Abstract
Tinto River (Huelva, Spain) is a natural acidic rock drainage (ARD) environment produced by the bio-oxidation of metallic sulfides from the Iberian Pyritic Belt. This study quantified the abundance of diverse microbial populations inhabiting ARD-related sediments from two physicochemically contrasting sampling sites (SN and JL dams). Depth profiles of total cell numbers differed greatly between the two sites yet were consistent in decreasing sharply at greater depths. Although catalyzed reporter deposition fluorescence in situ hybridization with domain-specific probes showed that Bacteria (>98%) dominated over Archaea (<2%) at both sites, important differences were detected at the class and genus levels, reflecting differences in pH, redox potential, and heavy metal concentrations. At SN, where the pH and redox potential are similar to that of the water column (pH 2.5 and +400 mV), the most abundant organisms were identified as iron-reducing bacteria: Acidithiobacillus spp. and Acidiphilium spp., probably related to the higher iron solubility at low pH. At the JL dam, characterized by a banded sediment with higher pH (4.2 to 6.2), more reducing redox potential (−210 mV to 50 mV), and a lower solubility of iron, members of sulfate-reducing genera Syntrophobacter, Desulfosporosinus, and Desulfurella were dominant. The latter was quantified with a newly designed CARD-FISH probe. In layers where sulfate-reducing bacteria were abundant, pH was higher and redox potential and levels of dissolved metals and iron were lower. These results suggest that the attenuation of ARD characteristics is biologically driven by sulfate reducers and the consequent precipitation of metals and iron as sulfides.
INTRODUCTION
The Tinto River is a natural acidic rock drainage (ARD) environment located in the Huelva region, in southwestern Spain. On its way from Peña de Hierro to the Atlantic Ocean, the Tinto River emerges from the core of the Iberian Pyritic Belt (IPB), one of the largest metal sulfide (pyrite, chalcopyrite, etc.) deposits on Earth. When exposed to air and water, the microbiologically enhanced oxidation of sulfides produces waters with low pH (pH 2.3) and high ferric iron (∼2 g liter−1) and sulfate (∼6 g liter−1) contents along the river course. Low pH and ferric iron levels facilitate metal solubilization; therefore, when these waters come into contact with metallic sulfides, they become highly metalliferous (Cu, ∼0.1 g liter−1; Zn, ∼0.2 g liter−1) (20).
ARD results in contamination of aquifer, with secondary effects like the death of fish, rodents, and livestock and reduced crop yields (36). These unpleasant consequences have led to efforts to remediate ARD environments (21). Besides the environmental concerns, there are biological, geochemical, and biotechnological reasons to analyze microbial populations in acidic environments and to understand their ecology. In ARD, acidophilic, chemolithotrophic, aerobic Bacteria and Archaea dissolve metallic sulfides by oxidizing the iron and sulfur components. Products resulting from these oxidation processes can be used as electron acceptors in dissimilatory reduction of ferric iron and sulfate under anaerobic conditions linked to the carbon and nitrogen cycles. So far, only ARD aquifers have been studied intensively (7, 8, 19, 22, 23); in contrast, little is known about the ARD sediments (17, 41).
At the Tinto River, knowledge of the diversity and abundance of benthic microbial populations is limited to a methanogen population study (43) and a description of the microbial ecology of two contrasting sampling sites (the SN and JL dams) (42). In the latter study, an extensive survey of the Tinto River sediment microbiota was performed using two culture-independent approaches: denaturing gradient gel electrophoresis (DGGE) and 16S rRNA gene sequencing. The taxonomic affiliation of the identified Bacteria showed a high degree of diversity, falling into 5 different phyla: Proteobacteria, Firmicutes, Bacteroidetes, Acidobacteria, and Actinobacteria; meanwhile, all the Archaea were affiliated with the order Thermoplasmatales. Microorganisms involved in the iron (Acidithiobacillus ferrooxidans, Sulfobacillus spp., and Ferroplasma spp.), sulfur (Desulfurella spp., Desulfosporosinus spp., and Thermodesulfobium spp.), and carbon (Acidiphilium spp., Bacillus spp., Clostridium spp., and Acidobacterium spp.) cycles were identified. However, the abundance of these groups of organisms was unknown. In this study, the microbial communities of the Tinto River sediments were quantified for the first time and analyzed in relation to the physicochemical properties of the sediments with the objective of identifying the dominant and active organisms thriving in the sediments and inferring the main microbial activities taking place.
MATERIALS AND METHODS
Field site description and sampling.
The selected sediments are located in the Tinto River basin (Huelva). Samples were collected from two sampling sites (Fig. 1), the SN dam (37.72173°N, 6.557465°W) and the JL dam (37.691207°N, 6.560587°W), at different positions of the river. The SN dam is a spring feeding the main stream of the river, while JL is located in the main course of the river. Sediment cores (inner diameter, 7 cm; length, 45 cm) were taken with a sampler (Eijkelkamp Agrisearch equipment, The Netherlands). The cores were sliced at 5-cm intervals. The redox potential (E) and pH of the drill core samples were measured in situ with E and pH probes, connected to a Thermo Orion 290A potentiometer placed into the fresh sediment just after extraction of the core. Sediment samples for hybridization were fixed immediately in 4% (wt/vol) paraformaldehyde (PFA) in phosphate-buffered saline (PBS; 145 mM NaCl, 1.4 mM NaH2PO4, 8 mM Na2HPO4, pH 7.6) at 4°C for 4 h, washed twice with PBS, and then stored in 50% (vol/vol) ethanol-PBS at −20°C until further use.
Fig 1.
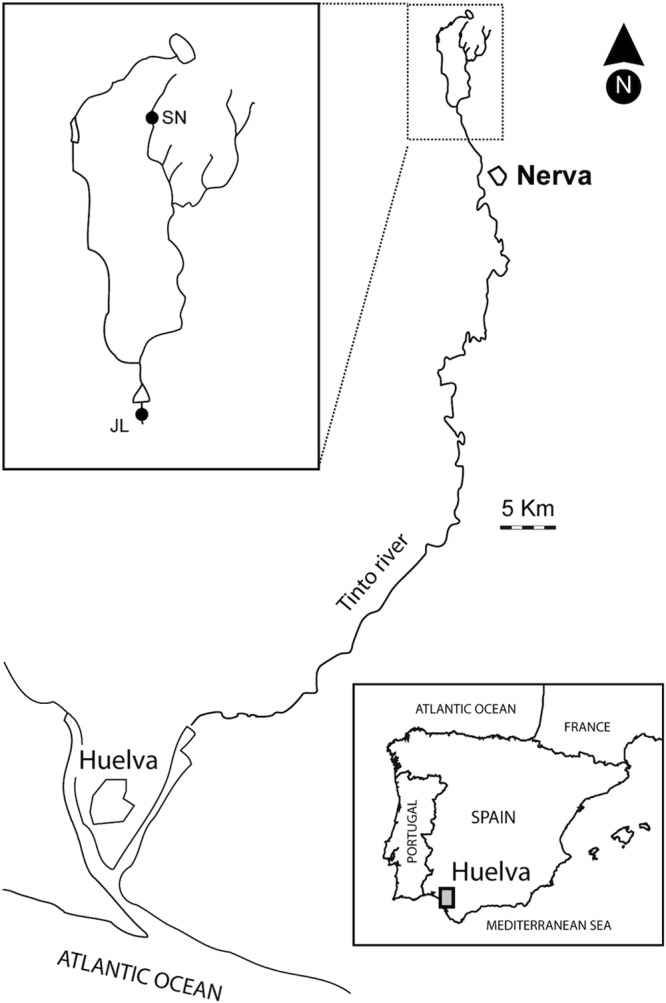
Map of Tinto River in Huelva (Spain) with the location of the studied dams. (Adapted from reference 16a with permission from Elsevier.)
Chemical analysis.
According to physicochemical parameters and visual appearance, the JL dam core was composed of two main layers, with black and brown colors, whereas the SN core looked homogeneous (Fig. 2a and e). The black (10 to 15 cm) and brown (25 to 30 cm) layers from the JL dam and two layers between 10 to 15 cm and 25 to 30 cm from the SN dam were excised and immediately stored in 15-ml tubes at 4°C until further processing in the laboratory (less than 1 week). Sediments were centrifuged at 13,000 × g for 5 min, and the supernatant was analyzed for the presence of heavy metals by inductively coupled plasma mass spectrometry (ICP-MS) and organic anions/volatile fatty acids (VFA) by ionic chromatography (IC) using an 861 Advanced Compact IC instrument. Water samples of both dams were also taken, kept at 4°C, and analyzed by total reflection X-ray fluorescence (TXRF) for S, P, Mn, Fe, Cu, Zn, As, and Ca; by ICP-MS for the rest of the elements; and by thermochemical oxidation (Thermostar LT200 of Hachlange) for total organic carbon (TOC).
Fig 2.
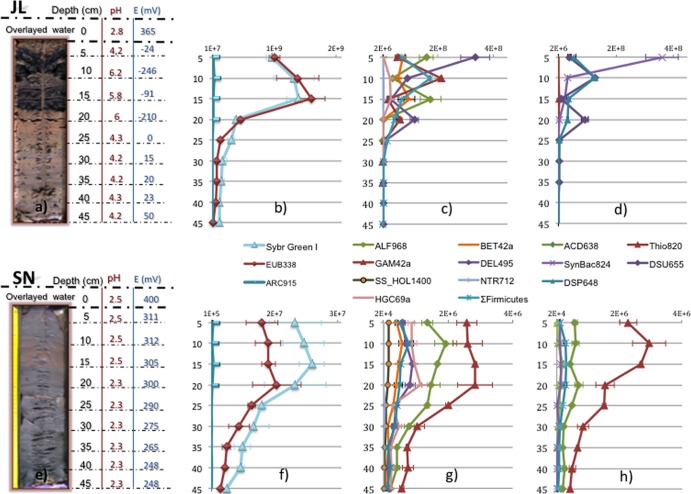
Depth profiles of pH and redox at JL (a) and SN (e), Sybr green I and domain-specific probes counts at JL (b) and SN (f), phylum-specific probes counts at JL (c) and SN (g), and genus-specific probes counts at JL (d) and SN (h).
Quantification of microorganisms. (i) Sybr green I direct counts.
Aliquots of fixed samples were sonicated to detach bacteria from the particles with a sterilized Sonic probe (Bandelin, Sonopuls HD200) for 20 s, 3 times at 20% intensity. Samples were kept on ice for 4 min to precipitate large sediment particles that could clog the filter, and then the supernatant was gently filtered through a black 0.2-μm polycarbonate membrane (Nuclepore; 25-mm diameter) (<0.2 bar). The filters were stained with Sybr green I in Moviol medium as described elsewhere (30). Samples were examined with an epifluorescence microscope (Axioplan 2; Carl Zeiss, Jena, Germany). A minimum of 1,000 Sybr green I-stained cells or at least 100 independent microscopic fields were counted.
(ii) CARD-FISH.
Samples were filtered following the same procedure as described above for the direct counts, except that white filters (0.2-μm pore size; Millipore GTTP) were used. In situ hybridizations with horseradish peroxidase (HRP)-labeled probes (biomers.net, Ulm, Germany) followed by catalyzed reporter deposition of fluorescently labeled tyramide were carried out as described elsewhere (38). Permeabilization was done with lysozyme (10 mg/ml) for 90 min at 37°C, followed by achromopeptidase (60 U/ml) for Gram-positive cells (44) and proteinase K for Archaea (15 μg/ml) (46). Probe sequences and formamide concentrations required for specific hybridization are given in Table 1. Hybridized samples were examined with an epifluorescence microscope (Axioplan 2). For each probe and sample, roughly 1,000 stained cells or at least 100 independent microscopic fields were counted. The signal obtained with probe NON338—a negative control—at each sampling site was subtracted from the specific counts. This signal was about 0.2% of the cell counts for each station.
Table 1.
Oligonucleotide probes used in this study
| Probe name | Sequence (5′ → 3′) | Target microorganism(s) | % FAa | Reference |
|---|---|---|---|---|
| NON338 | ACTCCTACGGGAGGCAGC | 0 | 47 | |
| EUB338 | GCTGCCTCCCGTAGGAGT | Bacteria | 35 | 3 |
| EUB338-II | GCAGCCACCCGTAGGTGT | Planctomyces | 35 | 9 |
| EUB338-III | GCTGCCACCCGTAGGTGT | Verrucomicrobiae (and others) | 35 | 9 |
| ALF968 | GGTAAGGTTCTGCGCGTT | Alphaproteobacteria, except of Rickettsiales | 20 | 35 |
| ACD840 | CGACACTGAAGTGCTAAGC | Acidiphilium spp. | 10 | 12 |
| BET42ab | GCCTTCCCACTTCGTTT | Betaproteobacteria | 35 | 32 |
| GAM42ac | GCCTTCCCACATCGTTT | Gammaproteobacteria | 35 | 32 |
| THIO820 | ACCAAACATCTAGTATTCATCG | Acidithiobacillus spp. | 10 | 37 |
| DELTA495a | AGTTAGCCGGTGCTTCCT | Most Deltaproteobacteria and Gemmatimonadetes | 30 | 27 |
| cDELTA495a | AGTTAGCCGGTGCTTCTT | Competitor for DEL495a | 31 | |
| DELTA495b | AGT TAG CCG GCG CTTCCT | Some Deltaproteobacteria | 30 | 27 |
| cDELTA495b | AGTTAGCCGGCGCTTC(T/G)T | Competitor for DEL495b | 28 | |
| DELTA495c | AATTAGCCGGTGCTTCCT | Some Deltaproteobacteria | 30 | 27 |
| cDELTA495c | AATTAGCCGGTGCTTCTT | Competitor for DEL495c | 28 | |
| SYNBAC824 | GTACCCGCTACACCTAGT | Syntrophobacter spp. | 10 | 4 |
| DSU655 | CGCTTGCTTTTCCCGAAC | Desulfurella spp. | 35 | This study |
| HGC69a | TATAGTTACCACCGCCGT | Gram-positive high GC content (Actinobacteria) | 25 | 40 |
| ARCH915 | GTGCTCCCCCGCCAATTCCT | Archaea | 35 | 45 |
| LGC354a | TGGAAGATTCCCTACTGC | Gram-positive low GC DNA content (Firmicutes) | 35 | 33 |
| LGC354b | CGGAAGATTCCCTACTGC | Gram-positive low GC DNA content (Firmicutes) | 35 | 33 |
| CLIT135 | GTTATCCGTGTGTACAGGG | Clostridium cluster XI | 0 | 15 |
| CLOST1 | TTCTTCCTAATCTCTACGCA | Clostridium clusters I and II | 30 | 24 |
| NTR712 | CGCCTTCGCCACCGGCCTTCC | Nitrospira group | 35 | 10 |
| NTR712c | CGCCTTCGCCACCGGTGTTCC | Competitor for NTR712 | 10 | |
| DSP648 | CTCTCCTGTCCTCAAGAT | Desulfosporosinus, Desulfitobacterium, Dehalobacter spp. | 30 | 18 |
| SS_HOL1400 | TTCGTGATGTGACGGGC | Acidobacteria | 20 | 34 |
| EUK516 | ACCAGACTTGCCCTCC | Eukarya | 30 | 3 |
Formamide concentration in the hybridization buffer to ensure specific detection of target organisms.
Used with unlabeled GAM42a as competitor.
Used with unlabeled BET42a as competitor.
Total cell numbers in the SN core were too low to allow specific cell counting. Thus, a density gradient centrifugation was carried out to increase the cell density for quantification of less abundant bacterial groups. Cell separation was performed by Histodenz (Sigma-Aldrich) density centrifugation. Subsamples (1 ml) were placed in 2-ml tubes; 1 ml of Hystodenz solution (60% [wt/vol] in PBS, density of 1.3) was carefully placed underneath using a syringe needle to avoid mixing. Centrifugation was performed at 14,000 × g for 90 min at 4°C. The supernatant above the Hystodenz layer was treated as explained above for the rest of the samples. Although there was significant cell loss during the procedure (30% compared to untreated samples), this method was shown to maintain representative ratios between communities (48); thus, it could be used for relative counts.
Design, evaluation and application of a Desulfurella sp. probe.
The high hybridization numbers shown for Deltaproteobacteria probe in this study, the abundant presence of Desulfurella clones in the former study of Tinto sediments (42), and the lack of a genus-specific probe for Desulfurella made it necessary to design a new probe for this genus. This was done using the probe design tool in the ARB software package (29). The target position of DSU655 probe (655 to 673) was chosen to be in a highly accessible region according to Behrens et al. (6) and to be fully specific for the genus Desulfurella (checked with the ARB Probe Match tool). Using the described CARD-FISH protocol, the stringent hybridization conditions of Desulfurella probe were determined with positive-control cells of Desulfurella kamchatkensis and negative-control cells of Syntrophobacter wolinii (DSM 2805) and Acidobacterium capsulatum (DSM 11244) cultures. Hybridization stringency was optimized by increasing formamide concentrations starting with 10% in 5% steps. A 35% formamide concentration was optimal, resulting in high signal intensity of D. kamchatkensis and no signal for the negative controls.
SEM.
Sediment samples were studied by scanning electron microscopy (SEM) as described elsewhere (2). Samples were fixed by immersion in glutaraldehyde (2.5%) for 2 h, washed twice in sodium cacodylate buffer (0.2 M, pH 7.1), and dehydrated in a graded series (10, 30, 50, 70, 90, and 100%) of ethanol-water mixtures for 20 min each. After dehydration, the samples were critical point dried and mounted on stubs. After gold shadowing, the samples were examined with a Phillips XL30 EDAX DX4i SEM.
RESULTS
Physicochemical properties of the sediments.
The redox potential and pH values of the sediment profiles were significantly different (P value of <0.001 with the Mann-Whitney U test) at the two sites (JL and SN) (Fig. 2a and b). JL cores were banded and varied from reducing (blackish) to oxidizing (brownish) zones, with a redox ranging from −246 to 50 mV and a pH range from 6.2 to 4.2. SN cores did not show strong variations with depth, the pH remained between 2.3 and 2.5, and the redox potential remained between +248 and +312 mV.
Comparing the black and brown layers of the JL dam, arsenic, heavy metals, and sulfate concentrations in the interstitial water were higher in the brown layers. In the black layers, there was a strong decrease (higher than 90%) for Co, Ni, Cu, Zn, and As and close to 50% for Fe, Mn, and sulfate (Table 2). A concentration of 79 mg liter−1 of acetate, an intermediary of the anaerobic digestion pathways and a substrate for some methanogens and sulfate reducers, was measured in the black band, and the concentration in the brown band was as low as 1 mg l−1. On the other hand, there was no stratification with depth at the SN dam, so the average of both studied layers is presented in Table 2. Compared to in JL, heavy metal concentrations varied strongly in SN: Co and Ni were under the detection limit, while the concentrations of Zn and Cu were much higher.
Table 2.
Heavy metals, metaloids, sulfate, and volatile fatty acid concentrations detected in the porewater sediments and percentage of reduction in JL layers
| Sample | Concn (mM)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Formate | Acetate | Sulfate | Fe | Co | Ni | Cu | Zn | As | Al | Mn | |
| SN | 1.1 × 10−2 ± 2.1 × 10−3 | 0.1 ± 2.1 ×10−2 | 20.4 ± 2.3 | 92.2 ± 2.1 | UDL | UDL | 1.0 ± 0.2 | 11.2 ± 0.4 | 1.1 × 10−2 ± 9 × 10−4 | 43 ± 3.5 | 2.9 ± 0.2 |
| JL brown | 1.3 ×10−2 ± 3.2 × 10−3 | 6.6 × 10−3 ± 3.2 × 10−4 | 25.5 ± 3.1 | 100.2 ± 1.2 | 1.3 × 10−1 ± 5 × 10−3 | 4.4 × 10−2 ± 8 × 10−4 | 7.9 × 10−3 ± 3 × 10−4 | 1.9 ± 0.1 | 1.4 × 10−2 ± 2.5 × 10−3 | 33 ± 1.4 | 4.1 ± 1.4 |
| JL black | 1.1 × 10−2 ± 2.1 × 10−3 | 1.3 ± 1.5 × 10−2 | 12.5 ± 1 | 52.2 ± 3.3 | 1.2 × 10−2 ± 2.1 × 10−3 | 1 × 10−3 ± 2.4 × 10−4 | 3.1 × 10−4 ± 1 × 10−5 | 6.1 × 10−3 ± 4.1 × 10−4 | 1.3 × 10−3 ± 4.5 × 10−4 | 3.3 × 10−2 ± 4 × 10−3 | 2.1 ± 0.1 |
| % reduction in JL black | 51.1 | 47.9 | 90.9 | 97.7 | 96.0 | 99.7 | 90.6 | 99.9 | 48.3 | ||
UDL, under the detection limit.
Chemistries of the overlaying waters at both dams were similar in pH and some metals (Table 3) but differed for K, Mn, Cd, P, S, and Fe. S and Fe are the elements that the oxidation of pyrite (the main substrate in the IPB) put into solution in ferric iron and sulfate forms. Both elements were less abundant at the JL dam, probably due to a dilution along the river of the concentrated ARD waters present at the SN dam.
Table 3.
Element concentration, TOC, redox potential, and pH values of SN and JL overlaid waters
| Sample | pH | E (mV) | TOC (mg l−1) | Concn (mM)a |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | Mg | Al | K | Mn | Fe | Cu | Zn | As | Ca | Cd | S | P | ||||
| SN | 2.5 | 400 | 9 ± 1.7 | 0.62 ± 0.1 | 8.3 ± 0.3 | 14.5 ± 0.7 | 0.23 ± 0.01 | UDL | 41.30 ± 2.4 | 0.43 ± 0.04 | 1.19 ± 0.3 | 0.07 ± 2.1 × 10−3 | 1.81 ± 0.07 | 2.7 × 10−3 ± 8 × 10−4 | 85.8 ± 6.4 | 2.3 ± 0.7 |
| JL | 2.8 | 365 | 20 ± 4 | 0.24 ± 0.02 | 12.3 ± 0.4 | 13.6 ± 0.4 | UDL | 26.07 ± 1.3 | 16.34 ± 1.7 | 1.94 ± 0.07 | 1.41 ± 0.05 | 0.04 ± 3.7 × 10−3 | 0.98 ± 0.05 | UDL | 54.3 ± 4.6 | UDL |
UDL, under the detection limit.
Total cell counts with the Sybr green I method.
In comparison to DAPI (4′,6-diamidino-2-phenylindole) staining, the use of the highly sensitive Sybr green I dye in combination with the Moviol embedding medium that lowered photobleaching and the use of black filters, which have less background, significantly improved the signal-to-noise ratio. This enabled reliable counting of total cell numbers.
The average of Sybr green I-stained cells of the JL core (Fig. 2b) was 5.5 × 108 cells g−1 wet weight of sediment, whereas in the SN dam sediments (Fig. 2f), the average was 1.4 × 107, which is 40-fold lower. A common feature among depth profiles of both sites is that cell numbers in both dams are higher in the 15-to-20-cm layer. After that, depth profiles followed a general trend observed in many sediments of a drastic decrease with depth (25), from 1.4 × 109 cells g−1 (wet weight) in the 15-to-20-cm layer to 1.3 × 108 in the 25-to-30-cm layer in the JL dam (10-fold lower) and from 2.4 × 107 to 3.7 × 106 in the SN dam (6-fold lower).
SEM.
Scanning electron microscopy (SEM) analysis of sediments revealed high densities of cells attached to the sediment particles in the black layer of site JL. The colonization of the brownish layer of JL and of sediment from site SN was scarce. This corroborated the direct counts obtained with the Sybr green I method.
CARD-FISH.
The sites investigated here had already been characterized in an earlier study by comparative 16S rRNA gene analysis, applying both fingerprinting by DGGE and comparative sequence analysis (42). FISH-based quantifications of particular taxa had first been hampered by high background due to the autofluorescence of sediment particles. These problems were solved in this study by applying the improved whole-cell hybridization technique CARD-FISH enabling the quantification of higher and lower taxa in a nested approach.
(i) Domain-specific probes.
First, oligonucleotide probes for the domains Bacteria, Archaea, and Eukarya were applied to the sediment samples. In all cases, Bacteria (>98%) dominated over Archaea (<2%) and Eukarya (below the detection limit) (Fig. 2b and f). As for the Sybr green I total cell counts, great differences were found between the two sampling sites. In the JL sediment, the average bacterial counts with probes EUB338 I to III (Fig. 2b) was 5.4 × 108 cells g−1 (wet weight) of sediment, ranging from 1.61 × 109 at 15 cm to 2.12 × 107 at 45 cm, whereas in the SN dam (Fig. 2f), the average was 8.9 × 106, ranging from 1.6 × 107 at 15 cm to 2.2 × 106 at 45 cm.
(ii) In-depth analysis of the bacterial community.
An earlier study of Tinto sediments (42) had retrieved sequences belonging to five phyla of the domain Bacteria: Proteobacteria of the classes Alpha-, Beta-, Gamma-, and Deltaproteobacteria; Acidobacteria; Nitrospira; Actinobacteria; and Firmicutes. The selection of the phylum- and class-specific probes used in this study was done according to these previous findings.
Depth profiles of the respective bacterial groups are shown in Fig. 2c and g. In JL dam sediments, Deltaproteobacteria was the most abundant group along the sediment core, as detected by probe DEL495a-c (average of 6.6 × 107 cells g−1 [wet weight]). This class of Proteobacteria showed a strong variation with depth with two population maxima. The first peak (3.3 × 108 cells g−1 [wet weight]) was detected within the uppermost 5 cm, and after a sharp decline, a second population maximum (1.1 × 108 cells g−1 [wet weight]) was found in the most reduced layer (15 to 20 cm). The second most abundant group was Firmicutes, detected with a probe mix consisting of LGC354a, LGC354b, CLIT135, CLOSTI, and DSP648, with an average along the profile of 4.3 × 107 cells g−1 (wet weight). Alphaproteobacteria as detected by probe ALF968 averaged 4.2 × 107 cells g−1 (wet weight), Gammaproteobacteria as detected by probe GAM42a averaged 4.0 ×107 cells g−1 (wet weight), and Betaproteobacteria as detected with probe BET42a averaged 2.6 × 107 cells g−1 (wet weight). In contrast, Acidobacteria (SS_HOL1400 probe) and Actinomicetales (HGC69a probe) appeared only in low numbers, 5.9 × 105 and 7.7 × 106 cells g−1 (wet weight), respectively. Signals for probe NTR712 (Nitrospira group) were below the detection limit.
The depth profiles of SN dam sediments did not show the variation found in JL. In this core, numbers decrease homogeneously with depth. Gammaproteobacteria dominated (average 1.7 × 106 cells g−1 [wet weight]) followed by Alphaproteobacteria with an average along the profile of 1.1 × 106 cells g−1 (wet weight). The rest of the groups were close to the detection limit, around 105 cells g−1 (wet weight), in decreasing order: Actinomycetales, Deltaproteobacteria, Firmicutes, Betaproteobacteria, and Acidobacteria.
(iii) Genus-specific probes.
All genus-specific probes applied in this study were targeting genera for which sequences had been frequently retrieved in an earlier study (42). This included the deltaproteobacterial genera Syntrophobacter (probe Synbac824) and Desulfurella (probe DSU655), the genus Desulfosporosinus (probe DSP648) within the phylum Firmicutes, the alphaproteobacterial genus Acidiphilium (probe ACD638), and the gammaproteobactrial genus Acidithiobacillus (Thio820).
The genus-specific probing confirmed the great differences between the two sampling sites (Fig. 2d and h). In JL, the most abundant genus detected was Syntrophobacter. Cells were rod-shaped and had a size of about 2 by 1 μm. The average along the profile was 4.7 × 107 cells g−1 (wet weight), but just in the first layer the signal counts were 3.6 × 108, representing 36% of the total Bacteria counts. Syntrophobacter spp. are propionate-degrading syntrophic bacteria that use sulfate as the electron acceptor. They typically occur in environments with neutral pH. The other abundant genera were Desulfurella (3.1 × 107 cells g−1 [wet weight], mostly rods of a size of 2 by 1 μm), and Desulfosporosinus (2.8 × 107 cells g−1 [wet weight], curved rods of a size of 2 to 4 by 0.7 μm). The latter genus is known to encompass spore-forming sulfate-reducing bacteria. Both genera showed higher numbers at those layers with higher pH and reducing redox potential (5 to 10 and 15 to 20 cm) (Fig. 2a and d). At SN, Acidithiobacillus spp. dominated with an average of 1.5 × 106 cells g−1 (wet weight) along the profile. The hybridized cells were coccoid rods with a size of 1 by 0.7 μm. Acidithiobacillus spp. derive energy from the oxidation of reduced sulfur compound and/or ferrous iron, or the reduction of ferric iron under anoxic conditions. Finally, Acidiphilium spp. were also present, with average numbers of 4.2 × 105 cells g−1 (wet weight) (rods of a size of 2 to 3 by 1 μm).
DISCUSSION
We used a nested-probe approach for the quantification of the microbial community composition in sediments of Tinto River. The generally low abundance of Archaea agreed with similar results found in the sediments of mine tailing dumps (23). In a previous study of Río Tinto sediments (42), the archaeal diversity had been restricted to two genera: Ferroplasma spp. and Thermoplasma spp. Although Sanz et al. (43) had described the presence of methanogens in the sediments of the Tinto basin, our data suggest that Archaea play a minor role in the ecology of these sediments. Both Ferroplasma and Thermoplasma are usually found in bioleaching operations at lower pH (around 2) and at much higher iron and sulfate concentrations than those prevailing in Río Tinto sediments (13). Although algae, ciliates, flagellates, amoebae, and fungi have been reported in the water column and the biofilms of the Río Tinto (1, 5), no Eukarya probe signal was detected by CARD-FISH; therefore, Bacteria seem to fully dominate the anoxic sediment layers of Tinto River.
When genus-specific probes were applied, a pronounced variability was detected between the two sampling sites. In SN sediments, where parameters such as pH and redox potential were similar to water column values, Bacteria related to the iron cycle prevailed, likely reflecting the higher availability of iron at low pH and oxidative redox potential. This included the genera Acidiphilium (ACD638 probe) and Acidithiobacillus (Thio820 probe), which both are capable of iron reduction under anoxic conditions. No members of the Leptospirillum genus, Nitrospira-like bacteria (Ntr712 probe), were detected. The common iron oxidizers in ARD environments are members of the aerobic genus Leptospirillum and the facultative anaerobe bacterium Acidithiobacillus ferrooxidans, which can oxidize iron and sulfur in aerobic conditions and reduce iron in anoxia. Our results confirm the dominance of the versatile Acidithiobacillus ferrooxidans in the sediments over the strictly aerobic Leptospirillum spp. that usually dominate in extremely acidic oxic ARD environments (7, 13).
At the JL dam, where the iron solubility is lower due to higher pH and reducing redox potential, microbes of the sulfur cycle were most abundant. Here, sulfate-reducing bacteria (SRB) of the genera Desulfosporosinus (DSP 648 probe) and Syntrophobacter (Syn824 probe) as well as sulfur reducers of the genus Desulfurella (DSU655 probe) predominated, showing the importance of sulfur cycling in these sediments. Syntrophobacter-affiliated 16S rRNA gene sequences (42) (HQ730668, HQ730666) have been retrieved in several acidic environments before (11, 17, 26), suggesting that not-yet-cultured relatives of Syntrophobacter might be well adapted to acidic conditions in contrast to the general neutral pH environments where they thrive.
The analyses of both brown and black layers in the JL sediments showed a significant reduction in the last ones for dissolved metals, iron, and sulfate in the black layers, which run parallel to the SRB abundance. Desulfurella and Desulfosporosinus abundance peaked perfectly with those black layers where the pH values and acetate concentration increased and the redox potential and the levels of the sulfate and the dissolved metals decreased. Our results suggest that the attenuation of ARD characteristics in the reduced black layers is biologically driven by SRB. The dissimilative reduction of sulfate, driven by identified sulfate- and sulfur-reducing bacteria, leads to a consumption of protons, thus increasing alkalinity, and to sulfide formation. In addition, there is a sulfide-mediated metal precipitation: the sulfide reacts with the dissolved iron and other heavy metals which can form black amorphous precipitates (FeS) and metallic sulfides (MS). These results agree with previous studies where remediation in AMD associated with SRB metabolism was observed (14, 16, 21, 39).
In summary, the CARD-FISH quantifications performed in this study underline the great differences between microbial communities of the two Río Tinto sediments studied that had been previously indicated by DGGE and comparative sequence analysis. These differences correlate with their physicochemical characteristics, which would correspond to different hydrology at both sites. In the JL dam, the location, thickness, and values of pH and redox potential of the oxidized and reduced zones vary temporally, but stratification has been routinely observed. In contrast, sediments in SN have been more homogeneous. Different interpretations can be put forward. On the one hand, the two sampling sites have different hydrology. Site SN is a stream located close to the source of the river with a constant water flow containing a high concentration of heavy metals, mainly iron. Therefore, the sediment characteristics are close to those of the water column strongly buffered by ferric iron. In contrast, the site JL is located 5 km downstream, with an increasing flow due to the addition of neutral tributaries which lower the iron concentration, albeit with a strong seasonal variability. One of the tributaries upstream of JL contains the waters from the municipal wastewater treatment plant of Nerva. This significantly increases the total organic content (TOC) of the waters. The combination of both factors could explain the differences detected in both types of sediments. At the JL dam, with seasonal variability, higher TOC, and lower iron concentration, microbial reduction processes such as sulfate reduction increase the pH. This effect would take place firstly in microniches and then expands to macroscopic conditions. That would explain the banded sediment at the JL dam, which would correspond to the fluctuating conditions of the input water. Within them, SRB communities reduce the dissolved sulfates to sulfides that precipitate iron and heavy metals, performing a local natural bioremediation of Tinto River.
ACKNOWLEDGMENTS
This research was supported by the Spanish “Ministerio de Ciencia e Innovación” grant CTM2009-10521 to J.L. Sanz and grant CGL2009-11059 to R. Amils and by the Max Planck Society. Irene Sánchez-Andrea is a predoctoral fellowship of the same agency.
We thank Nuria Rodríguez for her help with the fieldwork, Sara Kleindienst and Kyoko Kubo for their help introducing the CARD-FISH technique, and Ana Suárez for her invaluable help with the ARB program.
Footnotes
Published ahead of print 27 April 2012
REFERENCES
- 1. Aguilera A, Manrubia SC, Gomez F, Rodriguez N, Amils R. 2006. Eukaryotic community distribution and its relationship to water physicochemical parameters in an extreme acidic environment, Rio Tinto (Southwestern Spain). Appl. Environ. Microbiol. 72:5325–5330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alphenaar PA, Groeneveld N, Van Aelst AC. 1994. Scanning electron microscopical method for internal structure analysis of anaerobic granular sludge. Micron 25:129–133 [Google Scholar]
- 3. Amann RI, et al. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ariesyady HD, Ito T, Yoshiguchi K, Okabe S. 2007. Phylogenetic and functional diversity of propionate-oxidizing bacteria in an anaerobic digester sludge. Appl. Microbiol. Biotechnol. 75:673–683 [DOI] [PubMed] [Google Scholar]
- 5. Baker BJ, Tyson GW, Goosherst L, Banfield JF. 2009. Insights into the diversity of eukaryotes in acid mine drainage biofilm communities. Appl. Environ. Microbiol. 75:2192–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Behrens S, et al. 2003. In situ accessibility of small-subunit rRNA of members of the domains Bacteria, Archaea, and Eucarya to Cy3-labeled oligonucleotide probes. Appl. Environ. Microbiol. 69:1748–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bond PL, Druschel GK, Banfield JF. 2000. Comparison of acid mine drainage microbial communities in physically and geochemically distinct ecosystems. Appl. Environ. Microbiol. 66:4962–4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruneel O, Duran R, Casiot C, Elbaz-Poulichet F, Personné JC. 2006. Diversity of microorganisms in Fe-As-rich acid mine drainage waters of Carnoules, France. Appl. Environ. Microbiol. 72:551–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Daims H, Brühl A, Amann R, Schleifer KH, Wagner M. 1999. The domain-specific probe EUB 338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434–444 [DOI] [PubMed] [Google Scholar]
- 10. Daims H, Nielsen JL, Nielsen PH, Schleifer KH, Wagner M. 2001. In situ characterization of Nitrospira-like nitrite-oxidizing bacteria active in wastewater treatment plants. Appl. Environ. Microbiol. 67:5273–5284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dedysh SN, Pankratov TA, Belova SE, Kulichevskaya IS, Liesack W. 2006. Phylogenetic analysis and in situ identification of Bacteria community composition in an acidic Sphagnum peat bog. Appl. Environ. Microbiol. 72:2110–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Demanèche S, et al. 2008. Antibiotic-resistant soil bacteria in transgenic plant fields. Proc. Nat. Acad. Sci. 105:3957–3962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edwards KJ, Gihring TM, Banfield JF. 1999. Seasonal variations in microbial populations and environmental conditions in an extreme acid mine drainage environment. Appl. Environ. Microbiol. 65:3627–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fortin D, Rioux JP, Roy M. 2002. Geochemistry of iron and sulfur in the zone of microbial sulfate reduction in mine tailings. Water Air Soil Poll. 2:37–56 [Google Scholar]
- 15. Franks AH, et al. 1998. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 64:3336–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Friese K, et al. 1998. Biogeochemistry of iron and sulfur in sediments of an acidic mining lake in Lusatia, Germany. Water Air Soil Poll. 108:231–247 [Google Scholar]
- 16a. Garcia-Moyano A, Gonzalez-Toris E, Aguilera A, Amils R. 2007. Prokaryotic community composition and ecology of floating macroscopic filaments from an extreme acidic environment, Rio Tinto (SW, Spain). Syst. Appl. Microbiol. 30:601–614 [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez-Toril E, et al. 2011. Geomicrobiology of La Zarza-Perrunal acid mine effluent (Iberian Pyritic Belt, Spain). Appl. Environ. Microbiol. 77:2685–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. González-Toril E, Gómez F, Malki M, Amils R. 2006. The isolation and study of acidophilic microorganisms, p 471–510 In Rainey FA, Oren A. (ed), Extremophiles—methods in microbiology. Elsevier/Academic Press, London, United Kingdom [Google Scholar]
- 19. Gonzalez-Toril E, Llobet-Brossa E, Casamayor EO, Amann R, Amils R. 2003. Microbial ecology of an extreme acidic environment, the Tinto River. Appl. Environ. Microbiol. 69:4853–4865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson DB. 1998. Biodiversity and ecology of acidophilic microorganisms. FEMS Microbiol. Ecol. 27:307–317 [Google Scholar]
- 21. Johnson DB, Hallberg KB. 2005. Acid mine drainage remediation options: a review. Sci. Total Environ. 338:3–14 [DOI] [PubMed] [Google Scholar]
- 22. Johnson DB, Hallberg KB. 2009. Carbon, iron and sulfur metabolism in acidophilic micro-organisms. Adv. Microb. Physiol. 54:201–255 [DOI] [PubMed] [Google Scholar]
- 23. Kock D, Schippers A. 2008. Quantitative microbial community analysis of three different sulfidic mine tailing dumps generating acid mine drainage. Appl. Environ. Microbiol. 74:5211–5219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kusel K, Pinkart HC, Drake HL, Devereux R. 1999. Acetogenic and sulfate-reducing bacteria inhabiting the rhizoplane and deep cortex cells of the sea grass Halodule wrightii. Appl. Environ. Microbiol. 65:5117–5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Llobet-Brossa E, Rosselló-Mora R, Amann R. 1998. Microbial community composition of Wadden Sea sediments as revealed by fluorescence in situ hybridization. Appl. Environ. Microbiol. 64:2691–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loy A, Kusel K, Lehner A, Drake HL, Wagner M. 2004. Microarray and functional gene analyses of sulfate-reducing prokaryotes in low-sulfate, acidic fens reveal cooccurrence of recognized genera and novel lineages. Appl. Environ. Microbiol. 70:6998–7009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loy A, et al. 2002. Oligonucleotide microarray for 16S rRNA gene-based detection of all recognized lineages of sulfate-reducing prokaryotes in the environment. Appl. Environ. Microbiol. 68:5064–5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lücker S, et al. 2007. Improved 16S rRNA-targeted probe set for analysis of sulfate-reducing bacteria by fluorescence in situ hybridization. J. Microbiol. Methods 69:523–528 [DOI] [PubMed] [Google Scholar]
- 29. Ludwig W, Strunk O, Westram R, Richter L, Meier H. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lunau M, Lemke A, Walther K, Martens-Habbena W, Simon M. 2005. An improved method for counting bacteria from sediments and turbid environments by epifluorescence microscopy. Environ. Microbiol. 7:961–968 [DOI] [PubMed] [Google Scholar]
- 31. Macalady JL, et al. 2006. Dominant microbial populations in limestone-corroding stream biofilms, Frasassi cave system, Italy. Appl. Environ. Microbiol. 72:5596–5609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manz W, Amann R, Ludwig W, Wagner M, Schleifer KH. 1992. Phylogenetic oligodeoxynucleotide probes for the major subclasses of proteobacteria: problems and solutions. Syst. Appl. Microbiol. 15:593–600 [Google Scholar]
- 33. Meier H, Amann R, Ludwig W, Schleifer KH. 1999. Specific oligonucleotide probes for in situ detection of a major group of Gram-positive bacteria with low DNA G+C content. Syst. Appl. Microbiol. 22:186–196 [DOI] [PubMed] [Google Scholar]
- 34. Meisinger DB, et al. 2007. In situ detection of novel Acidobacteria in microbial mats from a chemolithoautotrophically based cave ecosystem (Lower Kane Cave, WY, U.S.A.). Environ. Microbiol. 9:1523–1534 [DOI] [PubMed] [Google Scholar]
- 35. Neef A. 1997. Anwendung der in situ Einzelzell-Identifizierung von Bakterien zur Populationsanalyse in komplexen mikrobiellen Biozönosen. Technische Universität München, Munich, Germany [Google Scholar]
- 36. Nordstrom DK, Alpers CN. 1999. Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund site, California. Proc. Natl. Acad. Sci. U. S. A. 96:3455–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peccia J, Marchand EA, Silverstein J, Hernandez M. 2000. Development and application of small-subunit rRNA probes for assessment of selected Thiobacillus species and members of the genus Acidiphilium. Appl. Environ. Microbiol. 66:3065–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pernthaler A, Pernthaler J, Amann R. 2002. Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl. Environ. Microbiol. 68:3094–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Praharaj T, Fortin D. 2004. Indicators of microbial sulfate reduction in acidic sulfide-rich mine tailings. Geomicrobiol. J. 21:457–467 [Google Scholar]
- 40. Roller C, Wagner M, Amann R, Ludwig W, Schleifer KH. 1994. In situ probing of Gram-positive bacteria with high DNA G+C content using 23S rRNA-targeted oligonucleotides. Microbiology 140:2849–2858 [DOI] [PubMed] [Google Scholar]
- 41. Rowe OF, JSánchez-España Hallberg KB, Johnson DB. 2007. Microbial communities and geochemical dynamics in an extremely acidic, metal-rich stream at an abandoned sulfide mine (Huelva, Spain) underpinned by two functional primary production systems. Environ. Microbiol. 9:1761–1771 [DOI] [PubMed] [Google Scholar]
- 42. Sánchez-Andrea I, Rodriguez N, Amils R, Sanz JL. 2011. Microbial diversity in anaerobic sediments at Rio Tinto, a naturally acidic environment with a high heavy metal content. Appl. Environ. Microbiol. 77:6085–6093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sanz JL, Rodríguez N, Díaz EE, Amils R. 2011. Methanogenesis in the sediments of Rio Tinto, an extreme acidic river. Environ. Microbiol. 13:2336–2341 [DOI] [PubMed] [Google Scholar]
- 44. Sekar R, et al. 2003. An improved protocol for quantification of freshwater Actinobacteria by fluorescence in situ hybridization. Appl. Environ. Microbiol. 69:2928–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stahl DA, Amann R. 1991. Development and application of nucleic acid probes, p 205–248 In Stackebrandt E, Goodfellow M. (ed), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Ltd., Chichester, United Kingdom [Google Scholar]
- 46. Teira E, Reinthaler T, Pernthaler A, Pernthaler J, Herndl GJ. 2004. Combining catalyzed reporter deposition-fluorescence in situ hybridization and microautoradiography to detect substrate utilization by bacteria and archaea in the deep ocean. Appl. Environ. Microbiol. 70:4411–4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wallner G, Amann R, Beisker W. 1993. Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry 14:136–143 [DOI] [PubMed] [Google Scholar]
- 48. Whiteley AS, Griffiths RI, Bailey MJ. 2003. Analysis of the microbial functional diversity within water-stressed soil communities by flow cytometric analysis and CTC+ cell sorting. J. Microbiol. Methods 54:257–267 [DOI] [PubMed] [Google Scholar]