Abstract
Soluble tumor necrosis factor receptors (TNFRs) are important modulators of TNF bioactivity. Proteolytic cleavage of the 28-kDa ectodomain of TNFR1 has been recognized as the mechanism by which soluble TNFR is shed. We now describe the release of exosome-like vesicles as a mechanism for the generation of soluble, full-length 55-kDa TNFR1. We found unexpectedly that the predominant form of soluble TNFR1 in human serum and lung epithelial lining fluid is a full-length 55-kDa protein. Furthermore, supernatants from human vascular endothelial cells contain only full-length 55-kDa TNFR1 that can be sedimented by high-speed centrifugation, floated on sucrose gradients at a density of 1.1 g/ml, and associated with vesicles that range in diameter from 20 nm to 50 nm. We conclude that the release of TNFR1 exosome-like vesicles represents a previously unrecognized mechanism by which constitutive production of soluble cytokine receptors may be regulated, independent of ectodomain cleavage by receptor sheddases.
Binding of tumor necrosis factor (TNF) to the 55-kDa, type I TNF receptor (TNFR1, TNFRSF1A, CD120a, p55) activates signaling pathways that regulate inflammatory, immune, and stress responses, as well as host defense and apoptosis (1). TNF signaling is negatively regulated at two levels to prevent excessive or inappropriate immune or inflammatory responses. First, constitutive TNFR1 signaling is prevented by the binding of silencer of death domains (SODD) to the TNFR1 intracytoplasmic domain (2). TNF binding to TNFR1 releases SODD, thereby allowing the formation of an active TNFR1 signaling complex. The second regulatory mechanism is shedding of cell surface TNF receptors to function as soluble TNF binding proteins that inhibit TNF bioactivity by competing with cell surface TNF receptors for free ligand. Soluble TNF receptors may also reversibly bind to and stabilize trimeric TNF, thereby prolonging its half-life and serving as a slow release reservoir for TNF when levels are low (3).
Soluble 27- to 30-kDa TNF-binding proteins, corresponding to the TNFR1 extracellular domain, were originally purified and isolated from human urine and serum (4–8). The demonstration by ELISA of TNFR1 molecules in culture supernatants from Chinese hamster ovary cells transfected with TNFR1 cDNA suggested that the soluble form is generated by proteolytic cleavage of the extracellular domain of cell surface receptors, rather than by alternative splicing (7). Sequence analysis identified the major TNFR1 cleavage site to be in the spacer region adjacent to the transmembrane domain between Asn-172 and Val-173, with a minor site between Lys-174 and Gly-175 (8–10). Further, the ability of hydroxamic-acid based metalloprotease inhibitors to block TNFR1 shedding suggested that proteolytic cleavage of cell surface TNFR1 receptors is mediated by a zinc metalloprotease (11). Consistent with this, TNF-α converting enzyme (TACE, ADAM 17), a member of the metalloprotease-disintegrin (ADAM) family of zinc metalloproteases, was identified as mediating TNFR1 shedding. This conclusion was based on the demonstration that TACE-deficient cells have lower ratios of shed to cell surface TNFR1 than cells reconstituted with TACE (12).
The goal of this study was to investigate further the mechanism of TNFR1 shedding. We unexpectedly found that the predominant form of soluble TNFR1 in human serum and lung epithelial lining fluid is the full-length 55-kDa protein. Therefore, we hypothesized that soluble TNFR1 could be generated by the release of vesicles containing full-length 55-kDa, membrane-associated TNFR1. We demonstrate that release of exosome-like vesicles is the predominant mechanism by which human vascular endothelial cells constitutively generate soluble TNFR1. We propose that exosome-like vesicle release represents a mechanism by which soluble cytokine receptors can be generated, independent of ectodomain cleavage by receptor sheddases.
Methods
Cells and Biological Fluids. Primary normal human umbilical vein vascular endothelial cells (HUVEC) were purchased from Cambrex (East Rutherford, NJ). Bronchoalveolar lavage fluid and serum samples were obtained from normal, healthy research volunteers who had provided informed consent and participated in research protocols (99-H-0076 and 96-H-100) that had been approved by the National Heart, Lung, and Blood Institute Institutional Review Board.
Isolation of Secreted Vesicles from Conditioned Medium by Sequential Centrifugation. Exosome-like vesicles were isolated as described, with minor modifications (13). HUVEC cells were grown in medium that contained FBS, which had been cleared of vesicles by centrifugation at 175,000 × g at 4°C for 16 h, and was determined not to contain soluble TNFR1 by ELISA and immunoblotting. Cell culture medium or bronchoalveolar lavage fluid was centrifuged at 200 × g, followed by 500 × g for 10 min to remove cells and debris. Samples were sequentially centrifuged for 20 min at 1,200 × g, followed by 35 min at 10,000 × g, in a SS34 rotor (Sorvall), and for 1.5 h at 100,000 × g, and for 16 h at 175,000 × g, in a S70Ti rotor to sediment small vesicles (Beckman-Coulter). Concentrated samples of HUVEC-conditioned medium and bronchoalveolar lavage fluid were prepared by initial centrifugation at 500 × g for 10 min and 10,000 × g for 35 min to remove cells and debris, followed by centrifugation in a Centriprep filtration device with a 3,000-kDa exclusion (Millipore).
For immunoblotting experiments, pellets were suspended in 50 mM Tris·HCl, pH 7.2/150 mM NaCl, pH 7.2/0.1% Triton X-100 with Complete protease inhibitor (Roche Diagnostics); protein concentrations were determined by using the bicinchoninic acid protein determination kit (Pierce). Proteins in sedimented vesicles (60 μg) were separated via SDS/PAGE by using 4–12% Bis–Tris Nupage gels (Invitrogen), electroblotted onto nitrocellulose membranes, and incubated overnight (4°C) with a murine IgG2b monoclonal antibody (2 μg/ml) that reacts with the extracellular domain of human TNFR1 (H-5, Santa Cruz Biotechnology). Membranes were stripped by using the Re-blot Western Blot Recycling Kit (Chemicon) and reacted with antibodies directed against rab5 (Santa Cruz Biotechnology) and lysosome-associated membrane protein 1 (LAMP-1) (BD Transduction Laboratories). The identity of exosome-associated TNFR1 was confirmed with a murine IgG1 monoclonal antibody (2 μg/ml) that also reacts with the TNFR1 extracellular domain (clone 16805.21, R & D Systems) and a goat polyclonal antibody (1 μg/ml) that reacts with the TNFR1 intracytoplasmic domain (C-20, Santa Cruz Biotechnology). IgG1 and IgG2b isotype controls were from Sigma. Immunoblots were also performed with antibodies that react with SODD (Santa Cruz Biotechnology), TNF receptor-associated death domain (TRADD) (Upstate Biotechnology), receptor-interacting protein (RIP) (BD Transduction Laboratories), TNF-receptor-associated factor 2 (TRAF2) (Imgenex, San Diego), and poly(ADP-ribose) polymerase (Clontech). The recombinant human TNFR1 extracellular domain protein was purchased from R & D Systems. Densitometry was performed by using nih image software.
Lipid Raft Isolation. Lipid rafts were isolated as described with minor modifications (14). Briefly, vesicles (2 mg) were isolated from HUVEC medium as described above and suspended in 1.5 ml of TNX buffer (50 mM Tris·HCl, pH 7.2/150 mM NaCl, pH 7.2 and 1% Triton X-100 with Complete protease inhibitor) and mixed with 1.5 ml of 90% sucrose in TNX buffer. Alternatively, HUVEC (T-175 flask) were lysed on ice with 1.5 ml of TNX buffer for 20 min, disrupted (10 strokes) with a glass dounce homogenizer, and mixed with 1.5 ml of 90% sucrose in TNX buffer. Samples were overlaid with 3 ml of 35% sucrose and 3 ml of 5% sucrose and centrifuged at 175,000 × g for 16 h at 4°C. Fractions (1 ml) were collected from the top and analyzed by immunoblotting. Cholera toxin B subunit–peroxidase conjugate (CTxHRP) was from Sigma.
Rate Zonal Centrifugation Through Continuous Sucrose Gradients. Samples of conditioned medium from HUVEC were centrifuged at 500 × g for 10 min and 10,000 × g for 30 min to remove cellular debris. Vesicles were sedimented by centrifugation at 175,000 × g for 16 h and suspended in endothelial cell media without FBS. Vesicles (2 mg) were overlaid on a continuous sucrose gradient (0.2–2.5 M in 20 mM Tris, pH 8.0) and centrifuged at 200,000 × g for 16 h. Fractions (0.5 ml) were collected from the bottom of the gradient, and proteins were quantified. Samples of proteins (60 μg) were precipitated with 10% trichloroacetic acid, as necessary, and analyzed by immunoblotting. A Palm Abbe Digital Refractometer (Misco, Cleveland) was used for densitometry.
Immunoelectron Microscopy. Immunoelectron microscopy was performed as described, with minor variations (13). Samples of HUVEC-conditioned medium or bronchoalveolar lavage fluid were centrifuged at 500 × g for 10 min and 10,000 × g for 30 min to remove cellular debris and concentrated by using a Centriprep filtration device with a 3,000-kDa exclusion. Droplets were applied to Formvar–carbon-coated nickel electron microscopy grids and fixed in 4% paraformaldehyde for 15 min. Samples were immunolabeled with either the murine monoclonal antibody directed against the human TNFR1 extracellular domain or the IgG2b isotype control (10 μg/ml), washed four times for 5 min in washing buffer (1% BSA in PBS), and reacted with a goat anti-mouse secondary antibody complexed with 5-nm gold particles (Amersham Pharmacia). Images were acquired with a 1200 EX transmission electron microscope (Jeol).
Exosome Binding of TNF-α. Exosome-like vesicles were isolated from HUVEC medium as described above, resuspended in serum-free medium, and incubated with 1 ng of recombinant human TNF-α (Promega) for 16 h at 4°C. Exosomes were sedimented by high-speed centrifugation at 175,000 × g for 16 h at 4°C and resuspended in SDS/PAGE loading buffer, and immunoblots were performed as described above by using a rabbit polyclonal antibody that reacts with TNF (Santa Cruz Biotechnology). HUVEC-derived exosomes were also selectively immunoprecipitated with a monoclonal antibody directed against the TNFR1 extracellular domain (clone 16805.21, R & D Systems) that was bound to a 96-well plate. The immunoprecipitated exosomes were washed with PBS three times and incubated with recombinant human TNF-α (rhTNF-α) for 2 h at room temperature, followed by washing. Bound rhTNF-α was measured by ELISA using a horseradish peroxidase-conjugated goat polyclonal anti-TNF-α antibody (R & D Systems). Nonspecific binding was controlled for by measuring TNF-α binding in the absence of exosomes.
Quantitation of Soluble TNFR1. HUVEC were grown to confluence in six-well plates and treated either with vehicle (0.1% DMSO), 25 μM benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD-FMK) (Calbiochem), or 50 μM TNF-α protease inhibitor (TAPI-2) (Peptides International) for 24 h. Z-VAD-FMK was dissolved in DMSO, whereas TAPI-2 was dissolved in endothelial cell medium. Cell viability was determined by using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega). Release of TNFR1 into HUVEC cell culture supernatants over a 24-h period was assayed by using a sandwich ELISA with a sensitivity of 7.8 pg/ml (R & D Systems). Statistical analysis was performed by a paired Student's t test with a Bonferroni correction for multiple comparisons. A P value <0.05 was considered significant.
Results
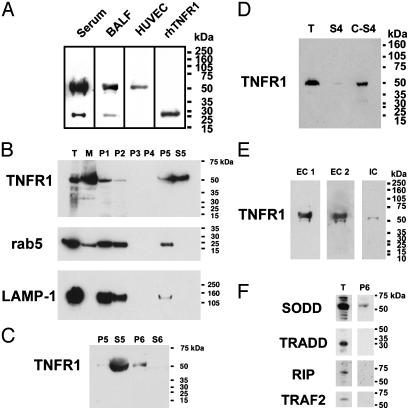
Full-Length 55-kDa Soluble TNFR1 Is Present in Human Serum and Lung Epithelial Lining Fluid. Immunoblots, using the H-5 murine monoclonal antibody directed against the human TNFR1 extracellular domain, were performed on samples of human serum and concentrated bronchoalveolar lavage fluid (BALF), as well as on conditioned HUVEC medium. As shown in Fig. 1A, soluble TNFR1 existed predominantly as a full-length 55-kDa protein in human serum and lung epithelial lining fluid. A 28-kDa species, consistent with the TNFR1 ectodomain generated by proteolytic cleavage, was also present. This pattern was present in all eight serum samples tested. In contrast, only the full-length 55-kDa TNFR1 was present in concentrated samples of conditioned medium from HUVEC. No 28-kDa TNFR1 was detected even after concentrating the medium 200-fold. A recombinant human TNFR1 ectodomain protein is shown for comparison as a positive control. Because the full-length 55-kDa TNFR1 is an integral type I membrane protein, we hypothesized that it might be associated with a membrane-containing structure, such as an exosome-like vesicle or a microvesicle generated by membrane vesiculation or blebbing. These findings led us to conclude that constitutive generation of soluble TNFR1 may occur by two distinct pathways in vivo; proteolytic cleavage of the TNFR1 ectodomain to generate a 28-kDa soluble isoform or sTNFR1, and the release of exosome-like vesicles containing the full-length, membrane-bound, 55-kDa TNFR1 or exosome-associated TNFR1 (eTNFR1).
Fig. 1.
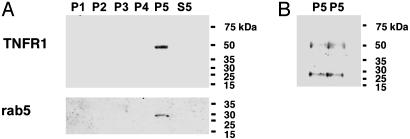
(A) Full length 55-kDa is the predominant form of soluble TNFR1 present in human serum and lung epithelial lining fluid. Samples of proteins (60 μg) of serum and BALF from healthy research volunteers, or medium from HUVEC, were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with a murine IgG2b monoclonal antibody (H-5) against the extracellular domain of human TNFR1. A recombinant human TNFR1 extracellular domain (rhTNFR1) is shown as a positive control. (B) Characterization of HUVEC-derived soluble TNFR1 as the full-length 55-kDa receptor by sequential centrifugation. Samples of proteins (60 μg) from the pellets [200 × g (P1), 500 × g (P2), 1,200 × g (P3), 10,000 × g (P4) or 100,000 × g (P5)], and the 100,000 × g supernatant (S5) from HUVEC medium, were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with the H-5 anti-TNFR1 antibody. Also shown are total cellular (T) and membrane (M) proteins. Nitrocellulose membranes were stripped and reacted with antibodies against rab5 and LAMP-1. (C) Characterization of HUVEC-derived soluble TNFR1. Sequential centrifugations were performed as described in B. Samples of proteins (60 μg) from the pellets [100,000 × g (P5), 175,000 × g (P6)] and supernatants [100,000 × g (S5), 175,000 × g (S6)] from HUVEC medium were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with the H-5 anti-TNFR1 antibody. These immunoblots are representative of three experiments demonstrating similar results. (D) Characterization of HUVEC-derived soluble TNFR1. Samples of proteins (40 μg) from HUVEC lysates (T) and medium that had been precleared of cells and debris by sequential centrifugation at 1,200 × g and 10,000 × g (S4) were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with the H-5 anti-TNFR1 antibody. C-S4 denotes a 30-fold concentration of the S4 supernatant. (E) Confirmation of TNFR1 in HUVEC-derived secreted vesicles. Samples of proteins (60 μg) from the 175,000 × g pellet (P6) were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with the H-5 (EC 1) and the clone 16805.21 (EC 2) murine monoclonal antibodies directed against the TNFR1 extracellular domain and the C-20 goat polyclonal antibody directed against the TNFR1 intracytoplasmic domain. (F) Characterization of the TNFR1 signaling complex I in HUVEC-derived exosome-like vesicles. Samples of proteins (60 μg) from whole cell lysates (T) and the 175,000 × g pellet (P6) were separated by SDS/PAGE, transferred to nitrocellulose and reacted with antibodies against SODD, TRADD, RIP, and TRAF2.
Human Vascular Endothelial Cell-Derived Soluble TNFR1 Is Constitutively Released as a Full-Length 55-kDa Protein. We used human vascular endothelial cells to characterize further the mechanism of release of soluble TNFR1. We reasoned that if the full-length 55-kDa TNFR1 isoform in HUVEC medium was associated with a membrane, it should be sedimented by high speed centrifugation, as had been described for exosomes (13). As shown in Fig. 1B, full-length 55-kDa TNFR1 was present in HUVEC total cell lysates (T) and in membrane fractions (M) by using the H-5 murine monoclonal antibody directed against the TNFR1 extracellular domain. Full-length 55-kDa TNFR1 was also sedimented by centrifugation of HUVEC medium at 200 × g and 500 × g, consistent with sedimentation of cellular debris, but was not detected in the 1,200 × g and 10,000 × g pellets. Soluble full-length 55-kDa TNFR1 was partially sedimented by centrifugation at 100,000 × g for 1 h and completely sedimented by centrifugation at 175,000 × g for 16 h (Fig. 1C). Although rab5, a small GTPase constituent of early endosomes, and LAMP-1, a membrane glycoprotein constituent of lysosomes, were both present in the 100,000 × g pellet, neither colocalized with TNFR1 in the 100,000 × g supernatant (Fig. 1B) or the 175,000 × g pellet (data not shown). These experiments demonstrate that HUVEC-derived soluble TNFR1 exists as a full-length 55-kDa receptor that can be sedimented by high-speed centrifugation at 175,000 × g, which is consistent with localization to a small, secreted vesicle.
Additional experiments were performed to confirm the presence of TNFR1 within exosome-like vesicles. First, we compared TNFR1 present in HUVEC whole cell lysates to 24-h conditioned medium that had been precleared of cells and debris by sequential centrifugation at 1,200 × g (10 min) and 10,000 × g (30 min), then concentrated 30-fold. Immunoblots revealed the presence of full-length 55-kDa TNFR1 in HUVEC whole cell lysates and concentrated medium, as well as a faint band in the unconcentrated medium (Fig. 1D). Experiments were also performed to confirm the identity of exosome-associated TNFR1. Exosome-like vesicles were sedimented by high-speed centrifugation at 175,000 × g from medium as described above. As shown in Fig. 1E, full-length 55-kDa TNFR1 was identified in exosome-like vesicles on immunoblots by using three different antibodies; murine IgG2b (H-5) and IgG1 (clone 16805.21) monoclonal antibodies that react with the TNFR1 extracellular domain and a goat polyclonal antibody (C-20) that reacts with the TNFR1 intracytoplasmic domain. No signal was detected by using IgG1 and IgG2b isotype controls. The H-5 anti-TNFR1 antibody was used for all subsequent experiments.
TNFR1 Exosomes Do Not Contain an Active Signaling Complex I. We next assessed whether exosome-associated TNFR1 contains an active signaling complex I, comprised of TRADD, RIP, and TRAF2 (15). As shown in Fig. 1E, neither TRADD, RIP, nor TRAF2 were present in the 175,000 × g pellet. In contrast, SODD, a negative regulatory protein that is associated with the death domain region of the TNFR1 intracytoplasmic domain, which prevents TRADD binding and maintains TNFR1 in an inactive state, was present in the 175,000 × g pellet (2). Taken together, these data suggest that exosome-associated TNFR1 does not possess intrinsic signaling capabilities because of the absence of an active TNFR1 signaling complex I.
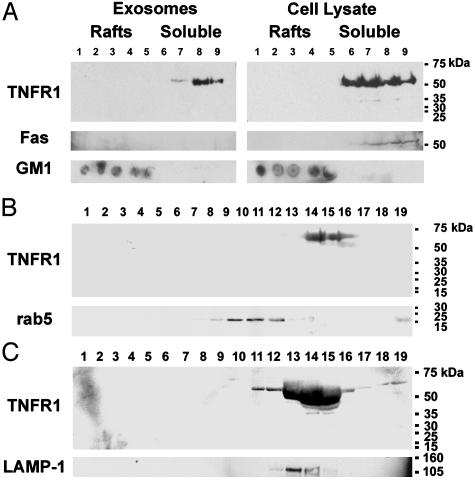
TNFR1 Exosomes Do Not Contain Lipid Raft Microdomains. TNFR1 is localized to Triton X-100 soluble cellular fractions under basal conditions and translocates to lipid raft microdomains upon TNF binding to form an active signaling complex I (14). Therefore, we assessed whether TNFR1 exosomes are also localized to Triton X-100 soluble fractions under basal conditions. Exosome-like vesicles from HUVEC medium and lysates were solubilized in 1% Triton X-100 and subjected to discontinuous sucrose density gradient centrifugation. TNFR1 from HUVEC lysates and TNFR1-exosomes partitioned to Triton X-100 soluble, nonlipid raft fractions (Fig. 2A). Fas, a marker of nonlipid raft fractions, colocalized with TNFR1 in the cellular lysates, but was not a constituent of exosomes. The lipid raft microdomain marker, glycosphingolipid GM1, floated to the light fractions and did not colocalize with TNFR1. Therefore, under basal conditions, eTNFR1 is inactive, based on its association with SODD, its localization to nonlipid raft membrane domains, and the absence of signaling complex I.
Fig. 2.
Characterization of HUVEC-derived exosome-associated TNFR1 by density gradient centrifugation. (A) HUVEC-derived TNFR1 exosomes are not comprised of lipid raft microdomains. Vesicles (2 mg) were isolated from HUVEC medium, suspended in 1% Triton X-100 at 4°C, and subjected to sucrose gradient centrifugation to isolate lipid raft microdomains. HUVEC were also lysed in 1% Triton X-100 at 4°C, and lipid raft microdomains were similarly isolated. Fractions (1 ml) were collected from the top, and 60 μg of protein from each fraction were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with antibodies against TNFR1. Nitrocellulose membranes were stripped and reacted with an antibody against Fas, a marker of Triton X-100 soluble fractions. The distribution of GM1, a marker of lipid raft microdomains, was detected by dot blotting 3 μl of each fraction onto nitrocellulose membranes and detected by using cholera toxin B subunit–peroxidase conjugate (CTxHRP). (B) Rate zonal centrifugation through continuous sucrose gradients. Vesicles in HUVEC medium were sedimented by centrifugation at 175,000 × g for 16 h (4°C) and resuspended in medium. A sample containing 2 mg of vesicles was layered on top of a continuous sucrose gradient (0.2–2.5 M in 20 mM Tris, pH 8) and centrifuged at 200,000 × g for 16 h. Fractions (0.5 ml) were collected from the bottom, and samples of proteins (60 μg) were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with antibodies against TNFR1. Nitrocellulose membranes were stripped and reprobed with antibodies against rab5 (B) or LAMP-1 (C). Lane numbers correspond to the fractions collected. Molecular mass markers are shown on the right of each immunoblot. These immunoblots are representative of duplicate experiments demonstrating similar results.
Localization of HUVEC-Derived Soluble TNFR1 to Secreted Exosome-Like Vesicles. To characterize further the localization of HUVEC-derived soluble TNFR1 to secreted exosome-like vesicles, we performed rate zonal centrifugation through continuous sucrose gradients. HUVEC-derived secreted vesicles (2 mg) were sedimented by high-speed centrifugation at 175,000 × g for 16 h, layered on top of a continuous sucrose gradient (0.2–2.5 M), and centrifuged at 200,000 × g for 16 h. As shown in Fig. 2, full-length 55-kDa TNFR1 floated at a peak density of 1.1 g/ml, consistent with its localization to an exosome-like vesicle. TNFR1, however, did not colocalize with rab5, and only partially colocalized with LAMP-1.
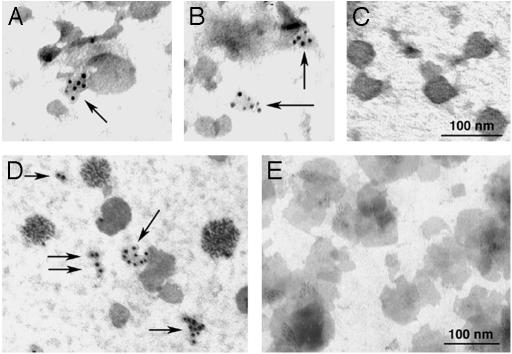
Electron microscopy was performed on BALF and HUVEC medium to visualize sTNFR1 in secreted vesicles. ImmunoGold labeling localized TNFR1 to small, irregularly shaped vesicles of ≈20–50 nm in diameter, in both BALF (Fig. 3 A and B) and HUVEC medium (Fig. 3D). No signal was detected with the IgG2b isotype control (Fig. 3 C and E). Taken together, these data demonstrate that TNFR1 is released as a full-length 55-kDa receptor that is associated with small, exosome-like vesicles in BALF and HUVEC medium.
Fig. 3.
Localization of TNFR1 to exosome-like vesicles by immunoelectron microscopy. Samples of bronchoalveolar lavage fluid (A–C) and HUVEC medium (D and E) were centrifuged at 500 × g for 10 min and 10,000 × g for 30 min to remove cellular debris. Samples were then concentrated by using a Centriprep filter with a 3,000-kDa exclusion, applied to Formvar-carbon-coated nickel electron microscopy grids, and immunostained with the antibodies against TNFR1. As shown by ImmunoGold labeling, TNFR1 was localized to small, irregularly shaped vesicles of 20–50 nm in diameter (arrows), consistent with exosome-like vesicles in both bronchoalveolar lavage fluid (A and B) and HUVEC-conditioned media (D). No ImmunoGold labeling was detected when ImmunoGold labeling was performed with an IgG2b isotype control antibody (C and E). These images are representative of duplicate experiments demonstrating similar results.
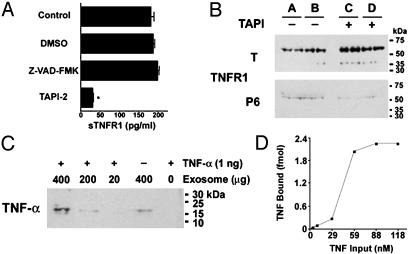
TNFR1 Exosome-Like Vesicle Release From Human Vascular Endothelial Cells Requires Zinc Metalloprotease Catalytic Activity. We next investigated whether eTNFR1 release requires zinc metalloprotease catalytic activity by treating HUVEC cells with the broad-spectrum zinc metalloprotease inhibitor, TAPI-2. TAPI-2 is known to attenuate the release of sTNFR1 and is thought to function by inhibiting the activity of TNFR1 receptor sheddases, such as TACE, thereby preventing the proteolytic cleavage of the TNFR1 ectodomain (12, 16). As shown in Fig. 4A, 50 μM TAPI-2 significantly inhibited the quantity of TNFR1 released into HUVEC medium over a 24-h period, as measured by ELISA. Similarly, treatment with 50 μM TAPI-2 for 24 h significantly decreased the quantity of full-length 55-kDa TNFR1 sedimented from HUVEC medium after centrifugation at 175,000 × g for 16 h (Fig. 4B). Reciprocally, there was an increase in cell-associated TNFR1 levels in TAPI-2-treated cells as compared to cells treated with media alone. These data demonstrate that TAPI-2-mediated inhibition of zinc metalloprotease activity decreases the release of eTNFR1. In contrast, the pan-caspase inhibitor Z-VAD-FMK had no effect on the quantity of TNFR1 released into HUVEC medium (Fig. 4A), which is consistent with the conclusion that the release of TNFR1-containing vesicles did not occur as a consequence of apoptotic membrane blebbing. Similarly, cleavage of poly(ADP-ribose) polymerase, an early marker of apoptosis, was not detected in cells treated with either TAPI-2 or control medium for 24 h (data not shown). Furthermore, there was no difference in cell viability or proliferation as determined in cells treated with TAPI-2 or control medium for 24 h (data not shown). Taken together, these experiments suggest that the release of eTNFR1 requires the catalytic activity of a zinc metalloprotease, but does not involve the proteolytic cleavage of TNFR1 ectodomain by a receptor sheddase.
Fig. 4.
(A) Zinc metalloprotease activity is required for soluble TNFR1 release. HUVEC were treated with 0.1% DMSO, 25 μM Z-VAD-FMK, or 50 μM TAPI-2 for 24 h, and concentrations of sTNFR1 present in culture supernatants were determined by ELISA (n = 6). *, P < 0.05 vs. DMSO-treated cells. (B) Effect of TAPI-2 on TNFR1 exosome-like vesicle release. HUVEC were treated with (+)(C and D) or without (–) (A and B) 50 μM TAPI-2 for 24 h, and medium was subjected to sequential centrifugation. Samples of proteins (60 μg), in duplicate, from whole cell lysates (T) and 175,000 × g (P6) pellets were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with antibodies against TNFR1. The average relative mean density of the TAPI-2-treated whole cell lysates was 1.56 as compared with 1.0 for the cells treated with medium alone, whereas the relative mean density of the TAPI-2-treated exosomes was 1.0 as compared with 1.75 for exosomes treated with medium alone. (C) HUVEC-derived exosomes bind TNF-α. Exosomes were isolated from HUVEC medium as described, resuspended in medium, and incubated with 1 ng of recombinant human TNF-α for 16 h at 4°C. Exosomes were recovered by centrifugation at 175,000 × g for 16 h at 4°C, separated by SDS/PAGE, transferred to nitrocellulose, and reacted with antibodies against TNF-α. (D) Binding of rhTNF-α to HUVEC-derived exosomes. TNFR1 exosomes (100 μg) were immunoprecipitated with a murine monoclonal antibody directed against the TNFR1 extracellular domain (clone 16805.21) and incubated with rhTNF-α, and the quantity of bound rhTNF-α, in duplicate samples, was measured by ELISA.
HUVEC-Derived Exosomes Bind TNF-α. Because the function of sTNFR1 is to serve as a TNF-binding protein, experiments were performed to assess whether exosome-associated TNFR1 is capable of binding rhTNF-α. HUVEC-derived exosomes were found to contain 1.9 pg of TNFR1 per μg of exosome protein by ELISA. As shown in Fig. 4C by immunoblotting, HUVEC-derived exosomes bound soluble rhTNF-α in a dose-dependent fashion. In addition, HUVEC-derived exosomes contain small quantities of soluble 17-kDa TNF bound under basal conditions. Furthermore, TNFR1 exosomes isolated by immunoprecipitation (Fig. 4D) demonstrate saturable binding of rhTNF-α in the nM range, similar to what has previously been reported for recombinant sTNFR1 (17). The shape of the binding curve, however, suggests that rhTNF-α binding by TNFR1 exosomes is complex and may involve more than one population of TNF receptors. Because of the heterogenous binding, a single Kd appears unlikely, however, the high-affinity Kd is in all probability <10 nM.
Characterization of TNFR1 Exosome-Like Vesicle Release in Pulmonary Epithelial Lining Fluid in Vivo. Experiments were performed to assess whether exosome-like vesicle release is a mechanism of sTNFR1 generation in pulmonary epithelial lining fluid in vivo. BALF was collected from healthy, normal volunteers and subjected to high-speed sequential centrifugation. In four of seven BALF samples, only full-length 55-kDa TNFR1 was sedimented by the 100,000 × g centrifugation, consistent with localization of TNFR1 in secreted exosome-like vesicles (Fig. 5A). In the other three BALF samples, both the full-length 55-kDa TNFR1 and the cleaved 28-kDa sTNFR1 ectodomain were sedimented by centrifugation at 100,000 × g (Fig. 5B). This finding demonstrates that soluble TNFR1 present in lung epithelial lining fluid is generated by two distinct mechanisms, ectodomain cleavage and release of exosome-like vesicles. Furthermore, these results demonstrate that the 28-kDa sTNFR1 ectodomain remains associated with vesicles after sedimentation at 100,000 × g.
Fig. 5.
(A) Characterization of soluble TNFR1 from human lung epithelial lining fluid. Samples of proteins (60 μg) from the sequential pellets of BALF, as described in Fig. 2, were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with antibodies against TNFR1. Nitrocellulose membranes were stripped and reacted with antibodies against rab5. This blot is representative of four of the seven normal volunteers. (B) Sequential centrifugation of BALF. Duplicate samples of proteins (60 μg) from the 100,000 × g (P5) pellet were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with antibodies against TNFR1. This blot is representative of three of the seven normal volunteers.
Discussion
Proteolytic cleavage of the TNFR1 ectodomain by a receptor sheddase, such as TACE, is recognized as the mechanism by which soluble TNF receptors are generated. Here, we describe the release of exosome-like vesicles as an alternative mechanism for generation of soluble TNFR1 that does not involve proteolytic cleavage of the TNFR1 ectodomain. We found that full-length 55-kDa TNFR1 was the predominant form present in both human serum and pulmonary epithelial lining fluid, whereas the 28-kDa cleaved TNFR1 ectodomain represented only a minor fraction of total immunoreactive TNFR1. Furthermore, we demonstrate that constitutive TNFR1 shedding from primary cultures of human vascular endothelial cells occurred via the release of exosome-like vesicles, because culture medium contained only full-length 55-kDa TNFR1, which could be sedimented by high-speed centrifugation. The presence of TNFR1 in exosome-like vesicles was confirmed by electron microscopy with ImmunoGold labeling, which localized soluble TNFR1 to small vesicles of ≈20–50 nm in diameter. Full-length 55-kDa TNFR1 floated at a specific gravity of 1.1 g/ml on a sucrose gradient, which is consistent with its localization in the membranes of small exosome-like vesicles. These data demonstrate that constitutive generation of soluble TNFR1 from human vascular endothelial cells occurs by the release of exosome-like vesicles. Therefore, the release of exosome-like vesicles represents a previously unrecognized mechanism by which soluble cytokine receptors can be generated. We propose that the full-length 55-kDa exosome-associated TNFR1 be designated as eTNFR1 to distinguish them from the soluble, cleaved, 28-kDa ectodomain or sTNFRI.
Although TNFR1 signaling is mediated by means of cell surface receptors, the majority of cellular TNFR1 in human vascular endothelial cells has been reported to reside in the Golgi apparatus, with a minority found on the plasma membrane (18, 19). The Golgi storage pool serves both to replenish cell surface TNFR1 receptors and as a source of shed receptors (20). It has recently been reported that histamine induces a redistribution of TNFR1 from the Golgi apparatus to a punctate pattern, consistent with localization to vesicles, and subsequently into the medium as soluble receptors, with a concomitant decrease in cell surface TNFR1 as measured by flow cytometry (20). Our data suggest that these intracellular TNFR1 vesicles may also serve as a reservoir for the release of full-length 55-kDa eTNFR1 into the extracellular milieu.
Two main pathways by which membrane vesicles are released from cells are via formation of exosomes and microvesicles (21, 22). Exosomes are small membrane-enclosed vesicles of 30–100 nm in diameter that correspond to the internal vesicles of endolysosome-related multivesicular bodies and are released from the cell via exocytic fusion with the plasma membrane (13, 23, 24). As is typical of lipid vesicles, exosomes float on sucrose gradients at densities that range from 1.08 to 1.22 g/ml (13, 23). Therefore, the soluble HUVEC-derived vesicles containing full-length 55-kDa TNFR1 that we have identified are most consistent with exosome-like vesicles. This conclusion is based on the small size of the vesicles (20–50 nm) and their floatation on sucrose density gradients at a density of 1.1 g/ml. The lack of colocalization of TNFR1 with either LAMP-1 or LAMP-2 (unpublished data) suggests that HUVEC-derived TNFR1 exosome-like vesicles may be similar to exosomes from B-lymphocytes, which also do not contain these lysosomal proteins (25). Therefore, although the HUVEC-derived TNFR1 vesicles demonstrate a size and morphology consistent with an exosomal origin, they do not colocalize with known exosome markers, which may be cell-type or vesicle specific.
In contrast to exosomes, microvesicles or microparticles are formed by plasma membrane vesiculation or blebbing. Microvesicles are typically larger than exosomes, with diameters ranging from 0.1 μm to >1 μm, and are associated with the translocation of phosphatidylserine from the inner to the outer cell membrane (21, 22, 26). Microvesicle release can occur during the process of apoptosis or cellular activation (21, 22, 27). The small size (20–50 nm) and constitutive release of HUVEC-derived TNFR1-containing vesicles suggest that they are not microparticles, but are instead more consistent with exosome-like vesicles. Similarly, the absence of poly(ADP-ribose) polymerase cleavage and the lack of effect of pan-caspase inhibition on the release of TNFR1-containing vesicles is consistent with the conclusion that their release is not a consequence of cellular apoptosis.
In summary, TNFR1 shedding has been previously recognized to be a consequence of proteolytic cleavage of the ectodomain by a receptor sheddase, such as TACE. We now report that exosome-like vesicles represent a mechanism for the constitutive release of TNFR1 into the extracellular compartment. This is an alternative pathway by which soluble cytokine receptors can be generated, independent of proteolytic cleavage of the receptor ectodomain.
Acknowledgments
We thank Drs. Martha Vaughan, Vincent Manganiello, and Joel Moss for their helpful advice and critical review of the manuscript.
Abbreviations: BALF, bronchoalveolar lavage fluid; TNF, tumor necrosis factor; TNFR, TNF receptor; eTNFR1, exosome-associated TNFR1; HUVEC, human umbilical vein endothelial cells; LAMP, lysosome-associated membrane protein; SODD, silencer of death domains; sTNFR1, 28-kDa cleaved TNFR1 ectodomain; TACE, TNF-α converting enzyme; rhTNF-α, recombinant human TNF-α; TAPI, TNF-α protease inhibitor; Z-VAD-FMK, benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone; RIP, receptor-interacting protein; TRADD, TNF receptor-associated death domain; TRAF, TNF receptor-associated factor.
References
- 1.Chen, G. & Goeddel, D. V. (2002) Science 296, 1634–1635. [DOI] [PubMed] [Google Scholar]
- 2.Jiang, Y., Woronicz, J. D., Liu, W. & Goeddel, D. V. (1999) Science 283, 543–546. [DOI] [PubMed] [Google Scholar]
- 3.Aderka, D., Engelmann, H., Maor, Y., Brakebusch, C. & Wallach, D. (1992) J. Exp. Med. 175, 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelmann, H., Aderka, D., Rubinstein, M., Rotman, D. & Wallach, D. (1989) J. Biol. Chem. 264, 11974–11980. [PubMed] [Google Scholar]
- 5.Olsson, I., Lantz, M., Nilsson, E., Peetre, C., Thysell, H., Grubb, A. & Adolf, G. (1989) Eur. J. Haematol. 42, 270–275. [DOI] [PubMed] [Google Scholar]
- 6.Seckinger, P., Isaaz, S. & Dayer, J. M. (1989) J. Biol. Chem. 264, 11966–11973. [PubMed] [Google Scholar]
- 7.Nophar, Y., Kemper, O., Brakebusch, C., Engelmann, H., Zwang, R., Aderka, D., Holtmann, H. & Wallach, D. (1990) EMBO J. 9, 3269–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schall, T. J., Lewis, M., Koller, K. J., Lee, A., Rice, G. C., Wong, G. H. W., Gatanaga, T., Granger, G. A., Lentz, R., Raab, H., et al. (1990) Cell 61, 361–370. [DOI] [PubMed] [Google Scholar]
- 9.Brakebusch, C., Varfolomeev, E. E., Batkin, M. & Wallach, D. (1994) J. Biol. Chem. 269, 32488–32496. [PubMed] [Google Scholar]
- 10.Wallach, D., Aderka, D., Engelmann, H., Nophar, Y., Kemper, O., Holtmann, H., Brakebusch, C., Villa, S., Gondi, F. G., Bucciarelli, U. & Brakebusch, C. (1991) in Tumor Necrosis Factor: Structure-Function Relationship and Clinical Application., eds. Osawa, T. & Bonavida, B. (Karger, Basel), pp. 47–57.
- 11.Mullberg, J., Durie, F. H., Otten-Evans, C., Alderson, M. R., Rose-John, S., Cosman, D., Black, R. A. & Mohler, K. M. (1995) J. Immunol. 155, 5198–5205. [PubMed] [Google Scholar]
- 12.Reddy, P., Slack, J. L., Davis, R., Cerretti, D. P., Kozlosky, C. J., Blanton, R. A., Shows, D., Peschon, J. J. & Black, R. A. (2000) J. Biol. Chem. 275, 14608–14614. [DOI] [PubMed] [Google Scholar]
- 13.Raposo, G., Nijman, H. W., Stoorvogel, W., Leijendekker, R., Harding, C. V., Melief, C. J. M. & Geuze, H. J. (1998) J. Exp. Med. 183, 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Legler, D. F., Micheau, O., Doucey, M. A., Tschopp, J. & Bron, C. (2003) Immunity 18, 655–664. [DOI] [PubMed] [Google Scholar]
- 15.Micheau, O. & Tschopp, J. (2003) Cell 114, 181–190. [DOI] [PubMed] [Google Scholar]
- 16.Cui, X., Hawari, F., Alsaaty, S., Lawrence, M., Combs, C. A., Geng, W., Rouhani, F. N., Miskinis, D. & Levine, S. J. (2002) J. Clin. Invest. 110, 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loetscher, H., Gentz, R., Zulauf, M., Lustig, A., Tabuchi, H., Schlaeger, E. J., Brockhaus, M., Gallati, H., Manneberg, M. & Lesslauer, W. (1991) J. Biol. Chem. 266, 18324–18329. [PubMed] [Google Scholar]
- 18.Bradley, J. R., Thiru, S. & Pober, J. S. (1995) Am. J. Pathol. 146, 27–32. [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, S. J., Ledgerwood, E. C., Prins, J. B., Galbraith, J., Johnson, D. R., Pober, J. S. & Bradley, J. R. (1999) J. Immunol. 162, 1042–1048. [PubMed] [Google Scholar]
- 20.Wang, J., Al-Lamki, R. S., Zhang, H., Kirkiles-Smith, N., Gaeta, M. L., Thiru, S., Pober, J. S. & Bradley, J. R. (2003) J. Biol. Chem. 278, 21751–21760. [DOI] [PubMed] [Google Scholar]
- 21.MacKenzie, A., Wilson, H. L., Kiss-Toth, E., Dower, S. K., North, R. A. & Surprenant, A. (2001) Immunity 15, 825–835. [DOI] [PubMed] [Google Scholar]
- 22.Heijnen, H. F., Schiel, A. E., Fijnheer, R., Geuze, H. J. & Sixma, J. J. (1999) Blood 94, 3791–3799. [PubMed] [Google Scholar]
- 23.Théry, C., Zitvogel, L. & Amigorena, S. (2002) Nat. Rev. Immunol. 2, 569–579. [DOI] [PubMed] [Google Scholar]
- 24.Denzer, K., Kleijmeer, M. J., Heijnen, H. F., Stoorvogel, W. & Geuze, H. J. (2000) J. Cell Sci. 113, 3365–3374. [DOI] [PubMed] [Google Scholar]
- 25.Escola, J. M., Kleijmeer, M. J., Stoorvogel, W., Griffith, J. M., Yoshie, O. & Geuze, H. J. (1998) J. Biol. Chem. 273, 20121–20127. [DOI] [PubMed] [Google Scholar]
- 26.Combes, V., Simon, A. C., Grau, G. E., Arnoux, D., Camoin, L., Sabatier, F., Mutin, M., Sanmarco, M., Sampol, J. & Dignat-George, F. (1999) J. Clin. Invest. 104, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aupeix, K., Hugel, B., Martin, T., Bischoff, P., Lill, H., Pasquali, J. L. & Freyssinet, J. M. (1997) J. Clin. Invest. 99, 1546–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]