Abstract
Higher termites are characterized by a purely prokaryotic gut microbiota and an increased compartmentation of their intestinal tract. In soil-feeding species, each gut compartment has different physicochemical conditions and is colonized by a specific microbial community. Although considerable information has accumulated also for wood-feeding species of the genus Nasutitermes, including cellulase activities and metagenomic data, a comprehensive study linking physicochemical gut conditions with the structure of the microbial communities in the different gut compartments is lacking. In this study, we measured high-resolution profiles of H2, O2, pH, and redox potential in the gut of Nasutitermes corniger termites, determined the fermentation products accumulating in the individual gut compartments, and analyzed the bacterial communities in detail by pyrotag sequencing of the V3-V4 region of the 16S rRNA genes. The dilated hindgut paunch (P3 compartment) was the only anoxic gut region, showed the highest density of bacteria, and accumulated H2 to high partial pressures (up to 12 kPa). Molecular hydrogen is apparently produced by a dense community of Spirochaetes and Fibrobacteres, which also dominate the gut of other Nasutitermes species. All other compartments, such as the alkaline P1 compartment (average pH, 10.0), showed high redox potentials and comprised small but distinct populations characteristic for each gut region. In the crop and the posterior hindgut compartments, the community was even more diverse than in the paunch. Similarities in the communities of the posterior hindgut and crop suggested that proctodeal trophallaxis or coprophagy also occurs in higher termites. The large sampling depths of pyrotag sequencing in combination with the determination of important physicochemical parameters allow cautious conclusions concerning the functions of particular bacterial lineages in the respective gut sections to be drawn.
INTRODUCTION
Termites contribute substantially to the turnover of carbon and nitrogen in tropical ecosystems (30). Their diet consists exclusively of lignocellulose in various stages of decomposition, ranging from sound wood to humus (4). The digestion of this recalcitrant diet relies on the metabolic activities of a dense and diverse intestinal microbiota (13). In the evolutionarily lower termites, flagellate protists hydrolyze the wood and ferment the resulting monomers, but in higher termites, these cellulolytic symbionts are lacking (see reference 13 and references therein). Although the endoglucanases in the midgut region are secreted by the host epithelium, the cellulolytic activities in the hindgut of higher termites seem to be of bacterial origin (70, 74).
In many higher termites, the hindgut is strongly compartmentalized (44), forming several consecutive microbial bioreactors, some of which are extremely alkaline (5, 12). The hindgut microbiota of wood-feeding Microcerotermes and Nasutitermes spp. is dominated by Spirochaetes, Fibrobacteres, and members of the candidate phylum TG3 (26, 27). A metagenomic analysis of the microbiota in the hindgut paunch (P3 compartment) of a Nasutitermes sp. implicated members of Spirochaetes and Fibrobacteres in the hydrolysis of wood (73). Although the presence of hydrogenase genes indicates the capacity of the gut microbiota to form or consume H2, the presence of H2 in the paunch or other sections of Nasutitermes spp., particularly the alkaline gut region (9), remains to be elucidated. The individual gut compartments of soil-feeding Cubitermes spp. (subfamily Termitinae) are colonized by different communities of bacteria and archaea (21, 61), and the alkaline P1 compartments of different higher termites harbor a similar bacterial microbiota (68). However, apart from a study of the bacteria colonizing the mixed segment of Nasutitermes takasagoensis (subfamily Nasutitermitinae) (71), information about the microbial communities in the different hindgut sections of wood-feeding higher termites and a highly resolved analysis of important physicochemical parameters in the different gut regions are lacking.
In this study, we combine microsensor measurements of physicochemical conditions (oxygen and hydrogen partial pressure, redox potential, and pH) with high-resolution profiles of the bacterial microbiota and their fermentation products in the different gut compartments of Nasutitermes corniger.
MATERIALS AND METHODS
Sample preparation.
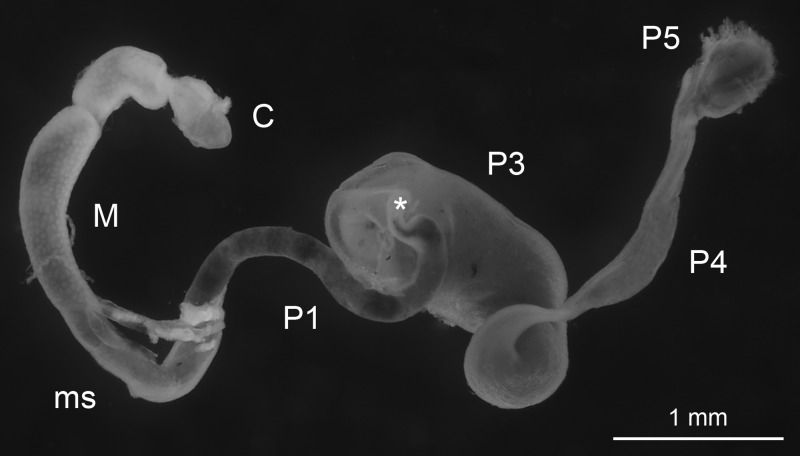
Nasutitermes corniger (Motschulsky) was taken from a laboratory nest (University of Florida) collected in Dania Beach, FL, from established field populations of this exotic arboreal termite, which is synonymous with Nasutitermes costalis (56, 57). Nasutitermes takasagoensis was collected on Iriomote Island, Japan, by Gaku Tokuda (University of the Ryukyus, Okinawa, Japan). Only worker caste termites were used for the experiments. After dissecting the termites with sterile, fine-tipped forceps, we used intact guts for microsensor studies of the individual compartments (Fig. 1). For metabolic profiles and pyrotag sequencing, we separated the guts, placed under a stereomicroscope, into six major sections, comprising the crop, the midgut, and the four major hindgut compartments (P1, P3, P4, and P5), and homogenized them with sterile micropestles (Eppendorf, Hamburg, Germany). Because they are difficult to delineate, the mixed segment (ms) was included with the P1 compartment. To increase sensitivity of detection and to account for intraspecific variations, we always pooled an indicated number of gut sections (see below).
Fig 1.
Intestinal tract of Nasutitermes corniger. The gut includes the crop (C), midgut (M), mixed segment (ms), and several hindgut segments (P1 to P5); the asterisk marks the position of the P2 (enteric valve).
Microsensor measurements.
All microsensors were purchased from Unisense (Aarhus, Denmark). Oxygen (OX-10) and hydrogen (H2-10) microsensors had tip diameters of ca. 10 μm and detection limits of ca. 0.02 and 0.04 kPa, respectively, and were polarized and calibrated as previously described (9, 18). The redox electrode (RD-10) had a tip diameter of ca. 10 μm, and the pH electrode (PH-10) had a tip diameter of 10 to 20 μm and a sensitive tip length of 100 to 150 μm; each was used together with an Ag-AgCl reference electrode and a high-impedance voltmeter. pH measurements were calibrated using standard curves obtained with commercial standard solutions of pH 4.0, 7.0, 9.0, and 11.0 (Carl Roth, Karlsruhe, Germany) as previously described (12). Redox measurements were calibrated using freshly prepared saturated quinhydrone solutions in pH standards at pH 4.0 and 7.0.
Microsensor profiles were measured in glass-faced microchambers as previously described (9). Freshly dissected guts of N. corniger were placed flat and fully extended onto a 4-mm layer of 2% (wt/vol) agarose and were covered with 0.5% (wt/vol) agarose (both made up with Ringer's solution). Microsensors were positioned using a manual micromanipulator (Märzhäuser, Wetzlar, Germany), and tip position was visually controlled with a horizontally mounted stereomicroscope (Zeiss, Jena, Germany); measurement commenced ca. 10 min after embedding and lasted less than 1 h.
Metabolite pools.
Forty gut sections each of N. corniger were homogenized in 80 μl NaOH (10 mM), and metabolites in the clarified supernatants were analyzed using a combination of gas chromatography (GC) (66) and high-performance liquid chromatography (HPLC) (52), as previously described in detail.
Microbial cell counts.
Twenty gut sections each of N. corniger were homogenized in 0.5 ml phosphate-buffered saline (PBS) (49) and fixed with 4% (vol/vol) formaldehyde at 4°C for 13 h. Microbial cells were counted using the procedure of Pernthaler et al. (50), but the sonication step was excluded. Samples were washed with PBS, and appropriate dilutions were filtered onto polycarbonate filters (pore size, 0.2 μm; GTTP; Millipore, Schwalbach/Ts., Germany) and stored at −20°C. For analysis, filters were stained with 4′,6′-diamidino-2-phenylindole (DAPI), washed with sterile water and then with 70% (vol/vol) ethanol, and embedded in Citifluor AF1 solution (Citifluor, London, United Kingdom). Microbial cells were counted at 1,000-fold magnification using a Zeiss Axiophot epifluorescence microscope equipped as previously described (61).
Primer design.
Primers 341F (40) and 787R (29), targeting the V3-V4 region of the bacterial 16S rRNA gene, were modified on the basis of the sequence information in the SILVA 100 database (53), focusing on an optimal coverage of the taxa known to prevail in termite guts. Modifications were tested using the probe match function of ARB software (version 5.1) (39). The resulting primer set, 343Fmod (TACGGGWGGCWGCA) and 784Rmod (GGGTMTCTAATCCBKTT), showed perfect matches to 87% of the sequences in the database (90.5%, allowing one mismatch), and coverage was even higher in the phyla relevant to the termite gut environment (see Fig. S1 in the supplemental material).
Pyrotag sequencing.
Twenty sections of each gut compartment of N. corniger, 10 hindguts (P1 to P5) of both N. corniger and N. takasagoensis, and 10 whole guts of N. corniger were each pooled and homogenized in PBS. DNA was extracted with phenol-chloroform using the bead-beating protocol described by Henckel et al. (25), precipitated with 2 volumes of polyethylene glycol, and amplified with the newly designed primers using a high-fidelity polymerase (Herculase II; Agilent, Waldbronn, Germany). The PCR conditions were initial denaturation (3 min at 95°C), 26 cycles of amplification (20 s at 95°C, 20 s at 48°C, and 30 s at 72°C), and terminal extension (3 min at 72°C). Both the forward and the reverse primers each had an additional, sample-specific 6-bp bar code at the 5′ end that differed by at least 2 bp between samples and contained no homopolymers. The amplicons were quantified photometrically (NanoDrop; Thermo Fisher Scientific, Schwerte, Germany) and mixed in equimolar amounts before further analysis. Adaptor ligation, subsequent amplification, and pyrosequencing (454 GS FLX Titanium; Roche, Mannheim, Germany) were done by a commercial service (GATC Biotech; Konstanz, Germany).
Sequence processing and classification.
Pyrotag data were preprocessed using the mothur software suite (version 1.15.0) (59), following the strategy described by Schloss et al. (58), with slight modifications. After sorting the sequences by their unique bar codes, all sequences that were shorter than 200 bp, contained ambiguous bases, had errors in the primer sequence, or showed homopolymer regions of more than 10 nucleotides were removed from the data sets. For phylotype analyses, the remaining sequences were denoised as previously described (58); for classification analyses, the sequences were aligned against the SILVA 102 nonredundant database (53) using a stand-alone version of the SINA aligner (http://www.arb-silva.de).
The sequences were assigned to taxonomic groups with the naïve Bayesian classifier implemented in the mothur software using a manually curated reference database and a confidence threshold of 60%. The reference database consisted of the SILVA 102 nonredundant database amended with numerous unpublished sequences from termite and cockroach guts obtained in our laboratory in Marburg. The existing classification of the SILVA database was extended and refined down to the genus level by introducing additional, termite-specific groups and renaming redundant or uninformative taxa. To allow processing in the mothur software environment and to improve the speed of the classifier, uninformative sequences from those taxa that contained no gut-related sequences were removed. The resulting reference database (82,400 sequences) contained all bacterial isolates, all uncultivated bacteria from intestinal environments, and at least three representative sequences from every other lowest-level group in the SILVA database. It is available from the authors upon request.
RESULTS
Physicochemical gut conditions.
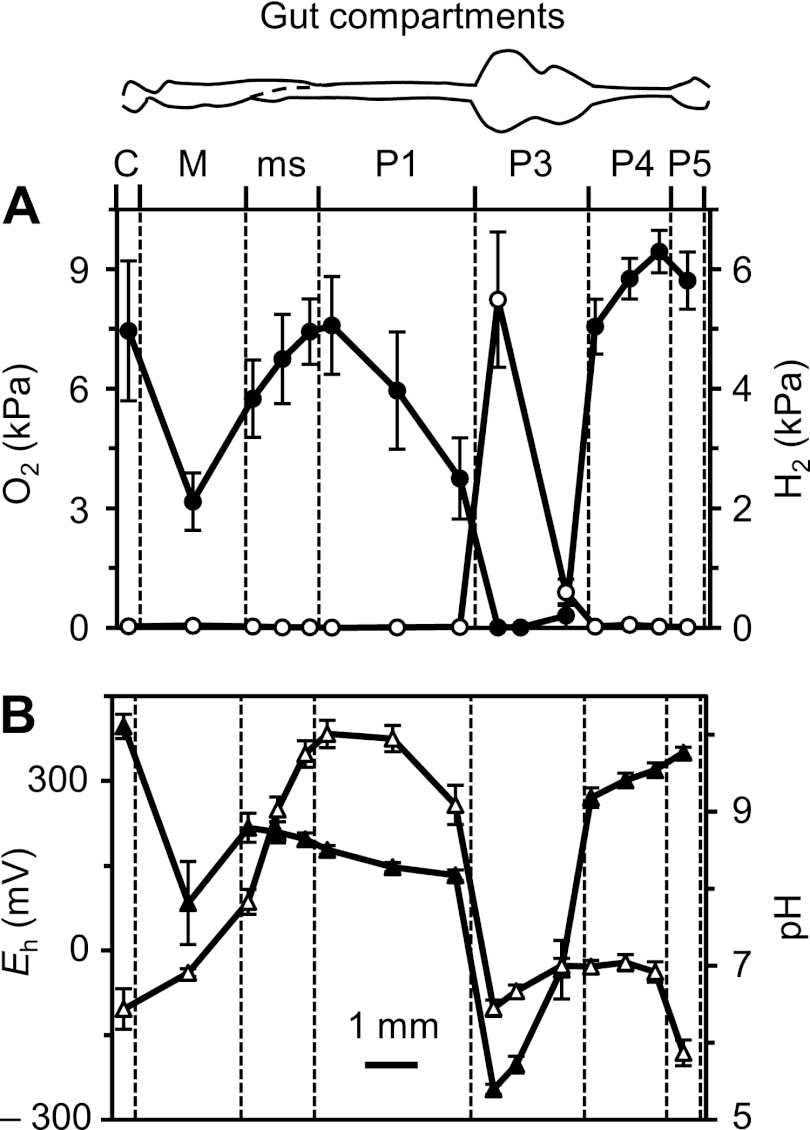
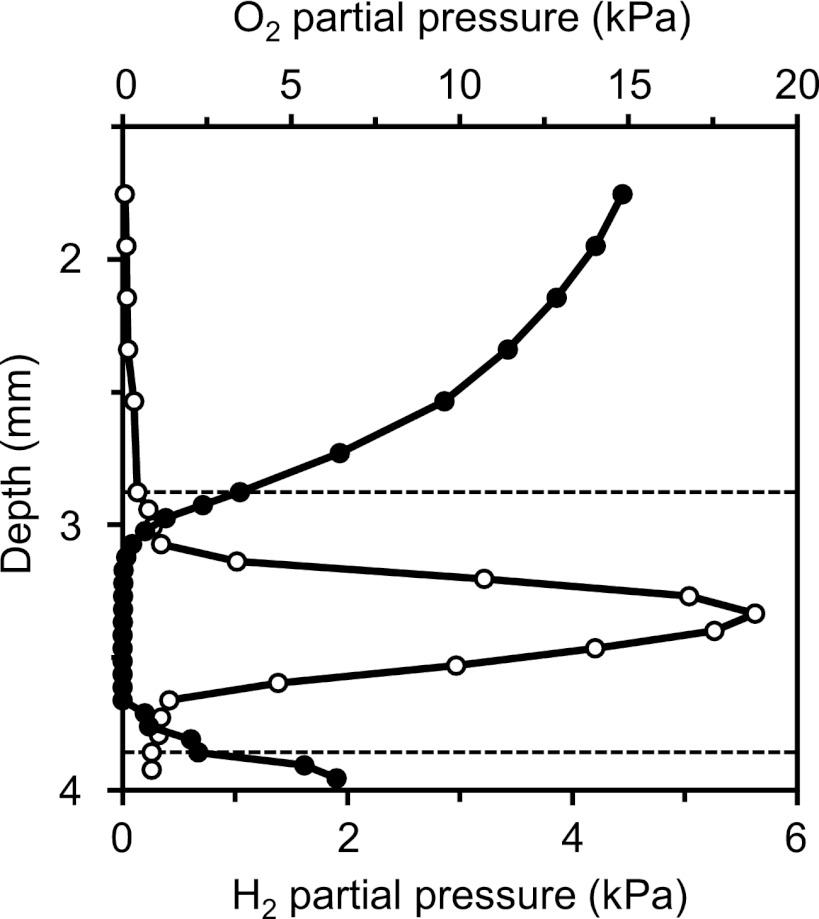
Microsensor profiles along the gut axis of N. corniger showed strong dynamics of oxygen concentration (Fig. 2A). Total anoxia was observed in the highly dilated hindgut paunch (anterior P3 compartment) but not in the less dilated, posterior part of the P3 compartment, where traces of oxygen at its center were often found, which suggested that a complete removal of oxygen depends on the diameter of the respective gut region. Radial oxygen profiles of the P3 compartment showed that the gut periphery acts as an oxygen sink, with the microoxic zone typically extending 200 to 300 μm below the gut wall (Fig. 3). However, the penetration depth of oxygen changed with the depth of embedding. If the agarose layer above the hindgut paunch was very shallow (<2 mm), the entire compartment occasionally became oxic.
Fig 2.
Axial profiles of oxygen (●) and hydrogen (○) partial pressure (A) and of redox potential (▲) and pH (▵) (B) along the gut of Nasutitermes corniger, measured at the gut center. Values are means ± standard errors obtained with 8 to 12 termites (except for the crop, which was lost in about half of the preparations). For definitions of the abbreviations of the gut compartments, see the legend to Fig. 1.
Fig 3.
Radial profiles of oxygen (●) and hydrogen (○) partial pressure in the agarose-embedded anterior P3 compartment of Nasutitermes corniger relative to the agarose surface. The dotted lines indicate the positions of the proximal and distal gut wall. The profiles were selected as being typical among six similar sets obtained with different termites.
The oxygen status corresponded to the redox conditions in the respective compartments; i.e., only the anoxic region (P3) had a negative redox potential (Fig. 2B). The accumulation of H2 was also restricted to the P3 compartment, with maximal values occurring in the anterior region (Fig. 2A). Radial hydrogen profiles of the anterior P3 compartment revealed steep H2 gradients from the gut center toward the gut wall (Fig. 3). However, hydrogen partial pressures in P3 varied over a wide range (from 0.02 to 12 kPa) and were quite sensitive to the depth of embedding. When intestinal hydrogen partial pressures were measured in situ (by inserting the microsensor through the dorsal cuticle into the abdomen of decapitated termites), hydrogen partial pressures ranged from 0.1 to 2.4 kPa. However, these values have to be regarded with caution, because the intransparency of the cuticle did not allow us to determine the exact location of the microsensor tip or to assess the damage to the intestines caused by the sensor.
The pH of the gut contents was also found to be highly dynamic along the gut axis. The intestinal pH was slightly acidic in the crop, was circumneutral in the midgut, and increased sharply in the mixed segment. The most alkaline values (pH 9.3 to 10.9) were found in the anterior P1 compartment. The pH decreased again in the P3 compartment, remained neutral in most of the posterior hindgut, and again turned slightly acidic in the P5 compartment (Fig. 2B).
Metabolite pools.
The metabolites accumulating in the different gut sections of N. corniger were determined by means of HPLC and GC (Table 1). Acetate was the predominant metabolite in all gut sections, except for the midgut, where succinate was more abundant. The highest proportion of acetate was present in the P3 section, which also contained the largest metabolite pool of all compartments. Lactate was detected only in the posterior gut, with the highest concentration being in the P5 section. Similar results have been previously reported for other Nasutitermes spp., except that the pool sizes of propionate, butyrate, and formate were smaller (66).
Table 1.
Pool sizes of major metabolites, fresh weight, and microbial cell counts for different gut sections of Nasutitermes cornigera
| Section | Pool size (nmol) |
Fresh wt (mg) | Cell count (106 section−1) | Cell density (109 g−1)b | |||||
|---|---|---|---|---|---|---|---|---|---|
| Acetate | Propionate | Butyrate | Succinate | Lactate | Formate | ||||
| Crop | 0.7 ± 0.1 | 0.2 ± 0.2 | —c | 0.4 ± 0.1 | — | 0.3 ± 0.1 | 0.7 ± 0.2 | 0.15 ± 0.03 | 0.21 ± 0.07 |
| Midgut | 0.9 ± 0.0 | 0.2 ± 0.2 | — | 2.0 ± 1.0 | — | 0.1 ± 0.1 | 0.6 ± 0.1 | 0.08 ± 0.02 | 0.13 ± 0.04 |
| ms/P1 | 1.4 ± 0.2 | 0.1 ± 0.1 | — | 1.1 ± 0.7 | — | 0.7 ± 0.1 | 0.8 ± 0.2 | 0.10 ± 0.04 | 0.13 ± 0.06 |
| P3 | 8.6 ± 1.8 | 0.7 ± 0.4 | 0.1 ± 0.1 | 1.0 ± 0.8 | 0.1 ± 0.1 | 0.5 ± 0.1 | 1.4 ± 0.3 | 15.2 ± 3.1 | 10.9 ± 3.2 |
| P4 | 2.1 ± 1.0 | 0.6 ± 0.2 | 0.1 ± 0.1 | 0.3 ± 0.1 | 0.1 ± 0.1 | 0.7 ± 0.1 | 0.4 ± 0.2 | 0.08 ± 0.01 | 0.20 ± 0.10 |
| P5 | 1.9 ± 1.2 | 0.4 ± 0.2 | — | 0.3 ± 0.2 | 0.7 ± 0.7 | 0.6 ± 0.2 | 0.6 ± 0.5 | 0.04 ± 0.01 | 0.07 ± 0.06 |
| Total gutd | 15.6 ± 2.4 | 2.2 ± 0.5 | 0.2 ± 0.2 | 5.1 ± 1.5 | 0.9 ± 0.7 | 2.9 ± 0.3 | 4.5 ± 0.7 | 15.6 ± 3.1 | 3.47 ± 0.87 |
Values are averages (± mean deviation) for two homogenates of 40 gut sections each.
Based on fresh weight, using error propagation.
—, below detection limit (ca. 0.02 nmol).
Calculated from the amount in each compartment.
Bacterial diversity.
The microbial cell counts in homogenates of individual gut sections of N. corniger differed greatly (Table 1). The highest absolute number was found in the P3 compartment (1.5 × 107 cells), surpassing those in the other gut regions by more than 2 orders of magnitude. The microbiota of the crop consisted mostly of cocci, whereas the midgut microbiota was dominated by short rods; cells with a spirochetal shape were rare in both compartments. In the P1 compartment, we observed mostly longer rods; cocci were less abundant, and the density of cells with a spirochetal shape began to increase. The density of spirochetes was highest in the P3 compartment but decreased again in the posterior sections, whose microbiota was dominated by coccoid cells.
Bacterial diversity in the different gut sections of N. corniger and in total hindguts of N. corniger and N. takasagoensis was determined by pyrotag sequencing of the V3-V4 region of the 16S rRNA genes in the DNA extracted from the different samples. Trimming and quality control removed 10 to 20% of the sequences from each data set, resulting in sequence libraries of 3,200 to 26,000 reads per sample (see Table S1 in the supplemental material).
Preliminary analysis using the classifier of the online platform of the Ribosomal Database Project (RDP; release 10) (72) yielded large fractions of unclassified sequences at all taxonomic levels (Table 2). Since most of the unclassified sequences were termite-specific bacterial groups that were not represented or poorly resolved in the reference database used by RDP, we prepared a manually curated reference database specifically adapted to the bacterial diversity in termite guts (see Materials and Methods). Reclassification of the samples using the mothur software suite (version 1.15.0) (59) resulted in a significantly improved classification at the phylum level, reducing the fraction of unclassified sequences in the different samples from 4 to 22% to 1 to 2%. The effect was even stronger at lower taxonomic ranks; at the genus level, the fraction of unclassified sequences in the samples decreased from 43 to 90% to 11 to 33%. The remaining sequences could be assigned only to higher taxa, mostly because closely related reference sequences were lacking. Closer inspection of 36 randomly selected sequences without phylum-level classification revealed that half of them were putative chimeras and the other half did not code for 16S rRNA.
Table 2.
Comparison of classification success at different taxonomic levels using the RDP online platform (release 10) and the curated reference database (this study)
| Section | %a |
|||||
|---|---|---|---|---|---|---|
| Phylum |
Family |
Genus |
||||
| RDP | This study | RDP | This study | RDP | This study | |
| Crop | 89 | 98 | 79 | 90 | 49 | 76 |
| Midgut | 96 | 99 | 17 | 95 | 10 | 89 |
| ms/P1 | 88 | 98 | 88 | 80 | 46 | 67 |
| P3 | 78 | 99 | 80 | 95 | 57 | 87 |
| P4 | 85 | 99 | 76 | 83 | 46 | 72 |
| P5 | 88 | 99 | 80 | 79 | 45 | 72 |
Values are based on the total number of sequences in the sample.
The individual gut sections of N. corniger each contained sequences from 200 to 300 different taxa (genus level), with the highest numbers being found in the crop, P3, and P4 (Table 3). Similarity-based clustering of the sequences into phylotypes with 5% (genus level) or 3% (species level) sequence divergence indicated that the genus/species richness in each sample was considerably higher than that indicated by hierarchical classification (many rare species were not classified in lieu of appropriate reference sequences); predictions of species richness based on the abundance of singletons in the different data sets (using the Chao1 estimator) were higher (Table 3).
Table 3.
Diversity and evenness of the bacterial communities in the different gut sections of Nasutitermes cornigera
| Section | No. of genus-level taxab | No. of phylotypes |
Diversity indexc |
|||
|---|---|---|---|---|---|---|
| 5% | 3% | Expected phylotypesd | Diversitye | Evennessf | ||
| Crop | 298 | 351 | 563 | 1,174 | 3.39 | 0.45 |
| Midgut | 217 | 285 | 511 | 1,231 | 1.54 | 0.20 |
| ms/P1 | 187 | 195 | 337 | 944 | 2.80 | 0.38 |
| P3 | 264 | 360 | 653 | 1,626 | 2.42 | 0.31 |
| P4 | 307 | 411 | 726 | 1,748 | 3.87 | 0.50 |
| P5 | 173 | 167 | 275 | 494 | 3.77 | 0.57 |
The number of taxa (hierarchical classification to genus level) is compared to the number of phylotypes (similarity-based classification, using a 3% or 5% dissimilarity threshold).
Lowest level of classification.
Diversity indices are based on 3% dissimilarity.
Chao1 estimator (14).
Nonparametric Shannon index (15).
Evenness was calculated as described elsewhere (34).
Diversity and evenness of the bacterial community were lowest in the midgut, which harbored a few very abundant groups. In the other gut sections, diversity was much higher and community structure was more balanced (Table 3); the same trends were also observed with similarity-based classification (5% cutoff) and hierarchical classification (genus level; details not shown). The relatively small number of phylotypes agrees with the results of Engelbrektson et al. (20), who observed less than 1,000 phylotypes in their rarefaction analyses when they tested several primer pairs for pyrotag sequencing using N. corniger luminal P3 hindgut compartment DNA as the template. Nevertheless, the composition of the communities differed substantially between the compartments (Table 4). High similarities were observed between the crop and hindgut (P4 and P5), whereas the midgut had only low similarities to other compartments.
Table 4.
Similarity indices of the bacterial communities in different gut sections of Nasutitermes corniger
| Section | Similarity indexa |
|||||
|---|---|---|---|---|---|---|
| Crop | Midgut | P1 | P3 | P4 | P5 | |
| Crop | 1.00 | |||||
| Midgut | 0.21 | 1.00 | ||||
| ms/P1 | 0.39 | 0.22 | 1.00 | |||
| P3 | 0.36 | 0.13 | 0.23 | 1.00 | ||
| P4 | 0.39 | 0.14 | 0.33 | 0.48 | 1.00 | |
| P5 | 0.35 | 0.17 | 0.33 | 0.20 | 0.20 | 1.00 |
Bray-Curtis coefficient (8), based on sequence similarity (3% dissimilarity threshold for phylotypes).
Community structure.
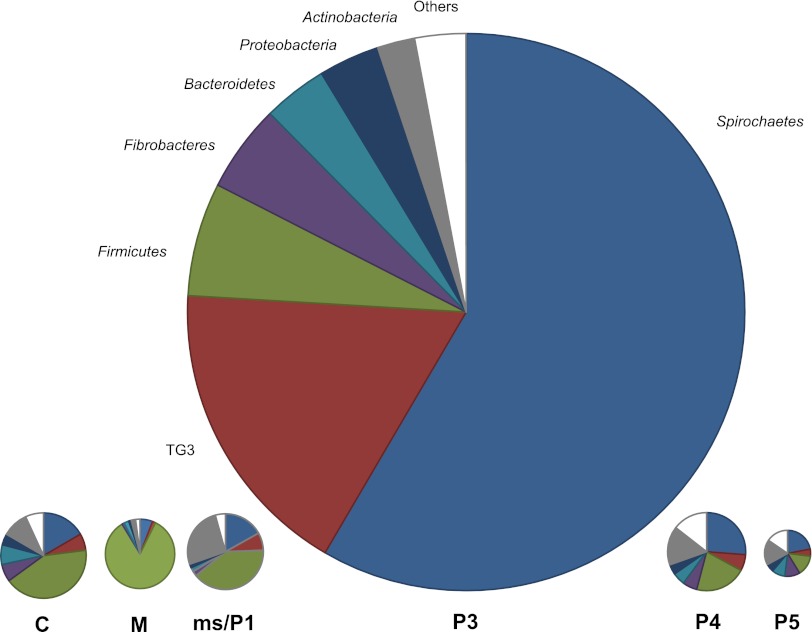
The major bacterial phyla consistently encountered in the different gut compartments of N. corniger were Spirochaetes, candidate phylum TG3, Firmicutes, Fibrobacteres, Bacteroidetes, Proteobacteria, and Actinobacteria (Fig. 4). Spirochaetes and members of the TG3 phylum were represented in all compartments but were most abundant in the hindgut paunch (P3). The phylum-level patterns in the posterior hindgut sections (P4 and P5) were similar to the pattern in the crop, except for an increased abundance of Firmicutes in all anterior sections.
Fig 4.
Relative abundance of major bacterial phyla in the different gut compartments of Nasutitermes corniger, based on pyrotag analysis of the V3-V4 region of the 16S rRNA genes. The area of the circles reflects the microbial cell counts in the respective gut sections (Table 1). For definitions of the abbreviations, see the legend to Fig. 1.
At higher taxonomic resolution, it becomes apparent that most phyla are represented by various lineages that are often unevenly distributed among the compartments. Figure 5 summarizes the relative abundance of the 50 major families represented in the different samples; detailed results for all taxonomic ranks can be found in interactive Table S1 in the supplemental material. A prominent example is the Firmicutes. In the midgut, most of the sequences of this phylum (80% of all sequences) are members of the order Clostridiales, consisting almost exclusively of a particular group of Lachnospiraceae (uncultured 67; see Table S1 in the supplemental material). Although this group was also present in the other compartments, it is outnumbered by other Clostridiales (Ruminococcaceae or family XIII incertae sedis) in the posterior hindgut (P4) and by Lactobacillales in the crop (here, mainly Streptococcaceae) and in the anterior hindgut (P1; here, mainly members of the insect group PeH08 and other, unclassified Lactobacillales). Many family-level taxa are highly represented in all gut sections (e.g., the termite clusters in Fibrobacteres and TG3 subphylum 1 or some Bacteroidetes). In some cases, the patterns in the posterior hindgut sections (P4 and P5) were similar to those of the crop, e.g., the Ruminococcaceae (Clostridiales), the Acidobacteriaceae (Acidobacteria), and the candidate divisions OP11, SR1, and TM7.
Fig 5.
Relative abundance of the major bacterial taxa in the different gut sections of Nasutitermes corniger (for definitions of the abbreviations, see the legend to Fig. 1) and in the total hindguts of N. corniger and N. takasagoensis. Classification is shown down to the family level (for genus level, see Table S1 in the supplemental material). The heat map uses a logarithmic scale to increase the visibility of low-abundance groups. The remaining sequences were extremely diverse (111 to 157 families), but each represented less than 1% of the community in the respective compartment (see Table S1 in the supplemental material). The families represented in previously published clone libraries of N. takasagoensis (26) (total gut) and a Nasutitermes sp. (73) (P3 lumen) are shown for comparison (shading of circles indicates relative abundance).
Many of the sequences obtained from the gut of N. corniger represent termite-specific lineages that have already been encountered in clone-based inventories of the gut microbiota of other Nasutitermes species (e.g., see references 26, 71, and 73). However, deep sequencing of the communities in the individual gut regions also revealed the presence of many lineages hitherto undetected in termite guts (e.g., from the phyla Lentisphaerae, Planctomycetes, and Firmicutes and candidate divisions OP11, TM7, and SR1), which underlines the high diversity of the gut microbiota also reflected by the high Shannon indices for most compartments (Table 3). Although 75% of the families detected each represent less than 1% of the sequences obtained from the different sections (see Table S1 in the supplemental material), many of these groups are numerically important because of the high density of the community (i.e., in the P3 compartment; Fig. 4) or because of their apparent specificity for termite guts. In any case, it should be considered that, especially in the P3 section, taxa that are close to the detection limit of the pyrotag analysis still form substantial populations.
Interspecific variation.
The bacterial community profiles of the P3 compartment are virtually identical to the artificial profiles for the hindgut and whole gut generated using cell density and the relative abundance of different families in the individual compartments (see Fig. S2 in the supplemental material), which illustrates that the community profiles of the total gut will always be dominated by the microbiota of the P3 compartment (Fig. 4). At the family level, these profiles were highly similar to replicate profiles of the hindgut and total gut of N. corniger (see Fig. S2), which were obtained with different batches of termites from the same nest. At the genus level, only 31% of the classified genera were shared among the profiles, but these genera represented 95% of the total sequence abundance. This documents the reproducibility of the profiles and the noise in the low-abundance taxa/singletons that leads to high species richness estimations (Table 3). The differences between the samples were exclusively in the low-abundance taxa. The similarities between the hindgut profiles of N. corniger and the closely related N. takasagoensis, an allopatric species from Japan, were slightly lower (23% shared genera), but the profiles showed striking similarities in the presence and abundance of family-level taxa (Fig. 5).
Comparison to clone libraries.
A comparison of the pyrotag data sets of N. corniger and N. takasagoensis to previous clone libraries of the bacterial 16S rRNA genes from the gut of Nasutitermes species showed that each of the major family-level lineages is represented in all Nasutitermes species, although their relative abundance differs (Fig. 5). A notable exception is a termite-specific lineage of Bacteroidetes (M2PB4-65) that is moderately abundant in the 454 data sets (0.6 to 1.7%) but not represented in the clone libraries. Strong differences between the data sets are encountered among the Fibrobacteres, TG3, Firmicutes, and Spirochaetes, particularly in the virtual absence of Fibrobacteres from the hindgut of the batch of N. takasagoensis used in this study.
When we compared at the genus level the bacteria in the total P3 section of N. corniger to the bacteria detected in the lumen of this compartment of a Nasutitermes sp. collected in Costa Rica (73), we found that 79% of the taxa in the pyrotag libraries were represented, which indicated that the bulk of the P3 compartment gut microbiota was already detected by a clone library of 1,252 sequences (73). However, the pyrotag library of P3 (24,029 reads) comprised 217 additional taxa. Many of them were also present in the pyrotag library of N. takasagoensis, which indicated that they are likely to occur also in other Nasutitermes species.
An interesting aspect became apparent when we compared the two data sets in the opposite direction. Since the pyrotag data set for N. corniger generated in this study was obtained from a homogenate of the complete P3 compartment and the clone library of Nasutitermes sp. was based only on its luminal content (73), any major taxa present in the analysis of the total compartment but missing from the luminal sample potentially represent wall-associated bacteria. To compensate for the lower sequencing depth of the luminal sample, a threshold for the larger amounts of pyrotag sequences was set by taking the noise signal (i.e., one sequence) multiplied by 3 (i.e., three sequences in the luminal data set corresponding to 0.24% in the pyrotag data set). Taking this threshold, we discovered 10 taxa that are strong candidates for gut wall-associated bacteria (see Table S1 in the supplemental material), including Sanguibacter spp. and other Actinobacteria, Bacteroidetes cluster V (Porphyromonadaceae 1), Arthromitus spp. (Lachnospiraceae), and some lineages of Spirochaetaceae specific for termite guts. Together, they formed 10% of the sequences from the P3 compartment. In contrast, taxa that were exclusively present in the luminal sample (see Table S1 in the supplemental material) were only a small fraction (0.6%) of the clones in the library. Moreover, two of these groups, OPB56 (Chlorobi) and Rs-H88 (Spirochaetes), were present in the total hindgut sample of N. corniger.
DISCUSSION
This is the first comprehensive analysis of the digestive tract of a wood-feeding higher termite from a microbiological perspective. It combines a microsensor study of physicochemical gut conditions with a highly resolved analysis of the bacterial microbiota in the individual gut compartments. The results revealed that the gut is a highly structured microenvironment, with differences in metabolic activities and microbial community structure. The dilated hindgut paunch (P3) contains the bulk of the microbiota, but other compartments, such as the alkaline P1 and the tubular P4, also harbor smaller communities distinct from those in other gut regions. The differences are already apparent at the phylum level, and a detailed analysis of relative abundance indicates that individual lineages preferentially colonize particular niches. The results allow us to draw cautious conclusions concerning the functions of particular bacterial lineages in the respective sections.
Hindgut paunch.
Since higher termites lack cellulolytic flagellates, wood fiber in the dilated hindgut paunch has to be digested by a purely prokaryotic microbiota (13). The hindgut of Nasutitermes takasagoensis and N. walkeri contains substantial cellulolytic activity (70), and metagenomic analysis of the luminal contents of the P3 compartment of another Nasutitermes species has identified numerous glycosyl hydrolases putatively involved in the degradation of cellulose and hemicelluloses. The genes have tentatively been assigned to members of the phyla Fibrobacteres and Spirochaetes on the basis of phylogenetic binning (73). Diverse members of these phyla and of the related TG3 phylum (included in the Fibrobacteres by Warnecke and colleagues [73]) have been documented to occur abundantly in the hindgut of Nasutitermes species (17, 48, 46, 26, 73). In accordance with these reports, the mentioned phyla were also highly represented in the pyrotag sequences of the total hindgut of N. corniger and N. takasagoensis. They dominate the microbiota in the P3 compartment of N. corniger (Fig. 4), the major microbial bioreactor in terms of anoxic status, microbial cell count, and concentration of fermentation products.
Interestingly, the P3 compartment is also the only gut region where H2 accumulated. Hydrogen partial pressures in the anterior P3 of N. corniger were in the same range as those in the paunch of Reticulitermes flavipes (18), where H2 production is largely attributed to the gut flagellates. Several higher termites, including Nasutitermes triodiae, have been reported to emit H2 in vivo (65), but microsensor profiles have so far been available only for soil-feeding Cubitermes spp., where the mixed segment and the P3 showed substantial accumulation of H2 (60). The bacterial populations responsible for hydrogen production have not been identified, but by means of phylogenetic analyses of conserved single-copy protein-coding genes, Warnecke et al. (73) could link the iron-only hydrogenases in the metagenome of a Nasutitermes sp. to members of the Spirochaetes. Molecular hydrogen is a major fermentation product of carbohydrates in many Spirochaeta spp. (35) and also in Treponema azotonutricium, an isolate from the lower termite Zootermopsis angusticollis (24). All isolates of termite gut treponemes possess several [FeFe] hydrogenases (2), and related hydrogenase genes are also present in other lower termites (1). It is therefore likely that spirochetes are—at least in part—also responsible for hydrogen production in Nasutitermes spp. It is possible that members of the Fibrobacteres and TG3, the other highly abundant bacterial phyla in the P3 compartment of N. corniger and hindguts of other Nasutitermes spp., also contribute to hydrogen production. While nothing is known about these uncultivated lineages, the genome of the distantly related Fibrobacter succinogenes does not encode any hydrogenases (64), and there were no hydrogenases binning with Fibrobacteres in the Nasutitermes sp. metagenome (73).
The steep radial profiles of H2 in the P3 compartment of N. corniger indicate the presence of a strong hydrogen sink, which is in agreement with the high rates of reductive acetogenesis in hindgut homogenates of several Nasutitermes species (7) and consolidates the accumulation of large amounts of H2 within the lumen of the hindgut paunch with the low rates of hydrogen emission by living termites (65). Analyses of the fhs gene, encoding formyl-tetrahydrofolate synthetase (FTHFS), a functional marker for the Wood-Ljungdahl pathway, have provided strong evidence that spirochetes are responsible for reductive acetogenesis from H2 and CO2 in the gut of lower termites (e.g., see references 32, 47, 51, and 55). A metagenomic survey of the hindgut microbiota of a Nasutitermes sp. has indicated that fhs genes in the hindgut community are highly similar to those in the hindgut of lower termites, including that of the genuine homoacetogen Treponema primitia (73). The cooS genes in the metagenome, encoding a catalytic subunit of the carbon monoxide dehydrogenase, have also been predicted to be encoded by treponemes (73).
It is not clear whether all spirochetal lineages present in the Nasutitermes gut are involved in reductive acetogenesis. Furthermore, not all fhs genes obtained by Warnecke et al. (73) are clustered with treponemal sequences. It is possible that members of the Ruminococaceae (Fig. 5) also contribute to reductive acetogenesis in the hindgut because many Ruminococcus species are homoacetogenic (33, 54). The same argument can be made for the Holophagaceae (Acidobacteria; Fig. 5) present in all gut compartments of N. corniger and the hindgut sample of N. takasagoensis, which are closely related to the homoacetogenic Holophaga foetida (37).
In view of the large surface-to-volume ratios of small guts (10), the gut wall emerges as an important microhabitat. Methanogenic archaea associated with the gut wall of lower termites have been implicated as a hydrogen sink, both in methanogenesis and owing to their capacity for hydrogen-dependent reduction of inflowing oxygen (67). However, methanogenesis is not as important in Nasutitermes spp. as in other termite species (see reference 11 and references therein). The situation is a bit more ambiguous in the case of sulfate-reducing microorganisms. About 1% of the sequences in the P3 compartment (Desulfovibrio 1; see Table S1 in the supplemental material) represent sulfate-reducing Deltaproteobacteria related to Desulfovibrio intestinalis, which—like other Desulfovibrio spp. isolated from termite guts—exhibits high rates of hydrogen-dependent oxygen reduction (22, 31). However, it is not known whether the Desulfovibrio spp. in Nasutitermes are located at the hindgut wall.
Clearly, the radial organization of the microbiota and the location of individual populations with respect to the oxygen gradient are important issues. Although the 454 data sets of the gut sections do not contain direct information about the localization of microorganisms within the respective compartments, the obvious absence of some bacterial groups from the purely luminal sample of the P3 gut compartment (73) compared to the total P3 sample allows us to make some careful inferences regarding peripheral localization. Among the possible gut wall colonizers are populations of the genus Sanguibacter, a genus comprising aerobic and facultatively anaerobic isolates (e.g., see reference 28), and other unclassified lineages of Actinobacteria. Several lineages of Trinervitermes cluster A and several other termite-specific Spirochaetaceae groups (see Table S1 in the supplemental material) are also abundant in the total P3 sample of N. corniger but absent from the luminal sample of a Nasutitermes sp. (73), which is in accordance with previous reports of an attachment of spirochetes to the gut wall of lower and higher termites (17, 41). An association with the gut wall of many lower termites has also been documented for relatives of the Bacteroidales cluster V (42), a group that is also abundantly encountered in gut homogenates of Nasutitermes spp. (26; this study). The frequent association of cluster V Bacteroidales also with the surface of cellulolytic protists in lower termites (43) suggests that the need for attachment is a strategy to prevent washout. The presence of oxygen-removing mechanisms among obligate anaerobes, necessary for the colonization of the microoxic gut periphery, has been previously documented for Methanobrevibacter species in lower termites (67) and is also encountered among the Bacteroidales (3).
Posterior hindgut.
Microbial cell counts decrease by 2 orders of magnitude and cell density drops 50-fold between the P3 and P4 section (Table 1), which suggests that the microbial biomass produced in P3 is digested after being transported into the posterior hindgut with the flow of the digesta. The distinct difference in community structure between the P3 and P4 sections (Fig. 5) indicates the presence of a microbiota specifically adapted to the environment of the posterior hindgut. Most obvious is the increase in relative abundance of Acidobacteriaceae and Coriobacteriaceae (see Table S1 in the supplemental material), but also, specific lineages of Lentisphaerae and members of candidate divisions OP11, SR1, and TM7 are enriched in the posterior hindgut. Distinct changes in diversity and community structure have also been observed between the alkaline P3 and the neutral P4 compartments of soil-feeding Cubitermes spp. (61, 62). Since both gut regions are neutral in Nasutitermes spp. (9; this study), it is likely that factors other than pH are responsible for this shift. Rather, the force driving community structure could be the increasing influence of oxygen in the tubular P4 section. The slightly acidic pH of the P5 compartment observed in N. corniger has also been found among several soil-feeding species (pH 5 to 6) (12).
Crop and midgut.
Since sound wood is a highly nitrogen-deficient diet, termites have developed the strategy to exploit the assimilatory capacities of their gut microbiota to acquire essential amino acids and vitamins (13). This is accomplished by digesting microbial biomass derived from the hindgut contents—either by coprophagy or by proctodeal trophallaxis. Although little is known about the behavior of Nasutitermes species, numerous similarities in the community patterns of the rectum (P5) and the anterior gut (crop) suggest that fecal material is consumed by the termites (Fig. 5). This agrees with observations of proctodeal feeding in N. corniger and other species (R.H.S., unpublished data). The strong shift in the bacterial community profiles (Fig. 5) and the reduction of microbial density between crop and midgut indicate that bacteria are digested in the midgut, which is in agreement with the presence of lysozyme and protease activities in this gut region (23). The community of the midgut is dominated by Firmicutes, particularly members of Lachnospiraceae (Fig. 5), which represent a lineage of uncultivated bacteria from intestinal environments, including termite guts (uncultured 67 group; see Table S1 in the supplemental material). The family Lachnospiraceae comprises many species with high proteolytic, xylanolytic, and also cellulolytic activities (e.g., Butyrivibrio and Pseudobutyrivibrio spp.) (16), but it remains to be clarified whether these bacteria contribute to the digestive capacities of the midgut of Nasutitermes spp. (69) or whether they are simply transient and inactive forms (e.g., spores) of bacteria residing in other gut regions.
Alkaline gut regions.
The anterior hindgut of many higher termites is highly alkaline (5). In soil-feeding Termitinae, the pH increases sharply in the mixed segment and reaches its maximum (pH > 12) in the P1 compartment (12). The alkalinity in the tubular P1 of N. corniger is considerably less pronounced (pH 10), and the pH already returns to neutral in P3, a compartment that remains strongly alkaline in the soil feeders. Since the profiles obtained for N. corniger (this study) were almost identical to previous profiles of N. nigriceps (9), it seems safe to conclude that they are typical, at least for the wood-feeding members of this genus.
A prevalent bacterial lineage in the alkaline P1 section of N. corniger is the genus Turicibacter (Firmicutes, Erysipelotrichaceae; abundance, 13%; see Table S1 in the supplemental material); their relative abundance in all other compartments is less than 1%. Representatives of this cluster have also been detected in the (putatively) alkaline P1 regions of the soil-feeding Pericapritermes latignathus and a grass-feeding Speculitermes sp. (68) and in the alkaline midgut of the humivorous larva of the scarab beetle Pachnoda ephippiata (19), which indicates an adaptation to high pH. The occurrence of Turicibacter spp. in the gut of a Microcerotermes sp. (27), which also comprises an alkaline P1 (9), is in agreement with this assumption. However, alkaliphily is not a typical trait for the whole genus; the next cultured relative, Turicibacter sanguinis, does not grow above pH 8 (6), and members of the Turicibacter clade are also present in mammals (36), which lack a highly alkaline gut.
Other bacterial groups prevailing in the P1 section are several lineages of Lactobacillales (Firmicutes). Sequences of cluster PeH08 (5% relative abundance) have been obtained from the alkaline compartments of other higher termites (68) and beetle larvae (19) but were also encountered in the posterior hindgut compartments of N. corniger (Fig. 5; see Table S1 in the supplemental material). The P1 section also contains a small number of sequences (1%) from a termite-specific lineage of Lachnospiraceae that is related to the sequences NT-1 and NT-2, which have previously been assigned to rod-shaped bacteria predominantly colonizing the mixed segment of N. takasagoensis (71). The presence of these bacteria in this sample is explained by the inclusion of the mixed segment in the P1 section, which was not separated for technical reasons.
Pyrotag sequencing of termite gut microbiota.
The diversity and community structure of the bacterial gut microbiota of termites have been addressed by numerous studies (see reference 45). Many of the more detailed analyses combined Sanger sequencing of 16S rRNA genes with terminal restriction fragment length polymorphism (T-RFLP) profiling, thus compensating for the shortcomings of the individual approaches (Sanger sequencing is notoriously undersampled, and T-RFLP analyses lack phylogenetic resolution). The application of high-throughput sequencing techniques in targeting the 16S rRNA gene opened a new dimension in microbial ecology studies, allowing the detection of as-yet-undetected microorganisms of very low abundance (63).
As in all PCR-based approaches, primer bias is an important issue. Our pyrotag sequencing data provided a good coverage of all lineages previously discovered by Sanger sequencing (73), which documented that the primers for the V3-V4 region did not introduce a serious bias over the 27F-1492R primers. The striking difference in the abundance of Fibrobacteres sequences between the clone library (26) and pyrotag library (this study) of N. takasagoensis (Fig. 5) may be rooted in the different batches of termites used in the respective studies.
The results of our study underline the suggestion that a comprehensive and well-curated database is crucial for a reliable sequence assignment (75). Refining the classification of the SILVA database by the introduction of additional, termite-specific groups significantly improved the assignment of pyrotag reads, especially at lower taxonomic levels (Table 2). This revealed the presence of rare taxa that were not discovered in previous, clone-based studies of Nasutitermes spp. An example are the sequences related to the fat-body-colonizing Blattabacterium (Flavobacteria, 0.5 to 0.9% relative abundance), which so far has been detected only in cockroaches and the primitive termite Mastotermes darwiniensis (38) and whose presence in higher termites requires further analysis.
The high resolution and fast sample treatment using pyrotag sequencing provide perfect tools for community profiling, combining the virtues of fingerprinting approaches with the benefit of exact taxonomic classification. The large sampling depths of pyrotag sequencing also decrease the detection limit of the analysis, which will help to investigate the existence of a core microbiota and other important questions concerning the evolution of the termite gut microbiota from a putative dictyopteran ancestor.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Max Planck Society. Tim Köhler received a doctoral fellowship from the International Max Planck Research School for Environmental, Cellular, and Molecular Microbiology.
We thank Gaku Tokuda for providing termites and Yuichi Hongoh and Moriya Ohkuma for sharing unpublished details of a previous study.
Footnotes
Published ahead of print 27 April 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Ballor NR, Leadbetter JR. 2012. Analysis of extensive [FeFe] hydrogenase gene diversity within the gut microbiota of insects representing five families of Dictyoptera. Microb. Ecol. 63:586–595 [DOI] [PubMed] [Google Scholar]
- 2. Ballor NR, Paulsen I, Leadbetter JR. 2012. Genomic analysis reveals multiple [FeFe] hydrogenases and hydrogen sensors encoded by treponemes from the H2-rich termite gut. Microb. Ecol. 63:282–294 [DOI] [PubMed] [Google Scholar]
- 3. Baughn AD, Malamy MH. 2004. The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature 427:441–444 [DOI] [PubMed] [Google Scholar]
- 4. Bignell DE. 2011. Morphology, physiology, biochemistry and functional design of the termite gut: an evolutionary wonderland, p 375–412 In Bignell DE, Roisin Y, Lo N. (ed), Biology of termites: a modern synthesis. Springer, Dordrecht, Netherlands [Google Scholar]
- 5. Bignell DE, Eggleton P. 1995. On the elevated intestinal pH of higher termites (Isoptera: Termitidae). Insect Soc. 42:57–69 [Google Scholar]
- 6. Bosshard PP, Zbinden R, Altwegg M. 2002. Turicibacter sanguinis gen. nov., sp. nov., a novel anaerobic, Gram-positive bacterium. Int. J. Syst. Evol. Microbiol. 52:1263–1266 [DOI] [PubMed] [Google Scholar]
- 7. Brauman A, Kane MD, Labat M, Breznak JA. 1992. Genesis of acetate and methane by gut bacteria of nutritionally diverse termites. Science 257:1384–1387 [DOI] [PubMed] [Google Scholar]
- 8. Bray JR, Curtis JT. 1957. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 27:325–349 [Google Scholar]
- 9. Brune A, Emerson D, Breznak JA. 1995. The termite gut microflora as an oxygen sink: microelectrode determination of oxygen and pH gradients in guts of lower and higher termites. Appl. Environ. Microbiol. 61:2681–2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brune A. 1998. Termite guts: the world's smallest bioreactors. Trends Biotechnol. 16:16–21 [Google Scholar]
- 11. Brune A. 2010. Methanogenesis in the digestive tracts of insects, p 707–728 In Timmis KN. (ed), Handbook of hydrocarbon and lipid microbiology, vol 8 Springer, Heidelberg, Germany [Google Scholar]
- 12. Brune A, Kühl M. 1996. pH profiles of the extremely alkaline hindguts of soil-feeding termites (Isoptera: Termitidae) determined with microelectrodes. J. Insect Physiol. 42:1121–1127 [Google Scholar]
- 13. Brune A, Ohkuma M. 2011. Role of the termite gut microbiota in symbiotic digestion, p 439–475 In Bignell DE, Roisin Y, Lo N. (ed), Biology of termites: a modern synthesis. Springer, Dordrecht, Netherlands [Google Scholar]
- 14. Chao A. 1984. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 11:265–270 [Google Scholar]
- 15. Chao A, Shen TJ. 2003. Nonparametric estimation of Shannon's index of diversity when there are unseen species in sample. Environ. Ecol. Stat. 10:429–443 [Google Scholar]
- 16. Cotta M, Forster R. 2006. The family Lachnospiraceae, including the genera Butyrivibrio, Lachnospira and Roseburia, p 1002–1021 In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E. (ed), The prokaryotes, 3rd ed, vol 4 Springer, New York, NY [Google Scholar]
- 17. Czolij R, Slaytor M, O'Brien RW. 1985. Bacterial flora of the mixed segment and the hindgut of the higher termite Nasutitermes exitiosus Hill (Termitidae, Nasutitermitinae). Appl. Environ. Microbiol. 49:1226–1236 [Google Scholar]
- 18. Ebert A, Brune A. 1997. Hydrogen concentration profiles at the oxic-anoxic interface: a microsensor study of the hindgut of the wood-feeding lower termite Reticulitermes flavipes (Kollar). Appl. Environ. Microbiol. 63:4039–4046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egert M, Wagner B, Lemke T, Brune A, Friedrich MW. 2003. Microbial community structure in midgut and hindgut of the humus-feeding larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Appl. Environ. Microbiol. 69:6659–6668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Engelbrektson A, et al. 2010. Experimental factors affecting PCR-based estimates of microbial species richness and evenness. Environ. Microbiol. 4:642–647 [DOI] [PubMed] [Google Scholar]
- 21. Friedrich MW, Schmitt-Wagner D, Lueders T, Brune A. 2001. Axial differences in community structure of Crenarchaeota and Euryarchaeota in the highly compartmentalized gut of the soil-feeding termite Cubitermes orthognathus. Appl. Environ. Microbiol. 67:4880–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fröhlich J, et al. 1999. Isolation of Desulfovibrio intestinalis sp. nov. from the hindgut of the lower termite Mastotermes darwiniensis. Can. J. Microbiol. 45:145–152 [DOI] [PubMed] [Google Scholar]
- 23. Fujita A, Abe T. 2002. Amino acid concentration and distribution of lysozyme and protease activities in the guts of higher termites. Physiol. Entomol. 27:76–78 [Google Scholar]
- 24. Graber JR, Leadbetter JR, Breznak JA. 2004. Description of Treponema azotonutricium sp. nov. and Treponema primitia sp. nov., the first spirochetes isolated from termite guts. Appl. Environ. Microbiol. 70:1315–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Henckel T, Friedrich M, Conrad R. 1999. Molecular analyses of the methane-oxidizing microbial community in rice field soil by targeting the genes of the 16S rRNA, particulate methane monooxygenase, and methanol dehydrogenase. Appl. Environ. Microbiol. 65:1980–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hongoh Y, et al. 2006. Phylogenetic diversity, localization and cell morphologies of the candidate phylum TG3 and a subphylum in the phylum Fibrobacteres, recently found bacterial groups dominant in termite guts. Appl. Environ. Microbiol. 72:6780–6788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hongoh Y, et al. 2005. Intra- and interspecific comparisons of bacterial diversity and community structure support coevolution of gut microbiota and termite host. Appl. Environ. Microbiol. 71:6590–6599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang Y, et al. 2005. Sanguibacter marinus sp. nov., isolated from coastal sediment. Int. J. Syst. Evol. Microbiol. 55:1755–1758 [DOI] [PubMed] [Google Scholar]
- 29. Hugenholtz P, Goebel BM. 2001. The polymerase chain reaction as a tool to investigate microbial diversity in environmental samples, p 31–41 In Rochelle PA. (ed), Environmental molecular microbiology: protocols and applications. Horizon Press Inc., New York, NY [Google Scholar]
- 30. Jouquet P, Traoré S, Choosai C, Hartmann C, Bignell D. 2011. Influence of termites on ecosystem functioning. Ecosystem services provided by termites. Eur. J. Soil Biol. 47:215–222 [Google Scholar]
- 31. Kuhnigk T, Branke J, Krekeler D, Cypionka H, König H. 1996. A feasible role of sulfate-reducing bacteria in the termite gut. Syst. Appl. Microbiol. 19:139–149 [Google Scholar]
- 32. Leadbetter JR, Schmidt TM, Graber JR, Breznak JA. 1999. Acetogenesis from H2 plus CO2 by spirochetes from termite guts. Science 283:686–689 [DOI] [PubMed] [Google Scholar]
- 33. Leaphart AB, Lovell CR. 2001. Recovery and analysis of formyltetrahydrofolate synthetase gene sequences from natural populations of acetogenic bacteria. Appl. Environ. Microbiol. 67:1392–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Legendre P, Legendre L. 1998. Numerical ecology, 2nd ed. Elsevier, Amsterdam, Netherlands [Google Scholar]
- 35. Leschine S, Paster B, Canale-Parola E. 2006. Free-living saccharolytic spirochetes: the genus Spirochaeta, p 195–210 In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E(ed) The prokaryotes, 3rd ed, vol 7 Springer, New York, NY [Google Scholar]
- 36. Ley RE, et al. 2008. Evolution of mammals and their gut microbes. Science 320:1647–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liesack W, Bak F, Kreft JU, Stackebrandt E. 1994. Holophaga foetida gen. nov., sp. nov., a new, homoacetogenic bacterium degrading methoxylated aromatic compounds. Arch. Microbiol. 162:85–90 [DOI] [PubMed] [Google Scholar]
- 38. Lo N, Bandi C, Watanabe H, Nalepa C, Beninati T. 2003. Evidence for co-cladogenesis between diverse dictyopteran lineages and their intracellular endosymbionts. Mol. Biol. Evol. 20:907–913 [DOI] [PubMed] [Google Scholar]
- 39. Ludwig W, et al. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muyzer G, de Waal EC, Uitterlinden AG. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nakajima H, Hongoh Y, Usamib R, Kudo T, Ohkuma M. 2005. Spatial distribution of bacterial phylotypes in the gut of the termite Reticulitermes speratus and the bacterial community colonizing the gut epithelium. FEMS Microbiol. Ecol. 54:247–255 [DOI] [PubMed] [Google Scholar]
- 42. Nakajima H, et al. 2006. Phylogenetic and morphological diversity of Bacteroidales members associated with the gut wall of termites. Biosci. Biotechnol. Biochem. 70:211–218 [DOI] [PubMed] [Google Scholar]
- 43. Noda S, et al. 2006. Identification and characterization of ectosymbionts of distinct lineages in Bacteroidales attached to flagellated protists in the gut of termites and a wood-feeding cockroach. Environ. Microbiol. 8:11–20 [DOI] [PubMed] [Google Scholar]
- 44. Noirot C. 2001. The gut of termites (Isoptera). Comparative anatomy, systematics, phylogeny. II. Higher termites (Termitidae). Ann. Soc. Entomol. Fr. (N.S.) 37:431–471 [Google Scholar]
- 45. Ohkuma M, Brune A. 2011. Diversity, structure, and evolution of the termite gut microbial community, p 413–438 In Bignell DE, Roisin Y, Lo N. (ed), Biology of termites: a modern synthesis. Springer, Dordrecht, Netherlands [Google Scholar]
- 46. Ohkuma M, Iida T, Kudo T. 1999. Phylogenetic relationships of symbiotic spirochetes in the gut of diverse termites. FEMS Microbiol. Lett. 181:123–129 [DOI] [PubMed] [Google Scholar]
- 47. Ottesen EA, Leadbetter JR. 2011. Formyltetrahydrofolate synthetase gene diversity in the guts of higher termites with different diets and lifestyles. Appl. Environ. Microbiol. 77:3461–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Paster BJ, et al. 1996. Phylogeny of not-yet-cultured spirochetes from termite guts. Appl. Environ. Microbiol. 62:347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pernthaler A, Pernthaler J, Amann R. 2004. Sensitive multi-color fluorescence in situ hybridization for the identification of environmental microorganisms, p 711–726 In Kowalchuk GA, de Brujin FJ, Head IM, Akkermans ADL, van Elsas JD. (ed), Molecular microbial ecology manual, 2nd ed, vol 1 Kluwer Academic Publishers, Dordrecht, Netherlands [Google Scholar]
- 50. Pernthaler J, Glöckner FO, Schönhuber W, Amann R. 2001. Fluorescence in situ hybridization with rRNA-targeted oligonucleotide probes, p 207–226 In Paul J. (ed), Methods in microbiology: marine microbiology, vol 30 Academic Press, London, United Kingdom [Google Scholar]
- 51. Pester M, Brune A. 2006. Expression profiles of fhs (FTHFS) genes support the hypothesis that spirochaetes dominate reductive acetogenesis in the hindgut of lower termites. Environ. Microbiol. 8:1261–1270 [DOI] [PubMed] [Google Scholar]
- 52. Pester M, Brune A. 2007. Hydrogen is the central free intermediate during lignocellulose degradation by termite gut symbionts. ISME J. 1:551–565 [DOI] [PubMed] [Google Scholar]
- 53. Pruesse E, et al. 2007. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 35:7188–7196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rieu-Lesme F, Morvan B, Collins MD, Fonty G, Willems A. 1996. A new H2/CO2-using acetogenic bacterium from the rumen: description of Ruminococcus schinkii sp. nov. FEMS Microbiol. Lett. 140:281–286 [DOI] [PubMed] [Google Scholar]
- 55. Salmassi TM, Leadbetter JR. 2003. Molecular aspects of CO2-reductive acetogenesis in cultivated spirochetes and the gut community of the termite Zootermopsis angusticollis. Microbiology 149:2529–2537 [DOI] [PubMed] [Google Scholar]
- 56. Scheffrahn RH, Cabrera BJ, Kern WH, Jr, Su N-Y. 2002. Nasutitermes costalis (Isoptera: Termitidae) in Florida: first record of a non-endemic establishment by a higher termite. Florida Entomol. 85:273–275 [Google Scholar]
- 57. Scheffrahn RH, Krecek J, Szalanski AL, Austin JW. 2005. Synonymy of the neotropical arboreal termites Nasutitermes corniger and N. costalis (Isoptera: Termitidae: Nasutitermitinae), with evidence from morphology, genetics, and biogeography. Ann. Entomol. Soc. Am. 98:273–281 [Google Scholar]
- 58. Schloss PD, Gevers D, Westcott SL. 2011. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS One 6:e27310 doi:10.1371/journal.pone.0027310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schloss PD, et al. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75:7537–7541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schmitt-Wagner D, Brune A. 1999. Hydrogen profiles and localization of methanogenic activities in the highly compartmentalized hindgut of soil-feeding higher termites (Cubitermes spp.). Appl. Environ. Microbiol. 65:4490–4496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schmitt-Wagner D, Friedrich MW, Wagner B, Brune A. 2003. Phylogenetic diversity, abundance, and axial distribution of bacteria in the intestinal tract of two soil-feeding termites (Cubitermes spp.). Appl. Environ. Microbiol. 69:6007–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schmitt-Wagner D, Friedrich MW, Wagner B, Brune A. 2003. Axial dynamics, stability, and interspecies similarity of bacterial community structure in the highly compartmentalized gut of soil-feeding termites (Cubitermes spp.). Appl. Environ. Microbiol. 69:6018–6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sogin ML, et al. 2006. Microbial diversity in the deep sea and the underexplored “rare biosphere.” Proc. Natl. Acad. Sci. U. S. A. 103:12115–12120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Suen G, et al. 2011. The complete genome sequence of Fibrobacter succinogenes S85 reveals a cellulolytic and metabolic specialist. PLoS One 6:e18814 doi:10.1371/journal.pone.0018814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sugimoto A, et al. 1998. Methane and hydrogen production in a termite-symbiont system. Ecol. Res. 13:241–257 [Google Scholar]
- 66. Tholen A, Brune A. 2000. Impact of oxygen on metabolic fluxes and in situ rates of reductive acetogenesis in the hindgut of the wood-feeding termite Reticulitermes flavipes. Environ. Microbiol. 2:436–449 [DOI] [PubMed] [Google Scholar]
- 67. Tholen A, Pester M, Brune A. 2007. Simultaneous methanogenesis and oxygen reduction by Methanobrevibacter cuticularis at low oxygen fluxes. FEMS Microbiol. Ecol. 62:303–312 [DOI] [PubMed] [Google Scholar]
- 68. Thongaram T, et al. 2005. Comparison of bacterial communities in the alkaline gut segment among various species of higher termites. Extremophiles 9:229–238 [DOI] [PubMed] [Google Scholar]
- 69. Tokuda G, et al. 2012. Cellulolytic environment in the midgut of the wood-feeding higher termite Nasutitermes takasagoensis. J. Insect Physiol. 58:147–154 [DOI] [PubMed] [Google Scholar]
- 70. Tokuda G, Watanabe H. 2007. Hidden cellulases in termites: revision of an old hypothesis. Biol. Lett. 3:336–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tokuda G, Yamaoka I, Noda H. 2000. Localization of symbiotic clostridia in the mixed segment of the termite Nasutitermes takasagoensis (Shiraki). Appl. Environ. Microbiol. 66:2199–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73:5261–5267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Warnecke F, et al. 2007. Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature 450:560–565 [DOI] [PubMed] [Google Scholar]
- 74. Watanabe H, Tokuda G. 2010. Cellulolytic systems in insects. Annu. Rev. Entomol. 55:609–632 [DOI] [PubMed] [Google Scholar]
- 75. Werner JJ, et al. 2012. Impact of training sets on classification of high-throughput bacterial 16S rRNA gene surveys. ISME J. 6:94–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.