Abstract
Glycogen is accumulated during the latter half of the diel cycle in Synechococcus sp. strain WH8103 following a midday maximum in glgA (encoding glycogen synthase) mRNA abundance. This temporal pattern is quite distinct from that of Prochlorococcus and may highlight divergent regulatory control of carbon/nitrogen metabolism in these closely related picocyanobacteria.
TEXT
Picoplanktonic cyanobacteria (Prochlorococcus and Synechococcus) contribute substantially to primary production in the world's oceans (4, 10, 12). Like other cyanobacteria, they accumulate storage polysaccharides (glycogen) during daylight hours, which provide an important source of carbon and energy to support nocturnal respiratory activity (11). Glycogen is synthesized by the product of glgA (glycogen synthase) as a linear molecule of α-1,4-linked glucose subunits which is modified by a branching enzyme to produce the mature reserve polymer.
Inactivation of glgA not only abolishes glycogen synthesis in Synechococcus strain PCC7942 but also enhances the sensitivity of mutants to salt and oxidative (H2O2) stress (23), an intriguing phenotype that suggests additional physiological roles for glycogen in cyanobacteria of potential ecological relevance. Here, we report on the temporal regulation of glgA expression and glycogen metabolism in Synechococcus sp. strain WH8103 and show that there are marked differences in the temporal patterns of glycogen metabolism in picocyanobacteria that may reflect divergent strategies in the assimilation of carbon and nitrogen over the diel cycle.
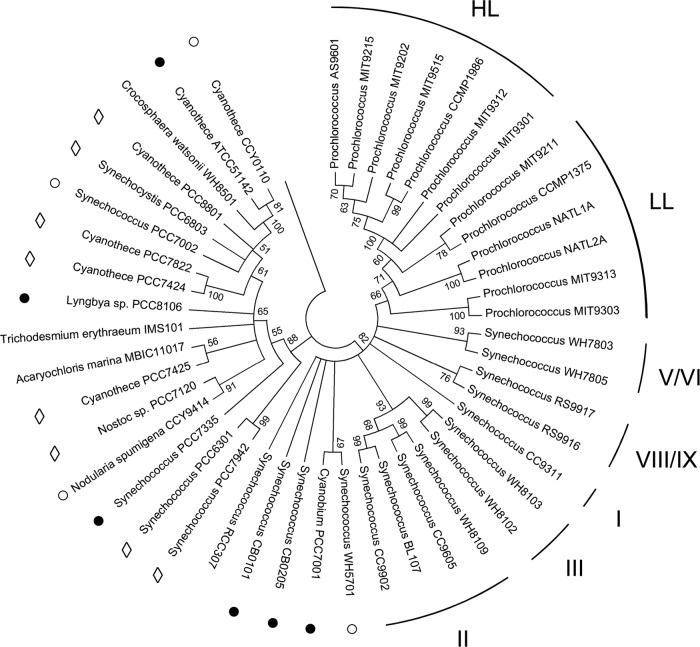
A fragment of Synechococcus strain WH8103 glgA was amplified by PCR using the primer pair GlgAFor/GlgARev (Table 1), cloned in pCR2.1-TOPO (Invitrogen, Paisley, United Kingdom), and sequenced bidirectionally (Source BioSciences LifeSciences, London, United Kingdom). Evolutionary analysis of the derived peptide sequence of glgA by the maximum likelihood method (24) placed most Synechococcus strains and all Prochlorococcus strains in a lineage distinct from other cyanobacteria of marine origin (Fig. 1). Picoplanktonic Synechococcus formed a monophyletic cluster encompassing subcluster 5.1 strains (5), including WH8103 in the previously designated clade III (3) and also subcluster 5.2, of which WH5701 is the type strain (5).
Table 1.
Oligonucleotide primers and PCR conditions used in this study
| Primer designation | Sequence (5′–3′) | Product size (bp) | Reaction conditions | Source or reference |
|---|---|---|---|---|
| GlgAFor | ATGATHCCNGTNTGGATGCA | 677 | 95°C, 2 min; (94°C, 30 s; 58°C, 30 s; 72°C, 45 s) × 25; 72°C, 10 min | This study |
| GlgARev | GGCTCGAANCKNAWNGGCAT | 677 | ||
| QGlgF | TTCACCATCCACAACCTCAA | 205 | 95°C, 10 min; (94°C, 15 s; 60°C, 30 s; 72°C, 60 s) × 40; increase from 55 to 95°C at 0.2°C s−1 | This study |
| QGlgR | CGAAATTGAGCAAACCATCC | 205 | ||
| QRNPB F | TGAGGAGAGTGCCACAGAAA | 238 | 95°C, 10 min; (94°C, 15 s; 60°C, 30 s; 72°C, 60 s) × 40; increase from 55 to 95°C at 0.2°C s−1 | 28 |
| QRNPB R | AAGAGGGTGGGTGGCTATCT | 238 |
Fig 1.
Consensus phylogram (500 bootstrap replicates) of cyanobacterial GlgA rooted with the orthologous peptide sequence from Streptococcus dysgalactiae subsp. equisimilis GGS124 (GenBank accession number YP_002996437). Sequences were aligned with MUSCLE using the UPGMB clustering method (2), and an evolutionary analysis based on 211 amino acid residues (gaps were removed) was conducted in MEGA5 using the maximum likelihood method based on the Dayhoff matrix model (24). All cyanobacterial taxa were isolated from open marine waters except those indicated with the following symbols: ○, coastal; ●, estuarine/intertidal; ♢, freshwater. Prochlorococcus ecotypes previously designated as either high-light (HL) or low-light (LL) adapted (19) are indicated. The Roman numerals correspond to the individual clades of Synechococcus spp. proposed by Fuller et al. (3), which are based on phylogenetic analyses of 16S rRNA gene sequences. The overall percentages of trees in which the designated branches received ≥50% support in the bootstrap test are indicated at the respective nodes.
Prochlorococcus formed a sister group in which high-light (HL) ecotypes were found in a single well-supported cluster, whereas low-light (LL) Prochlorococcus ecotypes, which are probably a paraphyletic grouping (30), were less clearly resolved from Synechococcus subcluster 5.1 strains. The lack of phylogenetic resolution among Prochlorococcus LL ecotypes has been attributed to introgression due to extensive horizontal gene transfer between Synechococcus and these organisms (30) and, in particular, the two isolates (MIT9303 and MIT9313) with the largest genomes that cluster most closely with Synechococcus (Fig. 1).
To investigate the diel regulation of glgA, light-limited continuous cultures of Synechococcus sp. strain WH8103 were grown in artificial seawater (ASW) medium (29) at 60 μmol photons m−2 s−1 and at 25°C in 1-liter water-jacketed vessels (Fig. 2) under a 16-h-light–8-h-dark cycle. Illumination was provided by a “Dusk till Dawn” self-dimming lighting system fitted with T5 Aquablue Plus bulbs (D-D The Aquarium Solution Ltd., Ilford, United Kingdom) programmed to deliver simulated 30-min-long “dawn” and “dusk” periods at the beginning and end of each light cycle. Samples of the culture suspension were obtained synoptically over 1-h periods, preserved with RNAlater (Invitrogen, Paisley, United Kingdom), and subsampled for the determination of the frequency of dividing cells (FDC) (1). The remaining samples were then centrifuged at 16,000 × g for 20 min, and the cell pellets were fractionated for the estimation of glycogen concentrations (15, 22), protein (DC protein assay kit; Bio-Rad, Hemel Hempstead, United Kingdom), and the extraction of RNA for cDNA synthesis (28). glgA mRNA abundance was determined by quantitative reverse transcription-PCR (qRT-PCR) using the primer pair QGlgF/QGlgR (Table 1) and normalized between samples using the housekeeping gene rnpB and QRNP primer pair as described previously (28).
Fig 2.
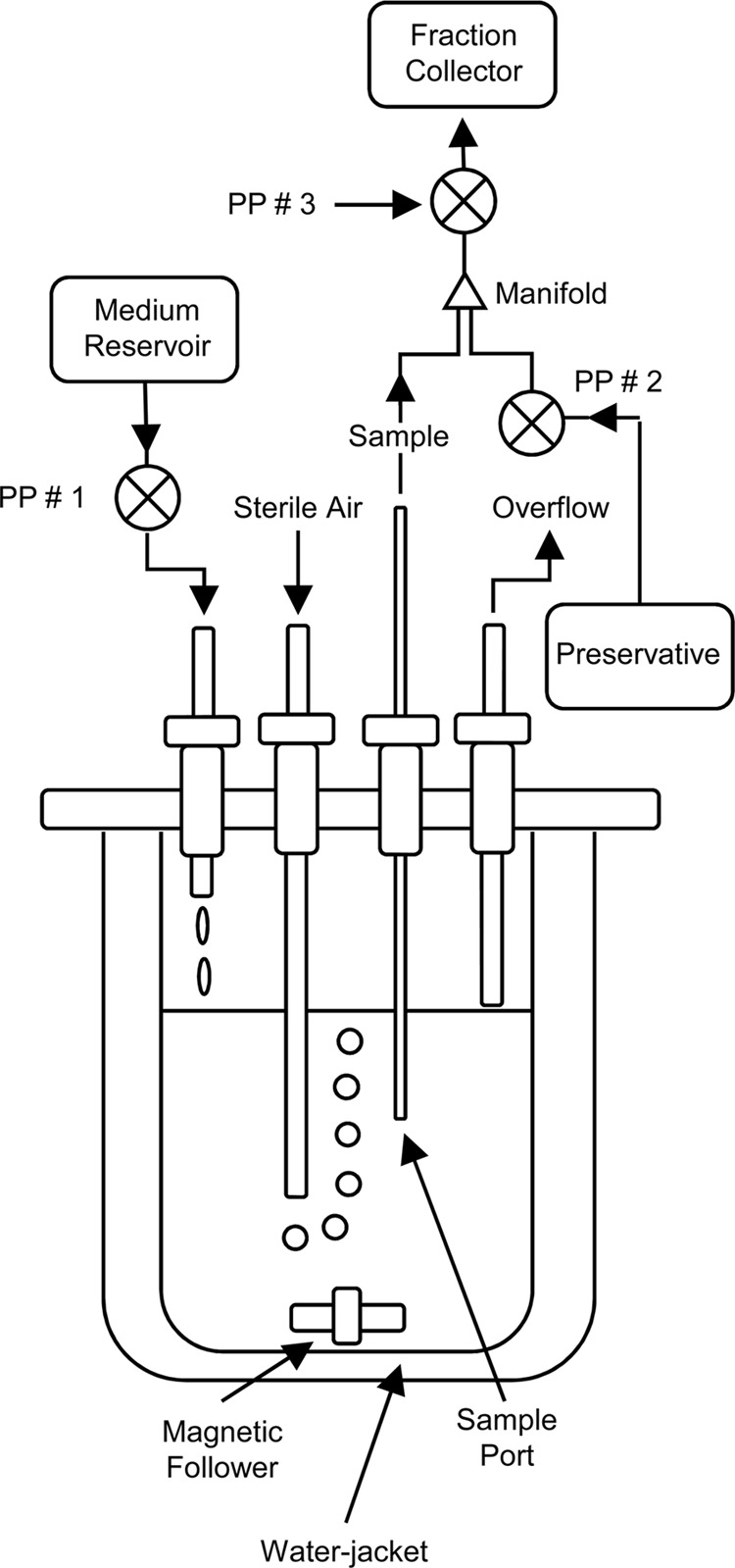
Diagram of the water-jacketed continuous culture apparatus used for the growth of Synechococcus sp. strain WH8103. Steady-state cultures were diluted with fresh medium introduced by a peristaltic pump (PP #1) at a flow rate of 14.2 ml hour−1 (μ = 0.341 day−1; g = 2.033 days). Preliminary experiments showed that the cultures were light limited; the specific growth rate was higher in more-dilute cultures during batch growth (μ = 0.659 day−1) and increased transiently following an increase in irradiance to 80 μmol photons m−2 s−1 until steady state was reestablished at a greater cell density. Experimental culture samples were obtained synoptically (in 1-h “bins”) by withdrawing cell suspension (∼5 ml hour−1) through a sampling port (transit time, ∼4 min inlet to outlet) into a three-way manifold in which the sample was combined with >2 volumes of the preservative RNAlater (Invitrogen, Paisley, United Kingdom) and then mixed further by passage through the rotor housing of the peristaltic pump labeled PP #3. The preserved samples were collected at 4°C in RNase-free tubes for 25 to 26 h using a programmable fraction collector.
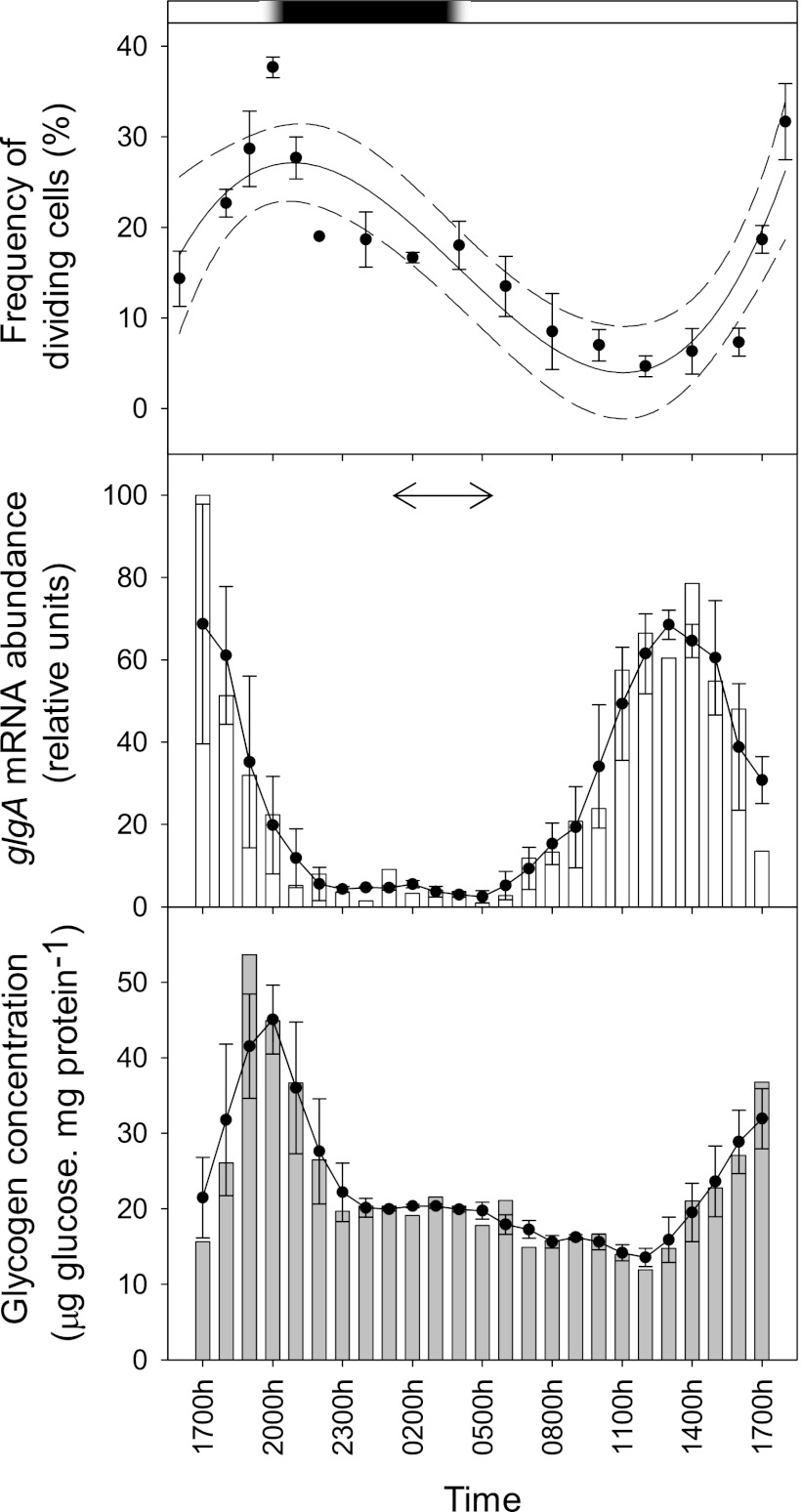
Cell cycle progression was synchronized to the photoperiod in Synechococcus sp. WH8103 (Fig. 3), with the peak in FDC appearing at subjective dusk with a similar temporal periodicity to that reported for batch cultures of this strain under a 12-h-light–12-h-dark cycle (6) and natural Synechococcus populations from a range of ocean provinces (e.g., see references 1, 25, and 27). Glycogen synthase expression was closely correlated with the division cycle; glgA mRNA abundance was at its minimum throughout the night but rose in the early part of the light phase to a midday peak coincident with the daily minimum in FDC. Following the upregulation of glgA, glycogen concentrations increased ∼3-fold over the second half of the light phase to reach a maximum at “dusk” just prior to the glgA transcriptional minimum and the nocturnal decline in FDC due to cell division (Fig. 3).
Fig 3.
(Top) Diel variability (mean ± 1 standard error [SE]; n ≥ 300 cells) in the frequency of dividing cells (FDC) (1) in Synechococcus sp. strain WH8103 grown in continuous cultures under a light-dark cycle. The data are fitted with a third-order linear regression curve (solid line; r2 = 0.766), and the 95% confidence intervals are shown by the dashed lines. The bar at the top of the figure shows the periodicity of the light (white) and dark (black) phases over the 24-h cycle, while the 30-min-long dusk and dawn intervals are indicated by the regions of shaded gradation. (Middle) Diel variability in glgA mRNA abundance (bars) normalized to the housekeeping gene rnpB (16, 28) and expressed as a percentage of the daily maximum. The closed circles and solid line show the 3-h central moving average (±1 SE) over the diel cycle. PCR efficiency was 96.45 to 98.92% (r2 = 0.997 to 0.999) and 95.11 to 102.78% (r2 = 0.998) for the QGlgF/QGlgR and QRNPB F/QRNPB R primer pairs, respectively. The temporal maximum in glgA mRNA reported for Prochlorococcus strain CCMP 1986 (31) is indicated by the double-headed arrow for comparison. (Bottom) Diel variability in glycogen concentration (bars) normalized to protein content. The closed circles and solid line show the 3-h central moving average (±1 SE) over the diel cycle.
Although the genes share an evolutionary origin (Fig. 1), the control of glgA expression that was observed was markedly different from that of Prochlorococcus strain CCMP 1986 (31). In this HL ecotype, glgA transcription peaks in concert with rbcLS (encoding RubisCO) and other photosynthesis genes during the dark-to-light transition much earlier in the cell cycle (Fig. 3). While comparisons between species grown under different experimental regimes (a 14-h-light–10-h-dark cycle for CCMP 1986 versus a 16-h-light–8-h-dark cycle for Synechococcus sp. WH8103) require caution, these observations suggest some divergence of the temporal patterns of carbon (and nitrogen) metabolism between these organisms.
The diel rhythm of rbcLS expression is similar to Synechococcus (17, 26, 27), but the temporal regulation of N assimilation in Prochlorococcus CCMP 1986 is unusual. Ammonium assimilation genes, including glnA (glutamine synthetase [GS]), have late-evening expression maxima, whereas in Synechococcus (27), Synechocystis strain PCC6803, and Synechococcus sp. PCC7942, glnA is expressed maximally during mid-light phase (8, 9, 13). N assimilation is strictly light dependent in Synechocystis PCC6803, and GS is rapidly inactivated following transfer to darkness (18). In contrast, the 2-oxoglutarate C skeletons required for N assimilation are probably derived from dark glycogen hydrolysis in Prochlorococcus CCMP 1986 (31), a metabolic arrangement that mimics the temporal separation of C and N metabolism in some diazotrophic cyanobacteria (20, 21). The additional nighttime demand for C skeletons in Prochlorococcus, therefore, may underpin why glgA is upregulated much earlier in the cell cycle than reported here for Synechococcus.
If such divergent metabolic organization is typical, temporal segregation of N uptake and assimilation of potential ecological relevance may occur in those waters where these picocyanobacteria cooccur. It is not clear what might have driven the adoption of distinct carbon/nitrogen assimilation strategies in these organisms, but if glycogen accumulation also enhances oxidative stress resistance in Prochlorococcus (23), then diverting fixed C to glycogen throughout the daylight hours may enhance the fitness of high-light ecotypes like CCMP 1986 that are somewhat less resistant to UV radiation than Synechococcus (7, 14).
Nucleotide sequence accession number.
The DNA sequence of glgA from Synechococcus sp. strain WH8103 has been deposited in GenBank under the accession number GU808826.
ACKNOWLEDGMENT
This work was supported by the Natural Environment Research Council (NERC) Post-Genomics and Proteomics program (grant NE/C507902/1).
Footnotes
Published ahead of print 20 April 2012
REFERENCES
- 1. Campbell L, Carpenter EJ. 1986. Die1 patterns of cell division in marine Synechococcus spp. (Cyanobacteria): use of the frequency of dividing cells technique to measure growth rate. Mar. Ecol. Prog. Ser. 32:139–148 [Google Scholar]
- 2. Edgar RG. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fuller NJ, et al. 2003. Clade-specific 16S ribosomal DNA oligonucleotides reveal the predominance of a single marine Synechococcus clade throughout a stratified water column in the Red Sea. Appl. Environ. Microbiol. 69:2430–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goericke R, Welschmeyer NA. 1993. The marine prochlorophyte Prochlorococcus contributes significantly to phytoplankton biomass and primary production in the Sargasso Sea. Deep Sea Res. 40:2283–2294 [Google Scholar]
- 5. Herdman M, Castenholz RW, Iteman I, Waterbury JB, Rippka R. 2001. Subsection I (formerly Chroococcales Wettstein 1924, emend. Rippka, Deruelles, Waterbury, Herdman and Stanier 1979), p 493–514 In Boone DR, Castenholz RW, Garrity GM. (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 1 Springer, New York, NY [Google Scholar]
- 6. Jacquet S, Partensky F, Lennon J-F, Vaulot D. 2001. Diel patterns of growth and division in marine picoplankton in culture. J. Phycol. 37:357–369 [Google Scholar]
- 7. Kolowrat C, et al. 2010. Ultraviolet stress delays chromosome replication in light/dark synchronized cells of the marine cyanobacterium Prochlorococcus marinus PCC9511. BMC Microbiol. 10:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kucho K, et al. 2005. Global analysis of circadian expression in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 187:2190–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Labiosa RG, et al. 2006. Examination of diel changes in global transcript accumulation in Synechocystis. J. Phycol. 42:622–636 [Google Scholar]
- 10. Li WKW. 1995. Composition of ultraphytoplankton in the central North Atlantic. Mar. Ecol. Prog. Ser. 122:1–8 [Google Scholar]
- 11. Lichtlé C, Thomas JC, Spilar A, Partensky F. 1995. Immunological and ultrastructural characterization of the photosynthetic complexes of the prochlorophyte Prochlorococcus (Oxychlorobacteria). J. Phycol. 31:934–941 [Google Scholar]
- 12. Liu H, Nolla HA, Campbell L. 1997. Prochlorococcus growth rate and contribution to primary production in the equatorial and subtropical North Pacific Ocean. Aquat. Microb. Ecol. 12:39–47 [Google Scholar]
- 13. Liu Y, et al. 1995. Circadian orchestration of gene expression in cyanobacteria. Genes Dev. 9:1469–1478 [DOI] [PubMed] [Google Scholar]
- 14. Morris JJ, Johnson ZI, Szul MJ, Keller M, Zinser ER. 2011. Dependence of the cyanobacterium Prochlorococcus on hydrogen peroxide scavenging microbes for growth at the ocean's surface. PLoS One 6:e16805 doi: 10.1371/journal.pone.0016805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ortega-Calvo JJ, Stal LJ. 1991. Diazotrophic growth of the unicellular cyanobacterium Gloeothece sp. PCC 6909 in continuous culture. J. Gen. Microbiol. 137:1789–1797 [Google Scholar]
- 16. Pfaffl MW. 2001. A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pichard SL, Campbell L, Kang JB, Tabita FR, Paul JH. 1996. Regulation of ribulose bisphosphate carboxylase gene expression in natural phytoplankton communities. I. Diel rhythms. Mar Ecol. Prog. Ser. 139:257–265 [Google Scholar]
- 18. Reyes JC, Crespo JL, Garcia-Dominguez M, Florencio FJ. 1995. Electron transport controls glutamine synthetase activity in the facultative heterotrophic cyanobacterium, Synechocystis sp. PCC 6803. Plant Physiol. 109:899–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rocap G, Distel DL, Waterbury JB, Chisholm SW. 2002. Resolution of Prochlorococcus and Synechococcus ecotypes using 16S-23S rRNA internal transcribed spacer ITS region sequences. Appl. Environ. Microbiol. 68:1180–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi T, Ilikchyan I, Rabouille S, Zehr JP. 2010. Genome-wide analysis of diel gene expression in the unicellular N2-fixing cyanobacterium, Crocosphaera watsonii WH 8501. ISME J. 4:621–632 [DOI] [PubMed] [Google Scholar]
- 21. Stöckel J, et al. 2008. Global transcriptomic analysis of Cyanothece 51142 reveals robust diurnal oscillation of central metabolic processes. Proc. Natl. Acad. Sci. U. S. A. 105:6156–6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Strickland JDH, Parsons TR. 1968. A practical handbook of seawater analysis. Fisheries Research Board of Canada, Ottawa, Ontario, Canada [Google Scholar]
- 23. Suzuki E, et al. 2010. Carbohydrate metabolism in mutants of the cyanobacterium Synechococcus elongatus PCC 7942 defective in glycogen synthesis. Appl. Environ. Microbiol. 76:3153–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamura K, et al. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaulot D, Lebot N, Marie D, Fukai E. 1996. Effect of phosphorus on the Synechococcus cell cycle in surface Mediterranean waters during summer. Appl. Environ. Microbiol. 62:2527–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watson GMF, Tabita FR. 1996. Regulation, unique gene organization, and unusual primary structure of carbon fixation genes from a marine phycoerythrin-containing cyanobacterium. Plant Mol. Biol. 32:1103–1115 [DOI] [PubMed] [Google Scholar]
- 27. Wyman M. 1999. Diel rhythms in ribulose-1,5-bisphosphate carboxylase/oxygenase and glutamine synthetase gene expression in a natural population of marine picoplanktonic cyanobacteria (Synechococcus spp.). Appl. Environ. Microbiol. 65:3651–3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wyman M, Bird C. 2007. Lack of control of nitrite assimilation by ammonium in an oceanic picocyanobacterium, Synechococcus sp. strain WH 8103. Appl. Environ. Microbiol. 73:3028–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wyman M, Gregory RPF, Carr NG. 1985. Novel role for phycoerythrin in a marine cyanobacterium, Synechococcus strain DC2. Science 230:818–820 [DOI] [PubMed] [Google Scholar]
- 30. Zhaxybayeva O, Doolittle WF, Papke RT, Gogarten JP. 2009. Intertwined evolutionary histories of marine Synechococcus and Prochlorococcus marinus. Genome Biol. Evol. 1:325–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zinser ER, et al. 2009. Choreography of the transcriptome, photophysiology, and cell cycle of a minimal photoautotroph, Prochlorococcus. PLoS One 4:e5135 doi: 10.1371/journal.pone.0005135 [DOI] [PMC free article] [PubMed] [Google Scholar]