Surprisingly, the combination of 4-1BBL and B7.1 did not show additive or synergistic effects over individual costimulatory molecules in any of the donors, even when analyzed at earlier time points (Fig. 4b).
Fig. 4.
Comparison of 4-1BBL versus B7.1-mediated costimulation of antiviral memory responses: 4-1BBL induces more rapid expansion of CD8 effector cells. (a) Expansion of tetramer+ T cells in response to influenza after 8 days of stimulation. Plots are gated on CD8+ events. The number in each plot indicates the percentage of CD8 T cells staining with tetramer. Data are representative of four donors (influenza) and two donors (EBV). (b) Kinetics of expansion of tetramer+ T cells in cultures with indicated recombinant adenoviruses as a function of stimulation time in days. Donor 1 in a and b represent two separate experiments with the same donor.
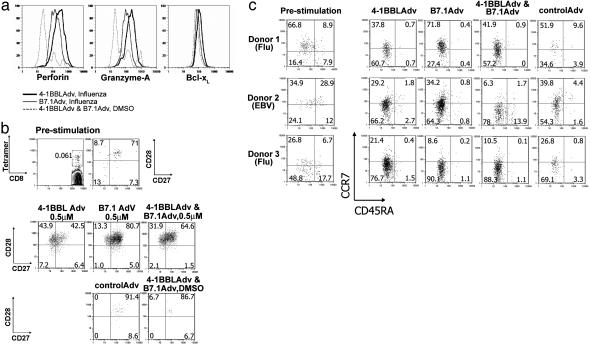
4-1BBL and B7.1 Costimulation and the Efficacy of Effector T Cells. The response to 4-1BBL, B7.1, or the combination was further analyzed by gating on the tetramer+ cells and visualizing the up-regulation of effector molecules. In six independent experiments on day 8 of stimulation, perforin and granzyme A were similar to or slightly higher in the 4-1BBL-costimulated cultures than in the B7.1 cultures (Fig. 5a and data not shown). Bcl-xL levels showed only a slight difference between the two costimulatory molecules. CTL responses in a standard chromium-release assay correlated with tetramer expansion (data not shown), indicating that the increase in perforin did not translate into an increase in CTL activity, at least in this assay. Analysis of four donors showed little or no difference between 4-1BBL- and B7.1-costimulated cultures in terms of IFN-γ production on day 8 (data not shown).
Fig. 5.
4-1BBL and B7.1 induce effective antiviral effector T cells. T cells were cultured with adherent monocytes preincubated with the indicated recombinant adenoviruses and pulsed with influenza matrix protein 1 peptide at 0.5 μM. Eight days later, cells were analyzed for intracellular levels of perforin, granzyme A, and Bcl-xL, measured by intracellular flow cytometry (a). 4-1BBL, B7.1, and no-antigen cultures are compared as indicated. (b) CD27 and CD28 expression, gated on CD8+ tetramer+ cells. Prestimulation profile is shown in Top and Middle.(c) Expression of CCR7 and CD45RA for three donors, before and after stimulation. Prestimulation expression is shown on the left, indicating central and effector memory T cell divisions. Data shown were gated on CD8+ tetramer+ cells, with the percentage of cells indicated in each quadrant.
Analysis of CD27 and CD28 expression on the activated T cells revealed that whereas the 4-1BBL-costimulated cultures showed the development of the more mature CD27– subset (Fig. 5b), this population was noticeably reduced in B7.1-stimulated cultures in four of seven experiments. The lack of a difference in some experiments appeared to be related to the time point analyzed, with day 7 showing a difference in all experiments, day 8 in most, and day 9 in none, even in the same donor. In most donors, effector T cells maintained the expression of CD28 for the 7–8 days of culture, but by 9 days higher numbers of CD28– T cells were observed in some donors, perhaps due to the accumulation of cytokines that are known to modulate the expression of CD28 (30–32). These data suggest that 4-1BBL can be more effective than B7.1 in driving human memory T cells into mature CD27– effector cells, at least under some conditions.
Freshly isolated tetramer+ cells from peripheral blood were mainly CD45RA– or CD45RAint (Fig. 5c), with either a predominantly CCR7+ population or a mixture of CCR7– and CCR7+ populations, suggesting they are comprised of both central memory and effector memory subsets (33). A donor with predominantly CCR7– effector memory T cells (donor 3) and a donor with predominantly CCR7+ central memory T cells (donor 1) both showed strong responses to 4-1BBL. Despite the apparent differences in some donors in CCR7 expression (compare donor 1 and 3 in Fig. 5c), the expanded tetramer+ T cells after 7–8 days of stimulation were of both CCR7+ and CCR7– phenotype, and the majority were CD45RA– (Fig. 5c). Thus, 4-1BBL costimulation can expand both CCR7+ and CCR7– effectors.
Discussion
The need to generate strong cell-mediated immunity, in particular a strong CD8 T cell response, is critical for eradication or control of many viral infections, including hepatitis C and HIV (34, 35). Both 4-1BBL and B7.1 are similar in their ability to costimulate expansion and development of effector function in memory T cells. Both 4-1BBL and B7.1 costimulation induced increased expression of perforin, granzyme A, and Bcl-xL, with some donors showing slightly higher increases in response to 4-1BBL compared with B7.1. 4-1BBL costimulation expanded human memory T cells with faster kinetics than B7.1 costimulation and was more effective in converting memory T cells into more mature CD27– effectors, particularly when analyzed earlier in the response. The ability to induce more efficient effector expansion may be critically important in chronic viral infections such as HIV, where HIV-specific effector T cells (as determined by tetramer staining) are unable to mature effectively and lack expression of perforin, an important cytotoxic mediator (36, 37).
The recent characterization of antiviral memory CD8 T cell subsets (26–28, 36), including the effector and central memory distinction (33), suggests not only that it will be important to expand effector cells, but also that it will be important for them to differentiate into memory/effector subset(s) able to act at the proper sites in the body to control disease. 4-1BBL costimulation was effective in donors where antiviral memory cells were predominantly CD45RA– CCR7hi (central memory) or CD45RA– CCR7low (effector memory) or a mixed population. At the end of the cultures, effector T cells in different donors had variable expression of CCR7 and the chemokine receptors CCR5, CXCR3, and CXCR4 (data not shown). Although not formally tested on isolated subsets, these results are consistent with a general costimulatory effect of 4-1BBL on human memory CD8 T cells, regardless of phenotype, yielding effector T cells with variable homing potential.
In the murine influenza model, 4-1BBL-mediated costimulation is important in recall responses (17, 18). The finding that 4-1BBL was able to expand memory virus-specific T cells with faster kinetics than B7.1-mediated costimulation (Fig. 4b) suggests that for human memory CD8 T cell responses signals through 4-1BB may also be important.
The apparent lack of synergy between 4-1BBL and B7.1 in our experiments was surprising. In contrast, we and others have observed increased effects by combining transfected 4-1BBL and anti-CD28 during polyclonal activation of human T cells (20, 23). The use of anti-CD28 avoids the inhibitory effect of B7.1/CTLA-4 interaction. In addition, in polyclonal activation studies, it is likely that naïve T cells are present in the cultures and respond to anti-CD3 plus anti-CD28, which may explain the differences observed. In the present study only primed antiviral memory CD8 T cells were likely to respond, because 7–9 days is not sufficient time to expand a naïve population. It is possible that memory T cells are unable to respond to both B7.1 and 4-1BBL simultaneously, perhaps due to competition for down-stream signaling intermediates (see ref. 38 for discussion). When both stimuli are given simultaneously, the kinetics of response parallels the response to B7 alone (Fig. 4b). Because CD28 is constitutively expressed and 4-1BB is inducible, it appears that the first interaction dominates the response.
The data presented here show that 4-1BBL expressed in a recombinant replication-defective adenovirus efficiently delivers costimulatory molecules to autologous APC. The viral delivery system has the added advantage of increasing MHC expression levels in the APC. Current methodologies for tumor vaccines often focus on the generation of dendritic cells from monocytes, a process that takes ≈7–10 days to accomplish (2). Recently, Wiethe et al. (39) modified dendritic cells with a combination of adenovirus-encoded 4-1BBL and adenovirus-encoded antigen and used the modified dendritic cells to induce antitumor CTL responses in mice. As shown here, we extend this approach by showing that autologous adherent monocytes can be converted into efficient APC with recombinant adenoviruses, a process that requires only an overnight incubation. Adenovirus-modified syngeneic APC could be used as immunogens directly (39, 40), or they could be used to activate patient T cells ex vivo for adoptive immunotherapy, an approach that is being developed for HIV therapy (41). As shown in Fig. 2, the inclusion of 4-1BBL in the stimulation reduces the effective antigen dose by >100-fold for both influenza- and EBV-specific responses. Thus, inclusion of 4-1BBL in a vaccine vector could serve to reduce the amount of vaccine required.
In conclusion, our results suggest that 4-1BBL delivered into syngeneic APC by recombinant adenovirus acts as an effective adjuvant for expansion and development of effector function in memory antiviral CTL originating from both acute (influenza) and latent (EBV) viral pathogens. Evidence suggests that 4-1BBL expands memory T cells more rapidly than B7.1 and in some cases generates more mature CD27– effectors. Based on the present results and analysis of anti-influenza responses in CD28–/– and 4-1BBL–/– mice (17), we suggest that B7.1 or innate inducers of B7 family members will be most useful during priming, whereas 4-1BBL will be most beneficial during the boost phase of a prime-boost regimen designed for CD8 T cell-mediated immunity. These results suggest that 4-1BBL should be given strong consideration for inclusion in antiviral therapeutic strategies.
Acknowledgments
We thank Kelly MacDonald for making available the HLA typing service and Rafick P. Sékaly for helpful discussion. This work was supported the Canadian Network for Vaccines and Immunotherapeutics funded by the Networks of Centers of Excellence Program. J.B. is funded by a Canadian Institutes for Health Research doctoral award.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Adv, adenovirus 5; 4-1BBL-Adv, recombinant replication-defective Adv expressing full-length human 4-1BBL ligand; PBMC, peripheral blood mononuclear cell; EBV, Epstein–Barr virus; APC, antigen-presenting cell; CTL, cytotoxic T lymphocyte.
References
- 1.Banchereau, J. & Steinman, R. M. (1998) Nature 392, 245–252. [DOI] [PubMed] [Google Scholar]
- 2.Schuler, G., Schuler-Thurner, B. & Steinman, R. M. (2003) Curr. Opin. Immunol. 15, 138–147. [DOI] [PubMed] [Google Scholar]
- 3.Steinman, R. M. & Pope, M. (2002) J. Clin. Invest. 109, 1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharpe, A. H. & Freeman, G. J. (2002) Nat. Rev. Immunol. 2, 116–126. [DOI] [PubMed] [Google Scholar]
- 5.Watts, T. H. & DeBenedette, M. A. (1999) Curr. Opin. Immunol. 11, 286–293. [DOI] [PubMed] [Google Scholar]
- 6.Croft, M. (2003) Cytokine Growth Factor Rev. 14, 265–273. [DOI] [PubMed] [Google Scholar]
- 7.Vinay, D. S. & Kwon, B. S. (1998) Semin. Immunol. 10, 481–489. [DOI] [PubMed] [Google Scholar]
- 8.Cannons, J. L., Lau, P., Ghumman, B., DeBenedette, M. A., Yagita, H., Okumura, K. & Watts, T. H. (2001) J. Immunol. 167, 1313–1324. [DOI] [PubMed] [Google Scholar]
- 9.Gramaglia, I., Cooper, D., Miner, K. T., Kwon, B. S. & Croft, M. (2000) Eur. J. Immunol. 30, 392–402. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin, R. G., Din, W. S., Davis-Smith, T., Anderson, D. M., Gimpel, S. D., Sato, T. A., Maliszewski, C. R., Brannan, C. I., Copeland, N. G., Jenkins, N. A., et al. (1993) Eur. J. Immunol. 23, 2631–2641. [DOI] [PubMed] [Google Scholar]
- 11.Chu, N. R., DeBenedette, M. A., Stiernholm, B. J., Barber, B. H. & Watts, T. H. (1997) J. Immunol. 158, 3081–3089. [PubMed] [Google Scholar]
- 12.DeBenedette, M. A., Shahinian, A., Mak, T. W. & Watts, T. H. (1997) J. Immunol. 158, 551–559. [PubMed] [Google Scholar]
- 13.Hurtado, J. C., Kim, Y. J. & Kwon, B. S. (1997) J. Immunol. 158, 2600–2609. [PubMed] [Google Scholar]
- 14.Takahashi, C., Mittler, R. S. & Vella, A. T. (1999) J. Immunol. 162, 5037–5040. [PubMed] [Google Scholar]
- 15.DeBenedette, M. A., Wen, T., Bachmann, M. F., Ohashi, P. S., Barber, B. H., Stocking, K. L., Peschon, J. J. & Watts, T. H. (1999) J. Immunol. 163, 4833–4841. [PubMed] [Google Scholar]
- 16.Tan, J. T., Whitmire, J. K., Murali-Krishna, K., Ahmed, R., Altman, J. D., Mittler, R. S., Sette, A., Pearson, T. C. & Larsen, C. P. (2000) J. Immunol. 164, 2320–2325. [DOI] [PubMed] [Google Scholar]
- 17.Bertram, E. M., Lau, P. & Watts, T. H. (2002) J. Immunol. 168, 3777–3785. [DOI] [PubMed] [Google Scholar]
- 18.Bertram, E. M., Dawicki, W., Sedgmen, B., Bramson, J. L., Lynch, D. H. & Watts, T. H. (2004) J. Immunol. 172, 981–988. [DOI] [PubMed] [Google Scholar]
- 19.Alderson, M. R., Smith, C. A., Tough, T. W., Davis-Smith, T., Armitage, R. J., Falk, B., Roux, E., Baker, E., Sutherland, G. R. & Din, W. S. (1994) Eur. J. Immunol. 24, 2219–2227. [DOI] [PubMed] [Google Scholar]
- 20.Wen, T., Bukczynski, J. & Watts, T. H. (2002) J. Immunol. 168, 4897–4906. [DOI] [PubMed] [Google Scholar]
- 21.Bukczynski, J., Wen, T. & Watts, T. H. (2003) Eur. J. Immunol. 33, 446–454. [DOI] [PubMed] [Google Scholar]
- 22.Laderach, D., Movassagh, M., Johnson, A., Mittler, R. S. & Galy, A. (2002) Int. Immunol. 14, 1155–1167. [DOI] [PubMed] [Google Scholar]
- 23.Maus, M. V., Thomas, A. K., Leonard, D. G., Allman, D., Addya, K., Schlienger, K., Riley, J. L. & June, C. H. (2002) Nat. Biotechnol. 20, 143–148. [DOI] [PubMed] [Google Scholar]
- 24.Altman, J. D., Moss, P. A. H., Goulder, P. J. R., Barouch, D. H., McHeyzer-Williams, M. G., Bell, J. I., McMichael, A. J. & Davis, M. M. (1996) Science 274, 94–96. [DOI] [PubMed] [Google Scholar]
- 25.Bett, A. J., Haddara, W., Prevec, L. & Graham, F. L. (1994) Proc. Natl. Acad. Sci. USA 91, 8802–8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamann, D., Baars, P. A., Rep, M. H., Hooibrink, B., Kerkhof-Garde, S. R., Klein, M. R. & van Lier, R. A. (1997) J. Exp. Med. 186, 1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hislop, A. D., Gudgeon, N. H., Callan, M. F., Fazou, C., Hasegawa, H., Salmon, M. & Rickinson, A. B. (2001) J. Immunol. 167, 2019–2029. [DOI] [PubMed] [Google Scholar]
- 28.Appay, V., Dunbar, P. R., Callan, M., Klenerman, P., Gillespie, G. M., Papagno, L., Ogg, G. S., King, A., Lechner, F., Spina, C. A., et al. (2002) Nat. Med. 8, 379–385. [DOI] [PubMed] [Google Scholar]
- 29.van Baarle, D., Kostense, S., van Oers, M. H., Hamann, D. & Miedema, F. (2002) Trends Immunol. 23, 586–591. [DOI] [PubMed] [Google Scholar]
- 30.Bryl, E., Vallejo, A. N., Weyand, C. M. & Goronzy, J. J. (2001) J. Immunol. 167, 3231–3238. [DOI] [PubMed] [Google Scholar]
- 31.Labalette, M., Leteurtre, E., Thumerelle, C., Grutzmacher, C., Tourvieille, B. & Dessaint, J. P. (1999) Int. Immunol. 11, 1327–1336. [DOI] [PubMed] [Google Scholar]
- 32.Borthwick, N. J., Lowdell, M., Salmon, M. & Akbar, A. N. (2000) Int. Immunol. 12, 1005–1013. [DOI] [PubMed] [Google Scholar]
- 33.Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. (1999) Nature 401, 708–712. [DOI] [PubMed] [Google Scholar]
- 34.Shoukry, N. H., Grakoui, A., Houghton, M., Chien, D. Y., Ghrayeb, J., Reimann, K. A. & Walker, C. M. (2003) J. Exp. Med. 197, 1645–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMichael, A. & Hanke, T. (2002) Nat. Rev. Immunol. 2, 283–291. [DOI] [PubMed] [Google Scholar]
- 36.Champagne, P., Ogg, G. S., King, A. S., Knabenhans, C., Ellefsen, K., Nobile, M., Appay, V., Rizzardi, G. P., Fleury, S., Lipp, M., et al. (2001) Nature 410, 106–111. [DOI] [PubMed] [Google Scholar]
- 37.Appay, V., Nixon, D. F., Donahoe, S. M., Gillespie, G. M., Dong, T., King, A., Ogg, G. S., Spiegel, H. M., Conlon, C., Spina, C. A., et al. (2000) J. Exp. Med. 192, 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cannons, J. L., Choi, Y. & Watts, T. H. (2000) J. Immunol. 165, 6193–6204. [DOI] [PubMed] [Google Scholar]
- 39.Wiethe, C., Dittmar, K., Doan, T., Lindenmaier, W. & Tindle, R. (2003) J. Immunol. 170, 2912–2922. [DOI] [PubMed] [Google Scholar]
- 40.Humrich, J. & Jenne, L. (2003) Curr. Top. Microbiol. Immunol. 276, 241–259. [DOI] [PubMed] [Google Scholar]
- 41.Levine, B. L., Bernstein, W. B., Aronson, N. E., Schlienger, K., Cotte, J., Perfetto, S., Humphries, M. J., Ratto-Kim, S., Birx, D. L., Steffens, C., et al. (2002) Nat. Med. 8, 47–53. [DOI] [PubMed] [Google Scholar]