Abstract
Symbiotic bacteria associated with midgut crypts of stinkbugs of the family Cydnidae, representing seven species and 13 populations, were investigated. All of the symbionts were species specific, and constituted at least four distinct lineages in the Gammaproteobacteria, indicating multiple evolutionary origins of the gut symbionts among the burrower bugs.
TEXT
The majority of plant-sucking stinkbugs are associated with symbiotic bacteria harbored in the lumen of a specialized region of the midgut (2, 4, 12). Previous studies have revealed remarkable diversity in the evolutionary host-symbiont relationships among major stinkbug groups. In the superfamily Pentatomoidea, the gut symbionts consist of a number of distinct lineages in the Gammaproteobacteria, and the host insects generally transmit their symbiont vertically by either of the posthatch transmission mechanisms, including egg surface contamination, coprophagy, symbiont capsule provisioning, etc. (12). Notably, different pentatomoid families may exhibit distinct patterns in their host-symbiont evolutionary intimacy. In the families Plataspidae and Acanthosomatidae, the symbiont phylogeny was highly congruent with the host phylogeny, and the symbiont genomes exhibit highly degenerative features such as AT-biased nucleotide compositions, accelerated molecular evolutionary rates, and drastic size reduction (6, 13, 19), reflecting the obligate and stable host-symbiont associations over evolutionary time (17, 26). In the family Pentatomidae, in contrast, the symbiont phylogeny does not mirror the host systematics, the symbionts consist of several distinct lineages in the Gammaproteobacteria, and the symbiont genes exhibit no or less AT-biased nucleotide compositions and less accelerated molecular evolutionary rates (14, 15, 20), suggesting occasional symbiont acquisitions and/or replacements in the evolutionary course of the Pentatomidae. As for the other pentatomoid families, such as Scutelleridae, Cydnidae, Parastrachiidae, etc., such host-symbiont coevolutionary aspects are elusive since only a few species have been inspected for their symbiotic bacteria (1, 7, 9, 10).
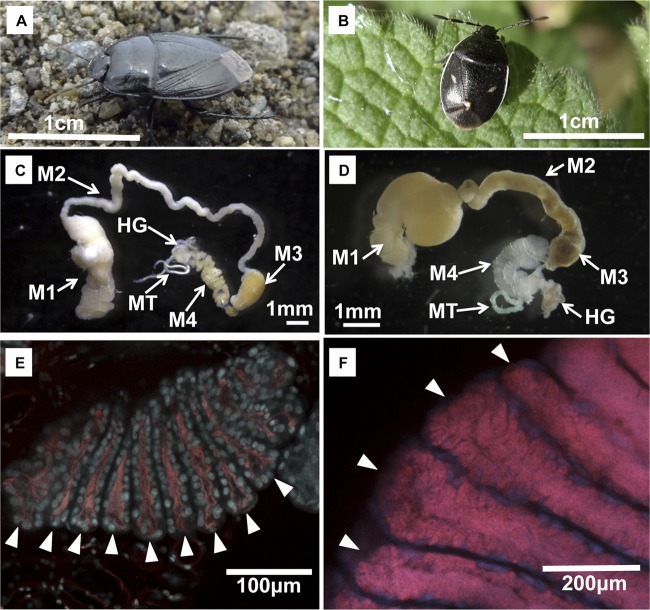
The family Cydnidae embraces over 110 genera and 600 species worldwide, many of which are characterized by their fossorial lifestyle and thus called “burrower bugs” (23). Here we investigated the symbiotic bacteria of Japanese cydnid stinkbugs representing 7 species and 13 populations (Fig. 1A and B; see Table S1 in the supplemental material). Each of the surface-sterilized adult insects was individually dissected in phosphate-buffered saline with fine forceps, by which a dissected midgut preparation was obtained from each insect (Fig. 1C and D). The symbiotic midgut fourth section with crypts (see M4 in Fig. 1C and D) was subjected to DNA extraction, PCR, cloning, and sequencing of the bacterial 16S rRNA gene as described previously (11). Sequencing of five to eight clones for each sample revealed that a single gammaproteobacterial species dominates in the symbiotic organ. The nucleotide sequences, 1,462 to 1,467 bp in size, exhibited 95% (1,386/1,465) to 99% (1,454/1,465) identities between the different cydnid species, while the sequences obtained from the same cydnid species were 100% identical to each other, except for those from local populations of Macroscytus japonensis, which had a slight difference (0.2% [3/1,463]). The dissected midgut preparations from M. japonensis and Adomerus triguttulus were subjected to fluorescent in situ hybridization targeting the 16S rRNA genes of their symbiotic bacteria, as described previously (16). The specific probe TCKM-A555 (5′-Alexa 555-CAC TTT GGT CTT GCG ACG-3′) detected the symbiont signals inside the lumen of the midgut crypts in M. japonensis (Fig. 1E), and so did the specific probe MTBS-A555 (5′-Alexa 555-GCC GTA TGG TCT TCT TCC-3′) in A. triguttulus (Fig. 1F).
Fig 1.
Adult insects and symbiotic organs of cydnid stinkbugs. (A) Adult insect of Macroscytus japonensis; (B) adult insect of Adomerus triguttulus; (C) dissected midgut from an adult female of M. japonensis; (D) dissected midgut from an adult female of A. triguttulus. M1, midgut first section; M2, midgut second section; M3, midgut third section; M4, midgut fourth section (symbiotic organ); HG, hindgut; MT, Malpighian tubule. (E) In situ hybridization of the gut symbiont in the midgut fourth section of M. japonensis; (F) in situ hybridization of the gut symbiont in the midgut fourth section of A. triguttulus. Each arrowhead indicates a crypt. Red and blue signals indicate the symbiont 16S rRNA and the host nuclear DNA, respectively.
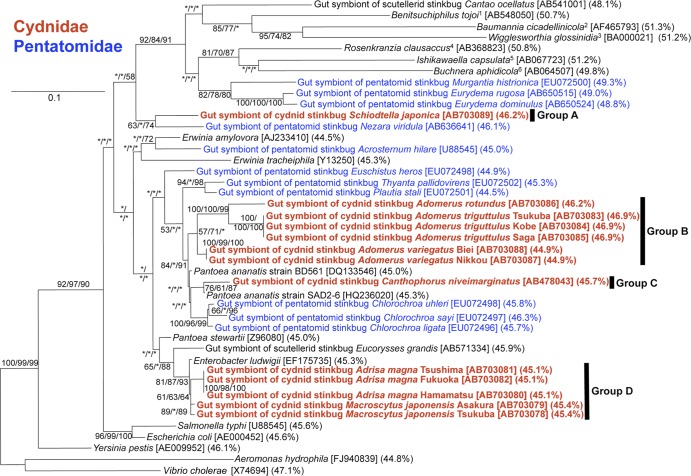
These symbiont gene sequences of the cydnid stinkbugs were subjected to phylogenetic analyses together with those of other pentatomoid stinkbugs (1, 6, 7, 9, 10, 13, 14, 20). Maximum likelihood, maximum parsimony, and neighbor-joining phylogenies were inferred from the 16S rRNA gene sequences using the programs PhyML 3.0 (5), PAUP 4.0b10 (24), and ClustalW (25), respectively. Figure 2 shows the phylogenetic placements of the cydnid gut symbionts in the Gammaproteobacteria. The cydnid gut symbionts were not monophyletic but formed at least four distinct phylogenetic groups: the gut symbiont of Schiodtella japonica clustered with the gut symbiont of a pentatomid Nezara viridula (group A); the gut symbionts of A. triguttulus, Adomerus rotundus, and Adomerus variegatus were allied with Pantoea ananatis strain BD561 (group B); the gut symbiont of Canthophorus niveimarginatus was allied with P. ananatis strain SAD2-6 (group C); and the gut symbionts of Adrisa magna and M. japonensis were allied with Enterobacter ludwigii (group D). Judging from the tree topologies and genetic distances within each of the groups, it was suggested that the group B and the group D might actually consist of two distinct lineages, respectively. These phylogenetic patterns indicate that the cydnid gut symbionts are of multiple evolutionary origins, wherein occasional acquisitions and/or replacements of the symbiotic associations must have occurred repeatedly. Meanwhile, on the grounds that samples from different populations of the same cydnid species are always associated with the same symbiont lineage (Fig. 2), the host-symbiont associations in the Cydnidae are evolutionarily stable to a considerable extent, at least at species levels.
Fig 2.
Phylogenetic relationship of the midgut symbionts in pentatomoid stinkbugs. A maximum likelihood phylogeny inferred from 1,178 aligned nucleotide sites of 16S rRNA genes is shown. On each of the nodes, bootstrap probabilities in the maximum likelihood (ML), the maximum parsimony (MP), and the neighbor-joining (NJ) analyses are depicted in the order ML, MP, and NJ. Asterisks indicate bootstrap probabilities lower than 50%. Accession numbers and AT contents of the nucleotide sequences are shown in brackets and parentheses, respectively. Red and blue indicate symbionts of cydnid and pentatomid stinkbugs, respectively. Superscript numbers indicate the following: 1, gut symbiont of the parastrachiid stinkbug Parastrachia japonensis; 2, bacteriome symbiont of the sharpshooter Homalodisca coagulate; 3, bacteriome symbiont of tsetse fly Glossina brevipalpis; 4, gut symbiont of the acanthosomatid stinkbug Elasmostethus humeralis; 5, gut symbiont of the plataspid stinkbug Megacopta punctatissima; 6, bacteriome symbiont of the aphid Acyrthosiphon pisum.
The 16S rRNA gene sequences of the cydnid gut symbionts exhibited AT contents ranging from 44.9% to 46.9%, which have not remarkably deviated from the values of free-living gammaproteobacteria of around 45% (Fig. 2). Meanwhile, relative rate tests using the program RRtree (21) detected significantly higher evolutionary rates in two of the four cydnid symbiont groups A to D in comparison with allied free-living gammaproteobacteria (see Table S2 in the supplemental material). These evolutionary patterns probably reflect a relatively young host-symbiont coevolution in each of the cydnid symbiont lineages.
In conclusion, the evolutionary host-symbiont relationship in the Cydnidae is similar to that in the Pentatomidae (14, 20) rather than to those in the Plataspidae and the Acanthosomatidae (6, 13). Comparative studies on these stinkbug groups would provide insights into coevolutionary aspects in the gut symbiotic associations. Here we point out that the anatomy of the symbiotic organ of the host stinkbugs might be relevant to the monophyly/polyphyly, or, in other words, the evolutionary stability of their gut symbiotic bacteria. In plataspid stinkbugs, oddly, their midgut is structurally disconnected in the middle, whereby the symbiotic region of the highly developed posterior midgut is completely isolated from the anterior midgut (6). In acanthosomatid stinkbugs, their midgut crypts are sealed off at the base, whereby the symbiotic region is structurally isolated from the midgut main tract (13). On the other hand, the midgut of pentatomid and cydnid stinkbugs is organized normally (2, 4, 14) (see Fig. 1C and D), which implies that their symbiotic bacteria in the posterior midgut may be constantly exposed to food-derived microbes and other types of bacterial contaminants, including potential bacterial symbionts derived from other insects. Many cydnid species of the subfamily Sehirinae are known as subsocial, in which adult females guard and take care of their eggs and nymphs in the nest (1, 3, 18). Hence, the gut symbiotic associations in the Cydnidae would provide intriguing opportunities to investigate the evolutionary connections between symbiosis and sociality (8, 22).
Nucleotide sequence accession numbers.
The nucleotide sequences of the 16S rRNA gene have been deposited in the DDBJ, EMBL, and GenBank databases under accession no. AB703078 to AB703089.
Supplementary Material
ACKNOWLEDGMENTS
We thank N. Baba, M. Hironaka, K. Inadomi, H. Mukai, S. Tani, H. Toju, and T. Yanagi for field collection of insect samples.
This study was supported by the Program for Promotion of Basic and Applied Research for Innovations in Bio-oriented Industry (BRAIN).
Footnotes
Published ahead of print 27 April 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Baba N, et al. 2011. Trophic eggs compensate for poor offspring feeding capacity in a subsocial burrower bug. Biol. Lett. 7:194–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buchner P. 1965. Endosymbiosis of animals with plant microorganisms. Interscience, New York, NY [Google Scholar]
- 3. Costa JT. 2006. The other insect societies. Harvard University Press, Cambridge, MA [Google Scholar]
- 4. Glasgow H. 1914. The gastric caeca and the caecal bacteria of the Heteroptera. Biol. Bull. 3:101–171 [Google Scholar]
- 5. Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696–704 [DOI] [PubMed] [Google Scholar]
- 6. Hosokawa T, Kikuchi Y, Nikoh N, Shimada M, Fukatsu T. 2006. Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol. 4:e337 doi:10.1371/journal.pbio.0040337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hosokawa T, et al. 2010. Phylogenetic position and peculiar genetic traits of the midgut bacterial symbiont in the stinkbug Parastrachia japonensis. Appl. Environ. Microbiol. 76:4130–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hosokawa T, et al. 2012. Mothers never miss the moment: a fine-tuned mechanism for vertical symbiont transmission in a subsocial insect. Anim. Behav. 83:293–300 [Google Scholar]
- 9. Kaiwa N, et al. 2010. Primary gut symbiont and secondary Sodalis-allied symbiont in the scutellerid stinkbug Cantao ocellatus. Appl. Environ. Microbiol. 76:3486–3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaiwa N, et al. 2011. Bacterial symbionts of the giant jewel stinkbug Eucorysses grandis (Hemiptera: Scutelleridae). Zool. Sci. 28:169–174 [DOI] [PubMed] [Google Scholar]
- 11. Kikuchi Y, Meng X-Y, Fukatsu T. 2005. Gut symbiotic bacteria of the genus Burkholderia in the broad-headed bugs Riptortus clavatus and Leptocorisa chinensis (Heteroptera: Alydidae). Appl. Environ. Microbiol. 71:4035–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kikuchi Y, Hosokawa T, Fukatsu T. 2008. Diversity of bacterial symbiosis in stinkbugs, p 39–63 In Dijk TV. (ed), Microbial ecology research trends. Nova Science Publishers, Inc., New York, NY [Google Scholar]
- 13. Kikuchi Y, et al. 2009. Host-symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol. 7:2 doi:10.1186/1741-7007-7-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kikuchi Y, Hosokawa T, Nikoh N, Fukatsu T. 2012. Gut symbiotic bacteria in the cabbage bugs Eurydema rugosa and Eurydema dominulus (Heteroptera: Pentatomidae). Appl. Entomol. Zool. 47:1–8 [Google Scholar]
- 15. Kobayashi H, Kawasaki K, Takeishi K, Noda H. 2011. Symbiont of the stink bug Plautia stali synthesizes rough-type lipopolysaccharide. Microbiol. Res. 167:48–54 [DOI] [PubMed] [Google Scholar]
- 16. Koga R, Tsuchida T, Fukatsu T. 2009. Quenching autofluorescence of insect tissues for in situ detection of endosymbionts. Appl. Entomol. Zool. 44:281–291 [Google Scholar]
- 17. Moran NA, McCutcheon JP, Nakabachi A. 2008. Genomics and evolution of heritable bacterial symbionts. Annu. Rev. Genet. 42:165–190 [DOI] [PubMed] [Google Scholar]
- 18. Mukai H, et al. 2010. Maternal-care behaviour in Adomerus variegatus (Hemiptera: Cydnidae). Can. Entomol. 142:52–56 [Google Scholar]
- 19. Nikoh N, Hosokawa T, Oshima K, Hattori M, Fukatsu T. 2011. Reductive evolution of bacterial genome in insect gut environment. Genome Biol. Evol. 3:702–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prado SS, Almeida RPP. 2009. Phylogenetic placement of pentatomid stink bug gut symbionts. Curr. Microbiol. 58:64–69 [DOI] [PubMed] [Google Scholar]
- 21. Robinson-Rechavi M, Huchon D. 2000. RRTree: relative-rate tests between groups of sequences on a phylogenetic tree. Bioinformatics 16:296–297 [DOI] [PubMed] [Google Scholar]
- 22. Schorr H. 1957. Zür Verhaltensbiologie und Symbiose von Brachypelta aterrima Först (Cydnidae, Heteroptera). Z. Morphol. Ökol. Tiere. 45:561–602 [Google Scholar]
- 23. Schuh RT, Slater JA. 1995. True bugs of the world (Hemiptera: Heteroptera). Cornell University Press, New York, NY [Google Scholar]
- 24. Swofford DL. 2001. PAUP* version 4.0b10. Sinauer, Sunderland, MA [Google Scholar]
- 25. Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wernegreen JJ. 2002. Genome evolution in bacterial endosymbionts of insects. Nat. Rev. Genet. 3:850–861 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.