Abstract
This review intends to provide an overview of historical and recent achievements in studies of microbial degradation of natural and synthetic rubber. The main scientific focus is on the key enzymes latex-clearing protein (Lcp) from the Gram-positive Streptomyces sp. strain K30 and rubber oxygenase A (RoxA) from the Gram-negative Xanthomonas sp. strain 35Y, which has been hitherto the only known rubber-degrading bacterium that does not belong to the actinomycetes. We also emphasize the importance of knowledge of biodegradation in industrial and environmental biotechnology for waste natural rubber disposal.
INTRODUCTION
Natural rubber (NR), or poly(cis-1,4-isoprene), is, by qualitative and quantitative criteria, one of the most important biopolymers. For almost a hundred years, millions of tons of NR-derived products have been produced by humankind. In addition, NR and other polyisoprenes are produced by thousands of plant species (4, 42). As biologically synthesized polyisoprene does not accumulate in the environment, natural degradation must occur. In spite of all of the efforts which have been made since 1914 to investigate microbial rubber degradation (58), the first genes involved in this process were identified and characterized only quite recently. However, the biochemical mechanisms of biological rubber degradation are still not widely known.
Analyses of degradation products of natural and synthetic rubber indicate an oxidative cleavage of the double bonds in the polymer backbone. A similar degradation mechanism was postulated for the cleavage of squalene, a biosynthetic precursor of steroids and triterpenoids. Aldehyde and/or carbonyl groups were detected in most of the analyzed degradation products of different rubber-degrading strains. The occurrence of degradation products of 104 to 106 Da from poly(cis-1,4-isoprene) in nearly all analyzed rubber-degrading strains without detection of intermediates remained to be elucidated. Data on rubber degradation on a molecular genetic basis and analysis of degradation products in detail have opened a gate to in-depth understanding of these new enzymatic reactions.
NR represents approximately 30 to 35% of the constituents of NR latex (4). About 99% of the commercially used NR is produced by Hevea brasiliensis, originally native to Brazil (72). A potential alternative source of NR is the Russian dandelion (Taraxacum kok-saghyz). This is a rubber dandelion plant that was specially cultivated in the former Soviet Union during World War II to satisfy the demand for NR.
Total world rubber production increased to 24.3 million tons in 2010, a rise of 11.9% from 21.7 million tons in 2009 (25). The products comprised 42.4% NR and 57.6% synthetic rubber. NR is an industrially important polymer and is used for many technical applications such as fabrication of automobile tires, for which most NR is used (53, 62). NR is a polymer composed of many C5H8 isoprene units (Fig. 1C), each containing one double bond in the cis configuration and linked at C1 and C4. Rubber is a material with properties that differ widely from those of the viscous sap of the rubber tree. Elasticity and extensibility are features most typical for rubber (7, 13, 20, 21).
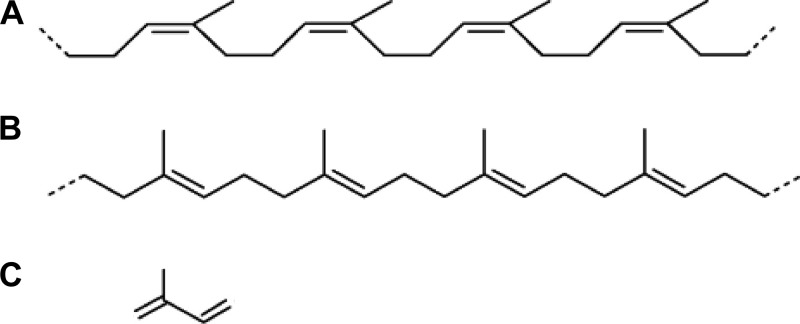
Fig 1.
Chemical structure of poly(cis-1,4-isoprene) (natural rubber, NR) (A), poly(trans-1,4-isoprene) (gutta-percha) (B), and isoprene (2-methyl-1,3-butadiene) (C).
As a consequence of the difficulties in reusing rubber material and of the widespread use of rubber products, huge amounts of waste rubber material are stockpiled all over the world. In the United States, about 2 to 3 billion used tires are currently stored in landfills (26). Due to the large volume produced and their durability, these tires are among the largest and most problematic sources of organic waste. The most common methods to cope with this problem are to burn the tires in cement kilns and power plants or to use them as artificial reefs. However, these methods induce further environmental pollution (19).
One way of overcoming the environmental problems is provided by microbial transformation of rubber into useful products (9, 12, 37, 49, 66, 67). There are some specific advantages of biotechnological processes compared to chemical and physical ones. Biotechnology does not produce any harmful or toxic chemicals and is normally not energy intensive. However, there are still some obstacles, most notably the sensitivity of microorganisms toward many chemical substances, including rubber additives, which are used to improve stability and function of tires over a wide temperature range.
Microbial deterioration of rubber products has attracted much interest (66), and many studies have been carried out on the degradation of both pure rubber elastomers and vulcanized rubber products. It is important to conduct further molecular and biochemical studies in the field of microbial degradation of natural and synthetic polyisoprenoids in order to find ways to solve the global problem of excess rubber material.
The scope of this review is to bring further details into the hardly understood field of rubber degradation.
NATURAL AND SYNTHETIC RUBBER
Two main types of polyisoprenoids that differ according to their isomerism are almost exclusively synthesized by plants; the first one is the cis isomer natural rubber (NR) [poly(cis-1,4-isoprene)] (Fig. 1A), and the second one is the trans isomer gutta-percha (GP) [poly(trans-1,4-isoprene)] (Fig. 1B).
The rubber tree, H. brasiliensis, grows throughout the tropics and is cultivated in plantations primarily in Southeast Asia; Malaysia and Indonesia are the most important sources. The sap is collected, and on exposure to air and mild heat it gives NR. NR has been used since the 11th century by the natives of Central and South America for the manufacture of balls. The present English term “rubber” for poly(cis-1,4-isoprene) was coined by Edward Nairne and Joseph Priestley due to its property as a pencil eraser. The economic importance of poly(cis-1,4-isoprene) is confirmed by its historical development. Until the late 19th century, Brazil held the absolute monopoly on NR and intended to protect it by an embargo on exports of scions and seeds. Sir Henry Wickham smuggled about 70,000 seeds from Brazil to England in 1876, whereas James Collins, the curator of the museum of the Pharmaceutical Society London, failed 3 years earlier. Between 1900 and 1913, rubber produced on plantations in Southeast Asia and East African countries captured the world market. The discovery of synthetic polyisoprene by the German chemist Fritz Hofmann in 1909 paved the way for large-scale production of synthetic poly(cis-1,4-isoprene) with a molecular structure similar to that of NR in the United States in 1954. Since all efforts to replace rubber from H. brasiliensis with rubber of endemic plants failed, all involved countries spent much effort on the development of synthetic rubbers during the First and Second World Wars. Germany produced some 2,500 tons of methyl rubber (polymer of 2,3-dimethyl-1,3-butadiene) during World War I but returned to hevea rubber at the end of the war. Between 1948 and 1951, the fabrication of synthetic rubbers was prohibited in Germany by the Allies. Nowadays, more than 15,000,000 tons of natural and synthetic rubber is produced annually and, as a consequence, an increasing amount of rubber-containing residual material emerges.
The trans isomer GP is in contrast to NR synthesized only by a few plants and occurs, e.g., in the Southeast Asian trees Palaquium gutta and Eucommia ulmoides, the European shrub Euonymus europaeus, and the South American tree Couma macrocarpa (Table 1). GP is utilized for a wide range of applications due to its resistance to biological degradation. This polymer has been used for the past century as insulation material for transatlantic telegraph cables, and it is still used for the production of conveyers, golf balls, decorative objects, and chewing gum. This implies that it does not readily react within the human body, and consequently, it is used for a variety of surgical devices and for dental applications during root canal therapy (Table 1).
Table 1.
Important rubber-producing organisms
| Organism type | Examples | Rubber type | Contents/applications | Reference(s) |
|---|---|---|---|---|
| Higher organisms | Hevea brasiliensis (rubber tree), Taraxacum kok-saghyz (Russian dandelion), Parthenium argentatum (guayule), Dyera costulata (jelutong) | cis isomer | Technical applications (e.g., fabrication of automobile tires) | 5, 30, 44, 62 |
| Lower organisms/two major classes of fungi | Ascomycetes, Basidiomycetes | Low-mol-wt rubber | 61 | |
| Plant species | Eucommia ulmoides (Tochu), Euonymus europaeus (Celastraceae), Manikara zapota (chicle), Mimusops balata (balata), Palaquium gutta (gutta-percha) | trans isomer | Cable insulation, belting, conveyors, decorative objects, golf ball covers, chewing gum | 1 |
FINE CHEMICAL STRUCTURES OF NATURAL POLYISOPRENOIDS AND LATEX PARTICLES
The average composition of latex milk is as follows: 25 to 35% (wt/wt) polyisoprene, 1 to 1.8% (wt/wt) protein, 1 to 2% (wt/wt) carbohydrates, 0.4 to 1.1% (wt/wt) neutral lipids, 0.5 to 0.6% (wt/wt) polar lipids, 0.4 to 0.6% (wt/wt) inorganic components, 0.4% (wt/wt) amino acids, amides, etc., and 50 to 70% (wt/wt) water (60).
Within the latex milk, NR occurs in the form of particles as an emulsion of droplets with a predominant size of 0.1 to 2 μm in diameter in water (43). These rubber particles are covered by a layer of proteins and lipids, which separate the hydrophobic rubber molecules from the hydrophilic environment.
13C-nuclear magnetic resonance (NMR) spectroscopy studies of natural polyisoprenes disclosed the detailed structure of the rubber molecules, unraveling deviations from the strict trans configuration. Due to these results, natural polyisoprenes are classified into three groups according to the structure at both chain ends, with the initiating start point of the chain being referred to as the ω-terminus and the opposite end as the α-terminus (63): (i) polyprenol type [ω-(trans)2-3-(cis)n-α], (ii) natural rubber type [ω′-(trans)2-(cis)n-α′], and (iii) wild rubber type [ω′-(cis)n-α]. NR of H. brasiliensis contains two trans-isoprene units and long-chain fatty acid groups linked to the rubber molecule through phospholipids (18, 62). Due to the structural pecularities of polyisoprenes, conclusions for the initiation and termination during biosynthesis were made. As a result of the presence of the terminal dimethylallyl group, followed by two to three isoprene units in trans configuration for rubber of the polyprenol type, it was concluded that the chain elongation starts from derivatives of farnesyl diphosphate and geranyl diphosphate, which are acting as primers for the subsequent addition of further isoprene moieties from isopentenyl diphosphate in cis configuration.
BIOSYNTHESIS OF ISOPRENE AND POLY(cis-1,4-ISOPRENE)
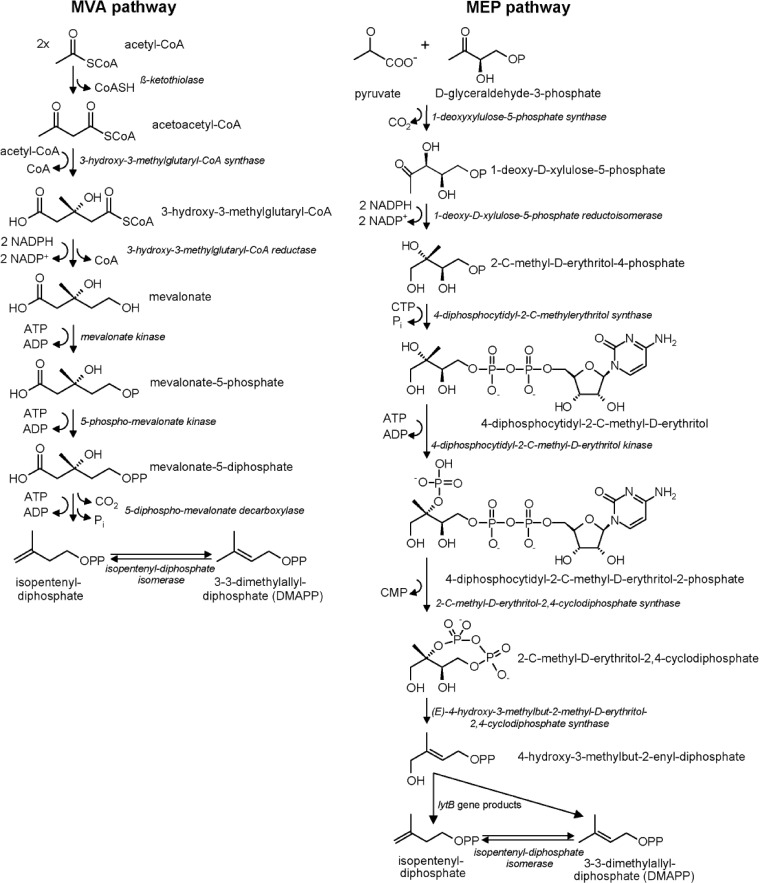
Isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) represent the basic modules for biosynthesis of all isoprenoids and were identified in all organisms, occurring as equivalent to isoprene. DMAPP is the starting subunit for the prenyltransferase [EC 2.5.1.20], which uses IPP for elongation yielding acyclic polyprenoles. Currently two autonomous biosynthesis pathways with different distribution processes are known to supply the needed precursors IPP and DMAPP.
Incorporation of radioactive labeled isotopes in precursors of cholesterol and ergosterol biosynthesis led to the discovery of the mevalonate (MVA) pathway for isoprenoid biosynthesis (6, 46, 57, 73). The activated form of acetate, acetyl coenzyme A (acetyl-CoA), serves as a starting compound (Fig. 2). The MVA pathway occurs particularly in archaea and eukaryotes, as, e.g., in the cytosol of higher plants (31). The alternative MVA-independent methylerythritol phosphate (MEP) pathway was discovered later in eukaryotes, algae, and higher plants by intensive incorporation experiments using radioactive isotopes (47). In contrast to the MVA pathway, isoprenoids synthesized via the MEP pathway represent carbohydrate metabolites and not metabolites synthesized derived from acetyl-CoA (Fig. 2). The first two precursors of the MEP pathway are pyruvate and glyceraldehyde phosphate, which are directly derived from glucose metabolism. The final key enzyme, IPP isomerase, which converts the two products IPP and DMAPP into each other, occurs in both the MEP and the MVA pathway. All essential isoprenoids, which are correlated with the photosynthetic apparatus (phytol of chlorophylls, carotenoids, and prenyl side chains of plastoquinones), as well as secondary metabolites such as isoprene and mono- and diterpenes, are synthesized via the MEP pathway, which occurs in many bacteria and in chloroplasts of all plants. Plants synthesize polyisoprenoids in the cytosol via the MVA pathway and in the chloroplasts via the MEP pathway. Streptomyces aeriouvifer also possesses both pathways, but they are not coevally expressed (54, 55).
Fig 2.
Schematic representation of the reaction mechanisms of the mevalonate (MVA) and the methylerythritol phosphate (MEP) pathways for isoprenoid biosynthesis. Both pathways possess the final key enzyme isopentenyl diphosphate (IPP) isomerase, which converts the two products, IPP and dimethylallyl diphosphate (DMAPP), into each other (modified according to reference 47).
BIOSYNTHESIS OF POLYISOPRENE AND NR VIA cis- AND trans-PRENYLTRANSFERASES
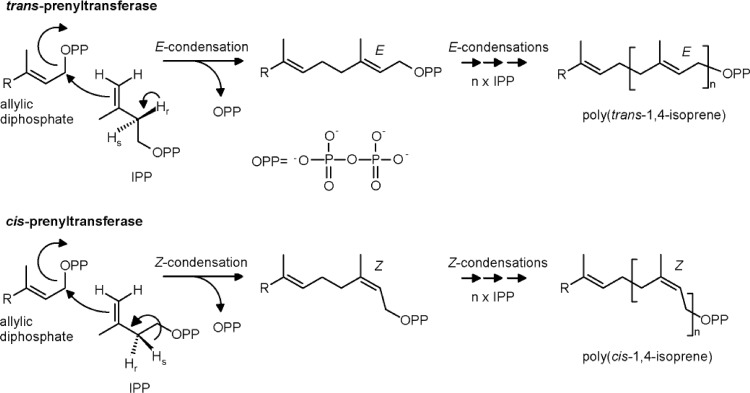
Biosynthesis of NR proceeds at the surface of rubber particles, where IPP is incorporated by a reaction with the terminal allyl diphosphate group of the rubber molecule. The enzyme catalyzing the rubber synthesis is designated rubber transferase or prenyltransferase (EC 2.5.1.20) (1, 14, 34, 35). It was proposed that the prenyltransferase synthesizes the hydrophobic rubber polymer out of the cytosol into the rubber particle, using the cytosolic hydrophilic substrate IPP (41). Due to their catalytic properties, prenyltransferases are divided into two different classes, cis- and trans-prenyltransferases (Fig. 3) (40). In both prokaryotes and eukaryotes, trans-prenyltransferases catalyze the formation of isoprenoid compounds, such as geranyl diphosphate (GPP) (C10), farnesyl diphosphate (FPP) (C15), and geranylgeranyl diphosphate (GGPP) (C20), which serve as initiating molecules to produce many other longer-chain isoprenoid compounds necessary for cellular growth and survival. Soluble trans-prenyltransferases occurring in plants are involved in NR biosynthesis, where they synthesize allylic diphosphates serving as initiator molecules for the polymerization process (14). Polymerization of NR is catalyzed by cis-prenyltransferases (EC 2.5.1.20), which require divalent cations such as Mg2+ and Mn2+ for their activity.
Fig 3.
Comparison of the reactions catalyzed by trans- and cis-prenyltransferases using allylic diphosphates and isopentenyl diphosphates (IPPs) (modified according to references 29 and 40).
Simplified, NR biosynthesis is divided into three biochemical processes: (i) initiation, where a trans-prenyltransferase synthesizes allylic diphosphates, (ii) elongation, where the cis-1,4 polymerization of isoprene units starting from allylic diphosphates and IPP is catalyzed by a cis-prenyltransferase (EC 2.5.1.20), and (iii) termination, where the synthesized polymer is released by a cis-prenyltransferase (EC 2.5.1.20).
The termination process occurs after a definite chain length, thus defining the molecular weight of the synthesized rubber (41). Therefore, cis-prenyltransferases are classified into three subfamilies with respect to product chain length, i.e., short-chain (C15), medium-chain (C50–55), and long-chain (C70–120) cis-prenyltransferases. Modification of the prenyl chain length determination mechanism of the undecaprenyl diphosphate (UPP) (C55) synthase of Micrococcus luteus strain B-P 26, which catalyzes cis condensation to synthesize UPP, through its structural manipulation was described by Kharel et al. (29). UPP is required as a lipid carrier of glycosyl residues in synthesis of the bacterial cell wall. Replacements of specific amino acid residues resulted in shorter ultimate products with C20–35, whereas insertion of specific residues originating from long-chain cis-prenyltransferases resulted in lengthening of the ultimate product chain, leading to C60–75. These results will help to understand the reaction mechanisms of cis-prenyltransferases, including regulation of the ultimate prenyl chain length, which have not yet been completely elucidated (29).
RUBBER-DEGRADING FUNGI
The first investigations on degradation of rubber by fungi were performed by De Vries (17). He cultivated different strains of Penicillium and Aspergillus in liquid medium containing NR and 10% (wt/vol) NaCl and documented an increase of biomass of 6% and a decrease in weight of the applied rubber material of 15.5 to 30.9% after incubation periods lasting from 19 months to 5 years. Schade observed good growth of the fungi Monascus rubber and M. purpureus on purified NR (50). Kalinenko described Aspergillus oryzae and different strains of Penicillium sp. as rubber-utilizing fungi, which was, however, not confirmed by other scientists (28, 39, 56). Further experiments revealed a decrease in weight of vulcanized rubber after incubation with Fusarium solani (32) and of NR and isoprene rubber (IR) after incubation with Penicillium variabile (71). Furthermore, strains of Cladosporium cladosporioides, Paecilomyces lilacinus, and Phoma eupyrena (10) were described as NR-degrading fungi, and three strains of Aspergillus (33) were found to be able to degrade vulcanized rubber.
RUBBER-DEGRADING BACTERIA
The most potent rubber-degrading bacteria are members of the CNM (Corynebacterium, Nocardia, Mycobacterium) group. They require direct contact with the rubber substrates (37) and do not produce translucent halos. The other group of rubber-degrading bacteria form clear zones on natural rubber latex agar plates and generally belong to the actinomycetes (Actinoplanes, Streptomyces, and Micromonospora) (24).
The Streptomyces strains were classified by Waksman and Henrici on the basis of morphology and cell wall chemotype (68). Today, the main emphasis is on 16S rRNA similarities, in addition to cell wall analysis, as well as fatty acid and lipid patterns (70, 71) (Table 2). Streptomycetes are Gram-positive, aerobic, spore-forming bacteria that show slow growth in soil or water as a branching substrate and aerial mycelia. The substrate hyphae are approximately 0.5 to 1.0 μm in diameter and often lack cross walls during the vegetative phase. Many members of the genus Streptomyces possess the ability to secrete enzymes, which are able to metabolize or cleave biopolymers (52).
Table 2.
List of rubber-degrading bacteria mentioned in this review
| Strain designation | Type of rubber degradationa | Reference |
|---|---|---|
| Actinomadura sp. strain E6 | B | 27 |
| Actinomyces candidus | ? | 39 |
| Actinomyces elastica | ? | 58 |
| Actinomyces fuscus | ? | 58 |
| Actinoplanes (three species) | B | 27 |
| Dactylosporangium sp. | B | 27 |
| Gordonia polyisoprenivorans VH2 | A | 37 |
| Gordonia polyisoprenivorans Y2K | A | 2 |
| Gordonia westfalica Kb1 | A | 36 |
| Micromonospora aurantiaca W2b | B | 37 |
| Micromonospora (five strains) | B | 27 |
| Mycobacterium fortuitum NF4 | A | 37 |
| Nocardia sp. strain 835 | ? | 66 |
| Nocardia farcinica S3 | A | 23 |
| Proactinomyces ruber | ? | 39 |
| Streptomyces (31 strains) | B | 27 |
| Streptomyces sp. strain K30 | B | 48 |
| Thermomonospora sp. strain E5 | B | 23 |
| Xanthomonas sp. strain 35Y | B | 67 |
A, rubber-degrading bacteria forming clear zone on latex overlay agar plates; B, adhesive-growing, rubber-degrading bacteria, which are not able to grow or form halos on latex overlay plates; ?, not known. Type of rubber degradation refers to reference 37.
Several bacteria are well known as poly(cis-1,4-isoprene)-degrading strains, such as a Gordonia sp. (2), Nocardia sp. strain 835A (66, 23), a Streptomyces sp. (49), and Xanthomonas sp. strain 35Y (27, 37) (Table 1). All these strains do not utilize the trans isomer of polyisoprene, i.e., gutta-percha (69). Even purified RoxA, which is responsible for poly(cis-1,4-isoprene) cleavage by Xanthomonas sp. 35Y, was incapable of cleaving poly(trans-1,4-isoprene) in vitro (11).
All hitherto known rubber-degrading bacteria had in common that they produced translucent halos on solid media containing poly(cis-1,4-isoprene) in the form of latex. The first gordoniae capable of utilizing poly(cis-1,4-isoprene) as a source for carbon and energy were described by Linos et al. (36). Surprisingly, these isolates did not produce clear zones on latex-containing solid media, but they nevertheless degraded the substrate very effectively. Presumably, this group of rubber-degrading bacteria had not been recognized earlier due to the method applied for enrichment and characterization. Later studies resulted in the description of the two novel species Gordonia polyisoprenivorans (36) and G. westfalica (36), which are distinguished by their adhesive growth during rubber degradation.
The molecular basis of rubber degradation is still only scarcely understood, but one can presume that the hydrophobicity of the cell surfaces of gordoniae affected by the presence of mycolic acids is significantly involved in this process. Probably, besides the occurrence of mycolic acids, the production of biosurfactants is important for the formation of biofilms, enabling direct contact with cis-1,4-polyisoprene in solid rubber materials, which is required for rubber degradation by these strains. In general, biosurfactants can be subdivided into low-molecular-weight compounds such as glycolipids and lipopeptides and high-molecular-weight polymeric compounds such as polysaccharides, lipoproteins, and lipopolysaccharides. Production of surface-active compounds has been reported for several Gordonia strains (3). For example, G. amarae is the most extensively studied species of the genus (2). By morphological and physiological means, it has been shown that G. amarae is strongly associated with foaming activated sludge in wastewater treatment plants, as also confirmed by phylogenetic hybridization-based experiments and comparative rRNA sequence analysis (16). The direct contact is required for rubber degradation by these two novel species Gordonia polyisoprenivorans (36) and G. westfalica (3, 36). Nocardia sp. strain 835A, which exhibited reasonable growth on natural and synthetic rubber, was one of the first strains that was investigated in detail with regard to rubber biodegradation, and it was postulated that oxidative cleavage of poly(cis-1,4-isoprene) occurs at the double bond (9, 12, 37, 49, 66, 67). Like Gordonia species, Nocardia spp. do not produce translucent halos and require direct contact to the rubber substrates.
A latex-clearing protein (Lcp) was identified in the Gram-positive Streptomyces sp. strain K30 by Rose et al. (48). In contrast, RoxA (rubber oxygenase A) was identified in the clear-zone-forming Xanthomonas sp. strain 35Y, which is the only known rubber-degrading bacterium that does not belong to the actinomycetes but is a Gram-negative bacterium (11, 27).
Imai et al. reported on the isolation of a Gram-negative rubber-degrading bacterium other than gammaproteobacteria (24). Three novel bacteria, Streptomyces sp. strain LCIC4, Actinoplanes sp. strain OR16, and Methylibium sp. strain NS21, were isolated. The lcp gene of LCIC4 showed 99% amino acid sequence identity with that of Streptomyces sp. strain K30. It is located next to oxiB. The results suggested that the lcp homologs are involved in rubber degradation in LCIC4 and OR16 (24).
DEGRADATION OF POLY(cis-1,4-ISOPRENE)
Gel permeation chromatography (GPC) analysis of the degradation products formed during the degradation of NR by the Gram-positive Nocardia sp. strain 835A (65) and by the Gram-negative Xanthomonas sp. strain 35Y (11) identified 12-oxo-4,8-dimethyl-trideca-4,8-dienal as a major and 8-oxo-4-methyl-4-nonenal as a minor component. Based on the location of 18O in the degradation products, the authors postulated an oxidative cleavage at the double bond in the polyisoprene backbone. Bode et al. (9) identified (6Z)-2,6-dimethyl-10-oxo-undec-6 enoic acid, (5Z)-6-methyl-undec-5-ene-2,9-dione, and (5Z,9Z)-6,10-dimethylpentadec-5,9-diene-2,13-dione as degradation products in a liquid culture of Streptomyces coelicolor strain 1A after cultivation of the cells on vulcanized rubber. This bacterium belongs to the first group of NR-degrading bacteria. Based on the postulated oxidative cleavage (45) and on the identified degradation products, a pathway for the degradation of NR was proposed, including (i) the oxidation of an aldehyde intermediate to a carboxylic acid, (ii) one cycle of β-oxidation, (iii) oxidation of the conjugated double bond yielding a β-keto acid, and (iv) its subsequent decarboxylation (8).
Purified RoxA degraded poly(cis-1,4-isoprene) by oxidative cleavage at the double bonds yielding 12-oxo-4,8-dimethyltrideca-4,8-diene-1-al as the main cleavage product; other minor cleavage products differed only in the number of repetitive isoprene units (8). In vitro experiments also revealed the occurrence of two 18O atoms in the reduced degradation product 12-hydroxy-4,8-dimethyltrideca-4,8-diene-1-ol, thereby disclosing a dioxygenase mechanism (8).
At the same time, Rose et al. (48) identified lcp in Streptomyces sp. strain K30, which belongs to the first group of NR-degrading bacteria (36). UV mutagenesis yielded mutants with a clear zone-negative phenotype on latex overlay agar plates and the inability to mineralize NR.
TWO IMPORTANT PROTEINS: Lcp AND RoxA
So far, two candidate proteins have been described that are involved in the attack on the polyisoprene carbon backbone. One is Lcp from Streptomyces sp. K30 (48), and the other one is RoxA from a Xanthomonas sp. (27). Lcp and RoxA are apparently different polypeptides devoid of any relevant amino acid similarity. Both bacteria belong to the so-called clear-zone-forming group of rubber-degrading bacteria, and obviously both bacteria are secreted into the extracellular medium leading to the formation of translucent halos on NR latex. Both enzymes cleave poly(cis-1,4-isoprene) by an oxidative reaction mechanism (12, 27) (details will be described in the following sections).
RUBBER OXYGENASE A (RoxA)
During growth on poly(cis-1,4-isoprene), Xanthomonas sp. strain 35Y secretes a heme-containing protein, rubber oxygenase A (RoxA), into the medium (12). RoxA is a dioxygenase, as it was shown by isotope labeling experiments (11). A large set of experiments was carried out to investigate the reactivity of the RoxA heme centers toward substrates, reductants, oxidants, inhibitors and well-known heme ligands, including imidazole and related compounds. Upon attack on rubber RoxA releases low-molecular-mass oligoisoprene units. As mentioned above, 12-oxo-4,8-dimethyltrideca-4,8-diene-1-al (ODTD) was identified as the major product under in vitro conditions together with a homologous series of minor compounds that differ from the major degradation product only in the number of repetitive isoprene units between terminal functions, CHO-CH2- and -CH2-COCH3 (12).
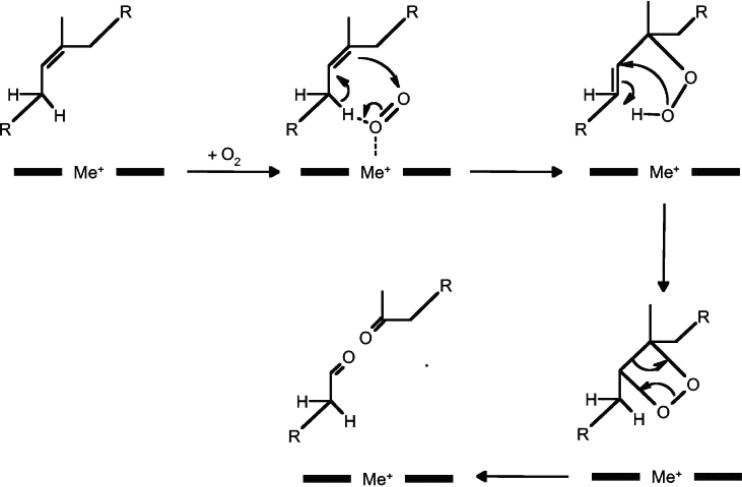
Until now only the oxidative reaction mechanism of RoxA is well understood (51). RoxA has two c-type heme centers. One of the two hemes is reduced by NADH, which is similar to the situation found in bacterial diheme peroxidases. Evidence for an electron transfer between the two hemes was provided by slow reduction of the second heme upon incubation of the partially reduced enzyme. In line with this result, RoxA did not show any peroxidase activity. Electron paramagnetic resonance (EPR) spectra of purified RoxA revealed two low-spin Fe(III) heme centers. A weak but clear signal in a region corresponding to high-spin Fe(III) heme was obtained; this signal disappeared in the presence of imidazole. Attempts to provide spectroscopic evidence for binding of the natural substrate (polyisoprene latex) to RoxA failed. However, experimental data showed that RoxA is able to subtract redox equivalents from its substrate or from model compounds. In conclusion, RoxA is a novel type of diheme dioxygenase, clearly different from classical cytochrome c peroxidases (51) (Fig. 4).
Fig 4.
Proposed dioxygenase reaction mechanism for cleavage of natural rubber by rubber oxygenase (RoxA). Black bars and Me+ (metal) indicate the heme reaction center of RoxA (12).
The roxA gene was cloned and transferred to Escherichia coli BL21(DE3). Significant expression of RoxA was not obtained (22). Unfortunately, all attempts to express RoxA in recombinant E. coli or in other Gram-negative bacteria have failed so far. Even coexpression of cytochrome maturation genes (ccm genes) from pEC86 did not result in significant expression of RoxA. However, formation of the diheme cytochrome Dhc2 from Geobacter sulfurreducens in E. coli was successfully obtained in the presence of pEC86 (22).
LATEX-CLEARING PROTEIN (Lcp)
Analysis of the amino acid sequence encoded by lcp of Streptomyces sp. strain K30 revealed a twin-arginine motif, thus indicating that Lcp is a substrate of the twin-arginine translocation (Tat) pathway (74). Clear evidence that Lcp is secreted into the culture medium was obtained from heterologous expression of lcp in E. coli (74). Transcriptional analysis revealed basal expression of Lcp in glucose-grown cells and induction of lcp in the presence of poly(cis-1,4-isoprene). In contrast, oxiB and oxiA, which are in Streptomyces sp. strain K30 located directly downstream of lcp and putatively encode a heteromultimeric aldehyde dehydrogenase oxidizing the primary cleavage products generated by Lcp from poly(cis-1,4-isoprene), were expressed only in the presence of poly(cis-1,4-isoprene) (Fig. 5). All three genes, lcp, oxiB, and oxiA, seem to constitute an operon, as a polycistronic mRNA comprising these genes was detected (74).
Fig 5.
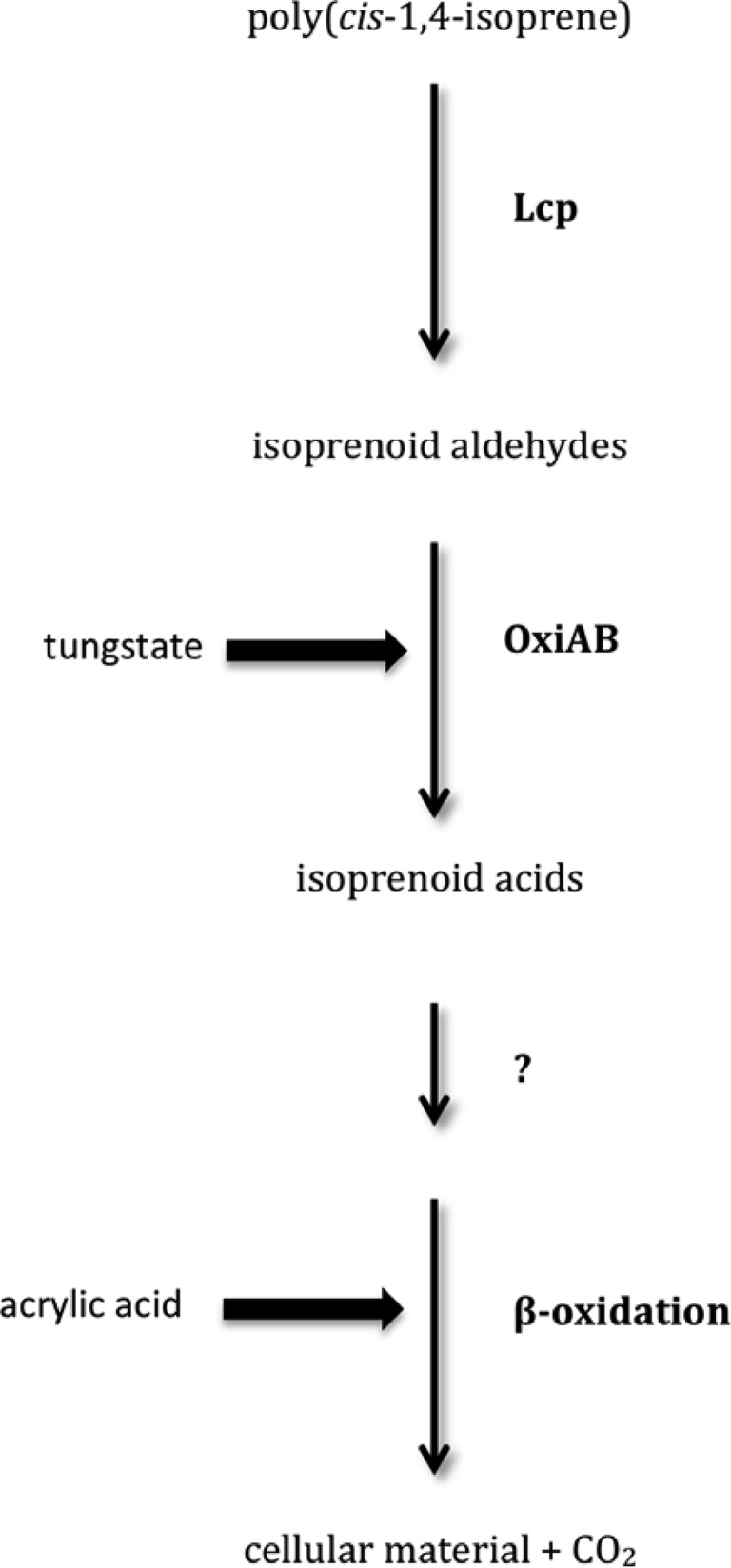
Degradation pathway of polyisoprene in Streptomyces sp. K30. Lcp, latex-clearing protein; OxiAB, rubber-oxidizing molybdenum hydroxylase. Polyisoprene rubber was degraded by Lcp to isoprenoid aldehydes; OxiAB probably subsequently oxidized these aldehydes to the corresponding acids, which can be further metabolized via β-oxidation.
Overexpression of Streptomyces genes in engineered expression hosts of the same or related species of this genus was successfully applied to the overproduction of different enzymes. Therefore, heterologous expression experiments with lcp derived from Streptomyces sp. K30 in different Streptomyces strains were performed to analyze to which extent the ability for rubber cleavage is transferable to other bacteria and also to clarify the role of Lcp in rubber degradation (75). For these analyses, Streptomyces strains were used, which are impaired to form clear zones on latex overlay agar plates. Previous experiments were done with S. lividans TK23 and plasmid pIJ702 harboring wild-type lcp (48). The parent strain S. lividans TK23 neither grows on IR nor forms clear zones on NR latex, whereas the recombinant strain S. lividans TK23 harboring pIJ702::lcp gained the ability to form clear zones on NR latex overlay plates.
New studies indicate that Lcp is responsible for clear zone formation on latex agar overlay plates or that it is at least an essential constituent of a protein complex forming clear zones by cleavage of poly(cis-1,4-isoprene). When Lcp from Streptomyces sp. strain K30 was heterologously expressed in strains TK23 and TK24 of S. lividans and in a strain of Saccharopolyspora erythraea (formerly Streptomyces erythraeus), the recombinant cells acquired the ability to cleave poly(cis-1,4-isoprene), thus confirming the participation of Lcp in initial polymer cleavage. By using the supernatant of these Lcp-expressing strains in vitro, it was clearly shown by halo formation that all three strains secreted a functional Lcp.
In order to verify the role of Lcp in rubber degradation, an lcp knockout mutant of Streptomyces sp. K30, Streptomyces sp. K30_lcpΩKm, was generated (75). Streptomyces sp. K30_lcpΩKm exhibited reduced growth in liquid mineral salts medium containing poly(cis-1,4-isoprene) as the sole carbon and energy source. Additionally, there was no detectable Lcp activity on latex overlay agar plates.
FUTURE WORK AND CONCLUDING REMARKS
Further studies are necessary to elucidate the rubber degradation pathway in Gram-positive and Gram-negative bacteria in more detail. More than 70 years after the first description of clear zone formation by bacteria on latex media (59), genetic data on the molecular basis of this observation are now available. One reason for the scarcity of knowledge on rubber degradation is the extremely long culture periods of bacteria on polyisoprene, but the major reason is the difficult expression of Lcp and RoxA in E. coli. Restriction of the genetic work with some members of the mycelium-forming actinomycetes, to which nearly all clear-zone-forming bacteria belong, contributes to the delay as well. The next step in the investigation of the rubber degradation pathway in Streptomyces sp. strain K30 is the purification of Lcp to unravel relevant biochemical features such as kinetic data and substrate specificity. The availability of detailed information about this enzyme involved in the cleavage of polyisoprene is a prerequisite for its technical application.
ACKNOWLEDGMENT
Financial support for this study by the Deutsche Forschungsgemeinschaft is gratefully acknowledged (grant STE-386/10-1).
Footnotes
Published ahead of print 13 April 2012
REFERENCES
- 1. Archer BL, Audley BG. 1987. New aspects of rubber biosynthesis. Bot. J. Linn. Soc. 94:181–196 [Google Scholar]
- 2. Arenskötter M, Baumeister D, Kalscheuer R, Steinbüchel A. 2003. Identification and application of plasmids suitable for transfer of foreign DNA to members of the genus Gordonia. Appl. Environ. Microbiol. 69:4971–4974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arenskötter M, Bröker D, Steinbüchel A. 2004. Biology of the metabolically diverse genus Gordonia. Appl. Environ. Microbiol. 70:3195–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Backhaus RA. 1985. Rubber formation in plants—a mini-review. Isr. J. Bot. 34:283–293 [Google Scholar]
- 5. Blanc G, Baptiste C, Oliver G, Martin F, Montoro P. 2006. Efficient Agrobacterium tumefaciens-mediated transformation of embryogenic calli and regeneration of Hevea brasiliensis Müll Arg. plants. Plant Cell Rep. 24:724–733 [DOI] [PubMed] [Google Scholar]
- 6. Bloch K, Rittenberg D. 1942. On the situation of acetic acid for cholesterol formation. J. Biol. Chem. 143:625–636 [Google Scholar]
- 7. Blow CM, Hepburn C. 1982. Rubber technology and manufacture, 2nd ed Butterworth Scientific, London, United Kingdom [Google Scholar]
- 8. Bode HB, Zeeck A, Plückhahn K, Jendrossek D. 2000. Physiological and chemical investigations into microbial degradation of synthetic poly(cis-1,4-isoprene). Appl. Environ. Microbiol. 66:3680–3685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bode HB, Kerkhoff K, Jendrossek D. 2001. Bacterial degradation of natural and synthetic rubber. Biomacromolecules 2:295–303 [DOI] [PubMed] [Google Scholar]
- 10. Borel M, Kergomard A, Renard MF. 1982. Degradation of natural rubber by fungi imperfecti. Agric. Biol. Chem. 46:877–881 [Google Scholar]
- 11. Braaz R, Armbruster W, Jendrossek D. 2005. Heme-dependent rubber oxygenase RoxA of Xanthomonas sp. cleaves the carbon backbone of poly(cis-1,4-isoprene) by a dioxygenase mechanism. Appl. Environ. Microbiol. 71:2473–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Braaz R, Fischer P, Jendrossek D. 2004. Novel type of heme-dependent oxygenase catalyzes oxidative cleavage of rubber (poly-cis-1,4-isoprene). Appl. Environ. Microbiol. 70:7388–7395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brydson JA. 1988. Rubber materials and their compounds, p 125–126 Elsevier Applied Science, London, United Kingdom [Google Scholar]
- 14. Cornish K. 1993. The separate roles of plant cis and trans prenyltransferases in cis-1,4-polyisoprene synthesis. Eur. J. Biochem. 218:267–271 [DOI] [PubMed] [Google Scholar]
- 15. Reference deleted. [Google Scholar]
- 16. de los Reyes MF, de los Reyes FL, Hernandez M, Raskin L. 1998. Identification and quantification of Gordona amarae strains in activated sludge systems using comparative rRNA sequence analysis and phylogenetic hybridization probes. Water Sci. Technol. 37:521 [Google Scholar]
- 17. De Vries O. 1928. Zersetzung von Kautschuk-Kohlenwasserstoff durch Pilze. Zentralbl. Bakteriol. Parasitenkd. Infekt. 74:22–24 [Google Scholar]
- 18. Eng AH, Tangpakdee J, Kawahara S, Tanaka Y. 1997. Distribution and origin of abnormal groups in natural rubber. J. Nat. Rubber Res. 12:11–20. [Google Scholar]
- 19. Fatta D, Papadopoulos A, Loizidou M. 1999. A study on the landfill leachate and its impact on the groundwater quality of the greater area. Environ. Geochem. Health 21:175–190 [Google Scholar]
- 20. Franta I. 1989. Elastomers and rubber compounding materials, p 302–315 American Elsevier Science Ltd., New York, NY [Google Scholar]
- 21. Gent AN. 1992. Engineering with rubber: how to design rubber components. Oxford University Press, New York, NY [Google Scholar]
- 22. Hambsch N, Schmitt G, Jendrossek D. 2010. Development of a homologous expression system for rubber oxygenase RoxA from Xanthomonas sp. J. Appl. Microbiol. 109:1067–1075 [DOI] [PubMed] [Google Scholar]
- 23. Ibrahim EMA, Arenskötter M, Luftmann H, Steinbüchel A. 2006. Identification of poly(cis-1,4-isoprene) degradation intermediates during growth of moderately thermophilic actinomycetes on rubber and cloning of a functional lcp homologue from Nocardia farcinica strain E1. Appl. Environ. Microbiol. 72:2275–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Imai S, et al. 2011. Isolation and characterization of Streptomyces, Actinoplanes, and Methylibium strains that are involved in degradation of natural rubber and synthetic poly(cis-1,4-isoprene). Enzyme Microb. Technol. 49:526–531 [DOI] [PubMed] [Google Scholar]
- 25. International Rubber Study Group (IRSG) 2010. Natural rubber: what has the future in store? Presentation at SICOM, Singapore, 20 June 2007 [Google Scholar]
- 26. Jang J-W, Yoo T-S, Oh J-H, Iwasaki I. 1998. Discarded tire recycling practices in the United States, Japan and Korea. Res. Conserv. Recycling 22:1–14 [Google Scholar]
- 27. Jendrossek D, Reinhardt S. 2003. Sequence analysis of a gene product synthesized by Xanthomonas sp. during growth on natural rubber latex. FEMS Microbiol. Lett. 224:61–65 [DOI] [PubMed] [Google Scholar]
- 28. Kalinenko VO. 1938. The role of Actinomyces and bacteria in decomposing rubber. Mikrobiologiia 17:119–128 [Google Scholar]
- 29. Kharel Y, Takahashi S, Yamashita S, Koyama T. 2006. Manipulation of prenyl chain length determination mechanism of cis-prenyltransferases. FEBS J. 273:647–657 [DOI] [PubMed] [Google Scholar]
- 30. Kim IJ, Ryu SB, Kwak YS, Kang H. 2004. A novel cDNA from Parthenium argentatum Gray enhances the rubber biosynthetic activity in vitro. J. Exp. Bot. 55:377–385 [DOI] [PubMed] [Google Scholar]
- 31. Kuzuyama T. 2002. Mevalonate and non-mevalonate pathways for the biosynthesis of isoprene units. Biosci. Biotechnol. Biochem. 66:1619–1627 [DOI] [PubMed] [Google Scholar]
- 32. Kwiatkowska D, Zyska BJ, Zankowicz LP. 1980. Microbial deterioration of natural rubber sheets by soil microorganisms. Biodeterioration 4:135–141 [Google Scholar]
- 33. Kwiatkowska D, Zyska B. 1988. Changes in natural rubber vulcanizates due to microbial degradation. Biodeterioration 7:575–579 [Google Scholar]
- 34. Light DR, Dennis MS. 1989. Purification of a prenyltransferase that elongates cis-polyisoprene rubber from the latex of Hevea brasiliensis. J. Biol. Chem. 264:18589–18597 [PubMed] [Google Scholar]
- 35. Light DR, Lazarus RA, Dennis MS. 1989. Rubber elongation by farnesyl pyrophosphate synthases involve a novel switch in enzyme stereospecificity. J. Biol. Chem. 264:18598–18607 [PubMed] [Google Scholar]
- 36. Linos A, Steinbüchel A, Spröer C, Kroppenstedt RM. 1999. Gordonia polyisoprenivorans sp. nov., a rubber degrading actinomycete isolated from an automobile tire. Int. J. Syst. Bacteriol. 49:1785–1791 [DOI] [PubMed] [Google Scholar]
- 37. Linos A, et al. 2000. Biodegradation of poly(cis-1,4-isoprene)rubbers by distinct actinomycetes: microbial strategies and detailed surface analysis. Appl. Environ. Microbiol. 66:1639–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reference deleted. [Google Scholar]
- 39. Nette IT, Pomortseva NV, Kozlova EI. 1959. Destruction of rubber by microorganisms. Mikrobiologiia 28:821–827 [PubMed] [Google Scholar]
- 40. Ogura K, Koyama T. 1998. Enzymatic aspects of isoprenoid chain elongation. Chem. Rev. 98:1263–1276 [DOI] [PubMed] [Google Scholar]
- 41. Ohya N, Koyama T. 2001. Biosynthesis of natural rubber and other natural poyisoprenoids, p 73–109 In Koyama TSteinbü, chel A. (ed), Biopolymers, vol 2 Polyisoprenoids. Wiley-VCH, Weinheim, Germany [Google Scholar]
- 42. Omo-Ikerodah EE, Omokhafe KO, Akpobome FA, Mokwunye MU. 2009. An overview of the potentials of natural rubber (Hevea brasiliensis) engineering for the production of valuable proteins. Afr. J. Biotechnol. 8:7303–7307 [Google Scholar]
- 43. Pendle TD, Swinyard PE. 1991. The particle size of natural rubber latex concentrates by photon correlation spectroscopy. J. Nat. Rubber Res. 6:1–11 [Google Scholar]
- 44. Polhamus LG. 1962. Rubber. Botany, production, and utilization. Leonard Hill Limited, London, United Kingdom [Google Scholar]
- 45. Rifaat HM, Yosery MA. 2004. Identification and characterization of rubber degrading actinobacteria. Appl. Ecol. Environ. Res. 2:63–70 [Google Scholar]
- 46. Rittenberg D, Schönheimer R. 1937. Deuterium as indicator in the study of intermediary metabolism. XI. Further studies on the biological uptake of deuterium into organic substances, with special reference to fat and cholesterol formation. J. Biol. Chem. 121:235–253 [Google Scholar]
- 47. Rohmer M, Knani M, Simonin P, Sutter B, Sahn H. 1993. Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem. J. 295:517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rose K, Tenberge KB, Steinbüchel A. 2005. Identification and characterization of genes from Streptomyces sp. strain K30 responsible for clear zone formation on natural rubber latex and poly(cis-1,4-isoprene) rubber degradation. Biomacromolecules 6:180–188 [DOI] [PubMed] [Google Scholar]
- 49. Rose K, Steinbüchel A. 2005. Biodegradation of natural rubber and related compounds: recent insights into a hardly understood catabolic capability of microorganisms. Appl. Environ. Microbiol. 71:2803–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schade AL. 1937. Observations on a Monascus isolated from rubber. Mycologia 29:295–302 [Google Scholar]
- 51. Schmitt G, Seiffert G, Kroneck MH, Braaz R, Jendrossek D. 2010. Spectroscopic properties of rubber oxygenase RoxA from Xanthomonas sp., a new type of diheme dioxygenase. J. Appl. Microbiol. 156:2537–2548 [DOI] [PubMed] [Google Scholar]
- 52. Schrempf H. 2007. The family of Streptomycetaceae. Part II: molecular biology, p 605–622 In Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E. (ed), The prokaryotes, vol 3 Springer, Berlin, Germany [Google Scholar]
- 53. Scrap Tire Management Council (STMC) 1997. Scrap tire use/disposal study, 1996 update. STMC, Washington, DC [Google Scholar]
- 54. Seto H, Watanabe H, Furihata K. 1996. Simultaneous operation of the mevalonate and non-mevalonate pathways in the biosynthesis of isopentenyl diphosphate in Streptomyces aeriouvifer. Tetrahedron Lett. 37:7979–7982 [Google Scholar]
- 55. Seto H, Orihara N, Furihata K. 1998. Studies on the biosynthesis of terpenoids produced by actinomycetes. Part. 4. Formation of BE-40644 by the mevalonate and nonmevalonate pathways. Tetrahedron Lett. 39:9497–9500 [Google Scholar]
- 56. Shaposhnikov VN, Rabotnova IL, Iarmola GA, Kuznetsova VM. 1952. On growths of moulds on natural rubber. Mikrobiologiia 21:280–282 [PubMed] [Google Scholar]
- 57. Skeggs HR, et al. 1956. Discovery of a new acetate-replacing factor. J. Bacteriol. 72:519–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Söhngen NL, Fol JG. 1914. Die Zersetzung des Kautschuks durch Mikroben. Zentralbl. Bakteriol. Parasitenkd. Infekt. 40:87–98 [Google Scholar]
- 59. Spence D, van Niel CB. 1936. Bacterial decomposition of rubber in Hevealatex. Ind. Eng. Chem. 28:847–850 [Google Scholar]
- 60. Subramaniam A. 1995. The chemistry of natural rubber latex. Immunol. Allergy Clin. N. Am. 15:1–20 [Google Scholar]
- 61. Stewart GA, Lucas SM. 1986. Potential production of natural rubber from guayule (Partheniumargentatum) in Australia. CSIRO, Melbourne, Australia [Google Scholar]
- 62. Tanaka Y, Sakdapipanich JT. 2001. Chemical structure and occurrence of natural polyisoprenes, p 1–25 In Koyama T, Steinbüchel A. (ed), Biopolymers, vol 2 Polyisoprenoids. Wiley-VCH, Weinheim, Germany [Google Scholar]
- 63. Tanaka Y, Kawahara S, Tangpakdee J. 1997. Structural characterization of natural rubber. J. Nat. Rubber Res. 50:6–11 [Google Scholar]
- 64. Reference deleted. [Google Scholar]
- 65. Towbin H, Staehelin T, Gordon J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 76:4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tsuchii A, Suzuki T, Takeda K. 1985. Microbial degradation of natural rubber vulcanizates. Appl. Environ. Microbiol. 50:965–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tsuchii A, Takeda K. 1990. Rubber-degrading enzyme from a bacterial culture. Appl. Environ. Microbiol. 56:269–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Waksman SA, Henrici AT. 1943. The nomenclature and classification of the Actinomycetes. J. Bacteriol. 46:337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Warneke S, Arenskötter M, Tenberge KB, Steinbüchel A. 2007. Bacterial degradation of poly(trans-1,4-isoprene) (gutta percha). Microbiology 153:347–356 [DOI] [PubMed] [Google Scholar]
- 70. Wellington EMH, Cresswell N, Herron PR. 1992. Gene transfer between streptomycetes in soil. Gene 115:193–198 [DOI] [PubMed] [Google Scholar]
- 71. Williams GR. 1982. The breakdown of rubber polymers by microorganisms. Int. Biodeterior. Bull. 18:31–36 [Google Scholar]
- 72. Wititsuwannakul D, Wititsuwannakul R. 2001. Biochemistry of natural rubber and structure of latex, p 151–202 In Steinbüchel A. (ed), Biopolymers, vol 2 Wiley-VCH, Weinheim, Germany [Google Scholar]
- 73. Wright LD, et al. 1956. Isolation of a new acetate-replacing factor. J. Am. Chem. Soc. 78:5273–5275 [Google Scholar]
- 74. Yikmis M, et al. 2008. Secretion and transcriptional regulation of the latex-clearing protein, Lcp, by the rubber-degrading bacterium Streptomyces sp. strain K30. Appl. Environ. Microbiol. 74:5373–5382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yikmis M, Steinbüchel A. 2012. Importance of the latex-clearing protein (Lcp) for poly(cis-1,4-isoprene) rubber cleavage in Streptomyces sp. K30. Microbiol. Open 1:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]