Abstract
Human neonates infected with herpes simplex virus 1 (HSV-1) develop one of three distinct patterns of infection: (i) infection limited to the skin, eye or mouth; (ii) infection of the CNS; or (iii) disseminated infection. The disseminated form usually involves the liver, adrenal gland, and lung, and resembles the clinical picture of bacterial sepsis. This spectrum of symptoms in HSV-1-infected neonates suggests that inflammatory cytokines play a significant role in the pathogenesis of the disease. Recent studies suggest that the Toll-like receptors (TLRs) may play an important role in the induction of inflammatory cytokines in response to viruses. TLRs are mammalian homologues of Toll, a Drosophila protein that is essential for host defense against infection. Engagement of TLRs by bacterial, viral, or fungal components leads to the production and release of cytokines and other antimicrobial products. Here, we demonstrate that TLR2 mediates the inflammatory cytokine response to HSV-1 by using both transfected cell lines and knockout mice. Studies of infected mice revealed that HSV-1 induced a blunted cytokine response in TLR2–/– mice. Brain levels of monocyte chemoattractant protein 1 chemokine were significantly lower in TLR2–/– mice than in either wild-type or TLR4–/– mice. TLR2–/– mice had reduced mortality compared with wild-type mice. The differences between TLR2–/– mice and both wild-type and TLR4–/– mice in the induction of monocyte chemoattractant protein 1, brain inflammation, or mortality could not be accounted for on the basis of virus levels. Thus, these studies suggest the TLR2-mediated cytokine response to HSV-1 is detrimental to the host.
Keywords: cytokines, viral pathogenesis
Herpesviruses are complex DNA viruses that are characterized by life-long latency. All herpesviruses are capable of reactivation to cause disease in both immunocompetent and incompetent individuals. Herpes simplex virus 1 (HSV-1) is the most commonly diagnosed cause of sporadic (nonepidemic) encephalitis in humans. Without early treatment, HSV-1 encephalitis is a devastating disease that is typically fatal. Among survivors, serious residual defects are commonly seen. Whereas HSV causes a variety of illnesses in immunocompromised hosts, including disseminated infection, pneumonia, and hepatitis, encephalitis is commonly seen in patients with normal immune responses. The role of inflammatory cytokines in viral encephalitis has not been well defined (1, 2).
In humans, HSV-1 is usually acquired in childhood and often presents as a self-limiting pharyngitis. Reactivation of HSV-1 infection is associated with perioral lesions, termed “fever blisters.” HSV in the neonate (either HSV-1 or HSV-2) is a far different disease, and may be characterized by a “sepsis-like” picture, which includes blood pressure instability, disseminated intravascular coagulation and shock, as well as lethal encephalitis (2, 3).
Toll-like receptors (TLRs) are transmembrane proteins that function as pattern-recognition receptors for the detection and response to microbial ligands (4, 5). To date, 10 TLRs have been identified in humans, and natural or synthetic ligands for at least nine TLRs have been identified (6–10). The extracellular regions of TLRs are diverse but contain variable numbers of leucinerich-repeat regions and conserved cysteine domains, which are thought to contribute to receptor structure and function. All of the TLRs share a signature intracellular signaling motif with the IL-1 receptor, called the TIR (Toll-IL-1-R) domain (6–8, 11). Activation of TLRs results in the recruitment of adaptor proteins, including MyD88 (all known TLRs) and Mal/TIRAP (TLR2 and TLR4) to the TIR domain. A series of phosphorylation/recruitment/activation events leads to the activation and translocation of NF-κB to the nucleus and the transcription of inflammatory and anti-inflammatory cytokine genes (12, 13). TLRs including TLR4, the lipopolysaccharide (LPS)-activated TLR, are the major signal transduction proteins associated with the pathophysiology of sepsis (14–16).
Although originally described as receptors for bacteria and fungi, it has now become clear that TLRs mediate the production of cytokines in response to a variety of viruses and viral ligands. A role for the TLRs TLR2, TLR3, TLR4, and TLR9, in the response to viruses has been established (17–22). Our previous experiments have demonstrated that the cytokine response to human cytomegalovirus is controlled by TLR2, whereas the response to respiratory syncytial virus depends on TLR4 (17–19). Here, we demonstrate that TLR2 mediates the induction of inflammatory cytokines in response to HSV-1. Remarkably, our studies indicate that TLR2 expression is not protective, but rather, is associated with lethal viral encephalitis on HSV-1 infection.
Materials and Methods
Human embryonic kidney (HEK)293 cells expressing human TLR2 were cloned as described (23). HEK293 expressing human TLR3, TLR9, TLR4, and MD2 were the kind gift of E. Latz (University of Massachusetts Medical School, Worcester) (24). All of the HEK293 cell lines were derived from the same parental HEK293 cells provided by D. Golenbock (University of Massachusetts Medical School) (25). Cells were transfected with an NF-κB firefly luciferase reporter plasmid and a control Renilla luciferase plasmid by using a GeneJuice (Novagen) transfection reagent. HEK293 cells were incubated overnight after transfection before challenge with virus or human IL-1β (100 ng/ml). Cells were lysed and luciferase activity was measured by using a Dual-Glo luciferase assay system (Promega). Luciferase activity was calculated in relative light units as a ratio of NF-κB-dependent firefly luciferase activity to NF-κB-independent Renilla luciferase activity.
Mice deficient in TLR2, TLR4, or TLR6 were the gift of S. Akira (Osaka University, Osaka). These mice were generated by gene targeting by Dr. Akira (16, 26–28) and were provided as F2 interbred 129 × C57BL/6 mice. Control mice were bred from C57BL/6 × 129 F2 mice obtained from The Jackson Laboratory (B6129F2/J). All mice were bred and housed for at least three generations (and housed within the same room in the Animal Facility at the University of Massachusetts Medical School) before their inclusion in these experiments to minimize the effects of the environment on their susceptibility to infection.
Mice were injected with 4% thioglycollate and peritoneal exudate cells (PECs) were harvested 4 days later. PECs were plated at 106 cells per well in 24-well plates and were challenged with virus, phenol-extracted LPS (10 ng/ml, a TLR4 ligand), zymosan (10 μg/ml a TLR2 ligand), IL-1β (100 ng/ml), or medium alone. IL-6 and monocyte chemoattractant protein 1 (MCP-1) levels were determined by ELISA using BD PharMingen (San Diego) OptEIA and R & D Systems DuoSet kits. All assays were performed in duplicate.
HSV-1 KOS strain was grown in Vero cells and collected from cell supernatants as described (29). Mice were infected by i.p. injection of 109 (adult) or 104 (neonate) plaque forming units (pfu) per mouse. Serum was collected in gel-separation tubes. Brains were homogenized in ice-cold sterile PBS containing 1% FCS and 0.1% glucose and virus titers were determined in plaque assays (29). For cytokine analysis, the homogenates were diluted 1:1 in PBS containing Complete protease inhibitors (Roche Biochem, Indianapolis) and were analyzed by ELISA. Homogenates were stored frozen at –70°C before analysis.
The survival curves were compared by using a generalized Wilcoxon–Breslow test as described in Stata Survival Analysis and Epidemiological Tables (stata statistical software 8.0., Stata Press, College Station, TX). Cytokine levels were compared by using the Kolmogorov–Smirnov test as described (stata statistical software).
Results
HSV-1 Induces Cytokines Through TLR2. We examined the ability of human cells with or without TLRs on their surface to respond to KOS strain HSV-1. Experiments with transfected human cells (HEK293) revealed that HSV-1 activates NF-κB through TLR2 (Fig. 1A). Stable TLR2 transfectants, but neither TLR3 nor TLR4 transfectants, activated NF-κB in response to HSV-1 challenge. TLR9 transfectants were also unresponsive to HSV-1 (Fig. 1B) although recent reports suggest that HSV-1 and HSV-2 may signal through TLR9 (22, 30).
Fig. 1.
HSV activates cells through TLR2. (A) HEK293 cells expressing human TLR2, TLR3, or TLR4 ± MD2 were transfected with an NF-κB-driven firefly luciferase reporter plasmid and were stimulated for 6 h with HSV-1 (KOS strain) at a moi of 100 or with IL-1β (100 ng/ml) as a positive control. Luciferase activity was calculated in relative light units as a ratio of NF-κB-dependent firefly luciferase activity to NF-κB-independent Renilla luciferase activity. The results are shown as the mean ± SD of triplicate wells. Each cell line was tested in 3–10 independent experiments. (B) HEK293 cells expressing human TLR2 or TLR9 were challenged with HSV-1 KOS (a moi of 3–100), CpG DNA (0.1–3 μM), GpC control DNA (0.1–3 μM), or medium alone. NF-κB luciferase activity was measured as above. (C) Peritoneal exudates cells from wild-type, TLR2–/–, or TLR4–/– mice were stimulated with medium alone or with HSV-1 KOS at mois of 1, 10, and 100. IL-6 levels were measured in 16-h supernatants. The results are shown as the mean ± SD of duplicate wells. Each mouse strain was tested in at least three independent experiments. (D) Wild-type, TLR6–/–, or TLR2–/– peritoneal exudates cells were challenged with HSV-1 KOS (a moi of 100), Pam2CSK4 (100 ng/ml, a TLR2/TLR6 ligand), or LPS (10 ng/ml, a TLR4 ligand). IL-6 levels were measured as above.
To further define the role of TLRs as signal transducers for HSV-1, we examined cytokine production by PECs from wild-type, TLR4 knockout, or TLR2 knockout mice (Fig. 1C). Wild-type and TLR4–/– mouse peritoneal macrophages produced IL-6 in response to challenge with HSV-1. In contrast, peritoneal macrophages from TLR2–/– mice produced very little IL-6 in response to HSV-1 challenge (Fig. 1C). In control cultures, TLR2–/– macrophages also failed to respond to zymosan (TLR2 ligand), but did secrete IL-6 when challenged with LPS, a TLR4 ligand.
TLR2 is thought to signal as a heterodimer in combination with either TLR1 or TLR6 (26, 27, 31–34). We compared the response of TLR6 knockout, TLR2 knockout, and wild-type PECs to HSV-1 (Fig. 1D). Both wild-type and TLR6–/– peritoneal macrophages secreted IL-6 when challenged with HSV-1, indicating the TLR6 is not required for HSV-1 induced signaling.
In addition to IL-6, challenge with HSV-1 induced MCP-1 secretion from peritoneal macrophages from wild-type and TLR4–/–, but not TLR2–/– mice (data not shown). The induction of cytokines by HSV-1 was dose-dependent at multiplicites of infection (mois) up to 100 (Fig. 1 B and C). Both live and UV-irradiated HSV-1 induced IL-6 secretion from murine macrophages, indicating that viral replication was not required for cytokine induction (data not shown).
TLR2-Deficient Mice Are Resistant to Lethal HSV-1 Challenge. We examined the role of TLR2 in vivo by using a murine model of lethal HSV-1 encephalitis. Mice were infected with HSV-1 KOS strain i.p. and were monitored for encephalitis or death. Moribund animals exhibiting total paralysis and/or seizures were killed. Whereas wild-type animals rapidly succumbed to infection, TLR2–/– mice had delayed death and an overall reduction in mortality. Five of eight TLR2–/– mice survived a challenge with 1–2 × 109 pfu of KOS virus, but only two of eight wild-type mice survived (Fig. 2, P = 0.03, Wilcoxon test). Symptoms were also reduced in TLR2–/– mice compared with wild-type or TLR4–/– mice. In a separate study (in which mice were killed at day 4 for brain cytokine levels), six of eight wild-type and six of eight TLR4–/– mice showed partial or total paralysis and/or seizures, whereas only three of eight TLR2–/– mice were symptomatic. The symptoms in the TLR2–/– mice were milder than that in the wild-type and TLR4–/– mice, because none of the TLR2–/– mice had either total paralysis or seizures.
Fig. 2.
Adult TLR2 knockout mice are resistant to lethal HSV-1 challenge. Groups of adult wild-type or TLR2–/– mice were challenged with 109 pfu of HSV-1 KOS virus i.p. Mice were observed for 1 week after challenge. Symptoms seen in mice included lethargy, ruffled fur, hindlimb paralysis, and seizures. All surviving mice were free of symptoms. (Eight mice per group, P ≤ 0.03, wild-type vs. TLR2–/– at day 4.)
Neonatal HSV-1 Mortality Is Greater in Wild-Type than in TLR2–/– Mice. Adult mice, like adult humans, are less likely to succumb to HSV-1 challenge. On the other hand, neonates are highly susceptible to lethal HSV-1. Therefore, we examined the response to 4-day-old mice to HSV-1 challenge. Neonates were injected with 104 pfu of HSV-1 i.p. and monitored for >14 days (Fig. 3). All neonates were well and developed normally for the first 4–5 days postinfection. Wild-type and TLR4–/– neonates succumbed to HSV-1 challenge on day 6 postinfection (>90% lethality). In contrast, >60% of the TLR2–/– neonates survived HSV-1 challenge. Remarkably, at least 50% of the TLR2–/– mice were symptom-free for the entire 2-week course of study and another 12% of the TLR2–/– neonates exhibited only mild, transient symptoms. TLR4–/– mice were indistinguishable from wild-type mice, with rapid onset of paralysis and death on day 6 postinfection (Fig. 3).
Fig. 3.
Neonatal TLR2 knockout mice are resistant to lethal HSV-1 challenge. Groups of 4-day-old mice were challenged with 104 pfu of HSV-1 KOS virus i.p. Mice were observed for 3 weeks after challenge. Symptoms seen in neonatal mice included spasmodic limb movement, hindlimb and total paralysis, and bloating. (Fourteen to 17 mice per group, P < 0.001, wild-type vs. TLR2–/– at day 6.)
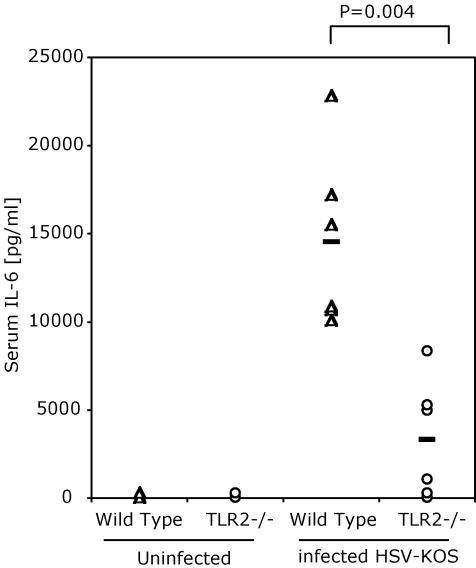
Wild-Type but Not TLR2 Knockout Mice Have Elevated Serum IL-6 Levels After HSV-1 Challenge. We next examined the effect of TLR2 deficiency on cytokine production in vivo in HSV-1-infected animals. Wild-type mice and TLR2–/– mice were challenged with HSV-1 (2 × 109 pfu of HSV-KOS i.p.). Elevated levels of IL-6 were found in the serum 1 day postinfection in wild-type mice, whereas TLR2–/– mice had very little IL-6 in their serum (Fig. 4, P = 0.004). Similarly, MCP-1 was detected in serum day 1 postinfection (data not shown). Serum cytokine levels returned to baseline by day 2 postinfection. These studies suggested that the acute production of inflammatory cytokines on HSV-1 infection depended on TLR2 expression in the infected host.
Fig. 4.
Wild-type mice produce higher levels of serum IL-6 cytokine than do TLR2 knockout mice in response to infection with HSV. Mice were infected with 109 pfu of HSV-KOS i.p. or uninfected. Blood was collected 24 h after infection, and serum IL-6 levels were determined by ELISA. (P = 0.004, wild-type vs. TLR2–/– at 24 h.)
MCP-1 Levels Are Elevated in the Brains of Wild-Type but Not TLR-2 Knockout Mice. In addition to cytokines, which are often essential to immune responses, interaction with TLRs also induces the production of chemokines such as MCP-1 from host cells. Wild-type and TLR4–/– mice demonstrated high MCP-1 levels in their brains at day 1 after challenge. In all mice, MCP-1 levels returned to baseline (<10 pg/ml) on days 2 and 3 after challenge. When symptoms of encephalitis appeared on day 4, a second wave of MCP-1 production in the brains was detected in wild-type and TLR4 knockout mice (Fig. 5A). Brain MCP-1 levels in TLR4–/– mice were indistinguishable from levels in wild-type mice (P = 1.00) In contrast, significantly lower levels of MCP-1 were detected in the brains of TLR2–/– mice (Fig. 5A, P = 0.02). At the same time as the increased MCP-1 production, significant hemorrhage was apparent in wild-type brain tissue, whereas the TLR2–/– brains had a normal gross appearance. HSV-1 virus was detected in the brains of TLR2–/–, as well as the wild-type and TLR4–/– mice with slightly higher levels of virus in the TLR2–/– mice, but the difference between groups did not reach statistical significance (Fig. 5B).
Fig. 5.
Wild-type mice produce higher levels of brain MCP-1 than do TLR2 knockout mice in response to infection with HSV. (A) Analysis of MCP-1 levels in the brains of HSV-1 KOS-infected mice. Wild-type, TLR2–/–, and TLR4–/– mice were infected with HSV-1 KOS i.p. (109 pfu of HSV i.p.). This dose was 50% lethal on day 4 in wild-type but not in TLR2–/– mice. Wild-type mice, but not TLR2–/– mice, were found to be moribund with brain hemorrhage at day 4. MCP-1 levels were measured in brain homogenates by ELISA. Levels in individual brains are shown. Geometric mean levels of MCP-1 are indicated by the bar. (B) Virus levels in brains on infected mice. Pfu of HSV-1 KOS in the brains of infected mice on day 4 after challenge with virus i.p. are shown. Levels in individual brains are shown. Geometric mean pfu is indicated by the bar.
Wild-Type and TLR4 Knockout but Not TLR2 Knockout Mice Have Inflammatory Lesions in the Brain After HSV-1 Challenge. Microscopic examination of the brains revealed mononuclear cell infiltrates and prominent perivascular cuffing in the medulla of wild-type and TLR4 knockout mice. In contrast, TLR2 knockout animals had normal appearing brain tissue (Fig. 6). The lack of apparent pathology in the TLR2 knockout brains was particularly remarkable, because virus titers in the brains of TLR2–/– mice did not differ significantly from those in the brains of TLR4–/– and wild-type mice but showed a trend toward being higher in TLR2–/– mice (Fig. 5B). Thus, the host response, rather than the virus itself, may be responsible for the pathologic changes in the brains.
Fig. 6.
TLR2 knockout mice have less brain inflammation than do wild-type mice after HSV-1 challenge. Histopathology in the brains of HSV-1 KOS infected mice. Hematoxylin/eosin-stained section of cerebellum of mice 4 days postinfection with HSV i.p. (Top) Meninges with mononuclear cell infiltrates in wild-type and TLR4 knockouts. TLR2 knockout meninges are normal. (Middle) Cerebellum with mononuclear cell infiltrates and activated glial cells in wild-type and TLR4 knockouts. TLR2 knockout cerebellar tissue is normal. (Bottom) Blood vessels with accumulating mononuclear cells along the endothelial surface as well as perivascular cuffing in wild-type and TLR4 knockout brain. Blood vessels in TLR2 knockout brain are normal, with no evidence of inflammatory mononuclear cell accumulation.
Discussion
We examined the role of TLRs in the response to HSV-1 in humans and mice. We demonstrate that TLR2 mediates the inflammatory cytokine response to HSV-1. Loss-of-function studies with macrophages demonstrate an essential role for TLR2 in the production of inflammatory cytokines after HSV-1 challenge, whereas gain-of-function studies demonstrated that expression of TLR2 in HEK293 cells was sufficient to confer responsiveness to HSV-1. Consistent with the role of TLR2 in the HSV-1-induced inflammatory response, infection with HSV-1 induced a blunted cytokine response in TLR2–/– mice compared with wild-type or TLR4–/– mice, both in the serum and within the brain. This finding of attenuated cytokine response was paralleled by a reduction in symptoms of encephalitis in TLR2–/– compared with wild-type and TLR4–/– mice. HSV-1-infected TLR2–/– neonates developed mild symptoms and mortality was <40% over a 21-day period. In contrast, wild-type and TLR4–/– neonates rapidly succumbed to HSV-1 infection with >90% mortality by day 6. Thus, our experiments suggest that the TLR2-mediated cytokine response to HSV-1 is detrimental to the host, particularly within the brain.
A large literature documents the greater susceptibility to HSV-1 infection of neonatal, as opposed to adult mice [a phenomenon similar to that observed in humans (35)]. Zawastsky et al. (36) showed that neonatal mice are at least 103 times more susceptible to fatal HSV-1 infection than adults. Resistance to HSV-1 infection develops 18–20 days after birth in mice (36). The reasons for this large discrepancy between neonatal animals and adults has never been clarified. Our studies suggest that neonate susceptibility, like adult susceptibility, is TLR2-dependent. The poor outcomes associated with infection in neonates have traditionally been thought to be due to some failure of the immature immune system to contain the virus. Our findings suggest a different interpretation may be appropriate. Rather than being less responsive than adults, the neonatal response to certain antigens, particularly those in which the innate immune response is through TLR2, may be even stronger than those seen in adults (37, 38). The data presented here suggest that the same phenomena occur in the case of viral infection and may explain the dramatically different diseases seen in neonates infected with HSV-1.
Both TLR9 and TLR3 have been suggested to function as innate immune receptors for viruses (21, 22). Lund et al. (22) noted that HSV-2-induced production of IFN-α in mouse dendritic cells through a TLR9-mediated pathway. Similarly, Krug et al. (30) found that the IFN response to HSV-1 depended on TLR9 and MyD88. Interestingly, TLR9 and MyD88 deficiency did not significantly affect viral replication when HSV-1 was administered either in the footpad or the cornea. It is notable that in our studies, HSV-1 replicated in the brains of TLR2-deficient mice, although these mice were protected from the lethal effects of this infection.
We have been unable to detect either TLR9- or TLR3-dependent (Fig. 1) responses to HSV-1 as measured by activation of NF-κB in transfected HEK293 cells. Our studies do not, however, rule out a role for TLR3 or TLR9 in the response to HSV-1. It is possible that HEK293 cells lack an essential accessory protein for TLR3- or TLR9-dependent responses to HSV-1. Alternatively, HSV-1 interaction with TLR3 or TLR9 may induce an IFN-response factor-dependent but not an NF-κB-dependent response. Nevertheless, in our studies, TLR2-deficient peritoneal macrophages failed to respond to HSV-1 challenge suggesting that, in this cell type, TLR2 is essential for HSV-1-induced cytokine secretion.
The role of the TLR9 and/or TLR3 pathways in the sensitivity of mice to HSV-1-induced mortality are not clear. Experiments with TLR9 knockout mice do not indicate that TLR9 plays a role in HSV-1 pathogenesis (30). In contrast, the knockout studies shown here clearly demonstrate a major, nonredundant role for TLR2 in susceptibility to HSV-1-induced lethal encephalitis.
Boivin et al. (39) have reported the expression of TLR2-positive cells in the brains of mice with HSV-2 encephalitis. In the brain, TLR2 is inducibly expressed on resident microglia and infiltrating monocytes (39–42). Interestingly, the levels of TLR2 expression in the brain were reported to increase in response to peripheral challenge with either TLR2 or TLR4 bacterial ligands. Oliveira et al. (43) have recently reported the presence of TLR2 on human Schwann cells. A prominent role for TLR4 in neuronal injury has been established by Vartanian and colleagues (44). By using a hypoxia-ischemia model, these investigators found that activation of TLR4 (expressed on microglia cells) exacerbated neurodegeneration and, further, mice expressing a mutant TLR4 were protected from neuronal loss (44).
The TLR2-expressing microglial cells, like the blood monocytes, are of myeloid origin (42). These microglial cells are the source of MCP-1 expressed in the inflamed brain (41). Expression of MCP-1 in the brain has been associated with brain damage. By using a model of ischemic brain injury, Chen et al. (45) noted that MCP-1 transgenic mice developed significantly larger areas of brain infarction than wild-type controls after cerebral artery occlusion. These authors also noted an increase in the infiltration of inflammatory cells into regions of brain injury in MCP-1-overexpressing mice (45).
TLR activation is a double-edged sword and may either lessen or worsen disease, depending on the pathogen and the location of the infection. Our experiments suggest that in the case of HSV-1, the induction of a TLR2-mediated cytokine response in the brain contributes to the death of the animal. In contrast, TLR4-dependent signals promote the rapid clearance of respiratory syncytial virus from the lungs of infected animals (17, 18) and the TLR2-dependent response to Staphylococcus aureus is protective (16). Overall, the surprising finding that TLR2-deficient mice are less likely to die of HSV-1 challenge than wild-type mice suggested the possibility that neonatal animals, rather than being less able to contain the virus, might die because of their exuberant cytokine responses to viral antigens. These data suggest that drugs or other therapies that dampen the innate immune response may decrease morbidity and mortality caused by HSV-1.
Acknowledgments
This work was supported by National Institutes of Health Grants RO1 AI51415 (to E.A.K.-J.), PO1 NS35138 and RO1 AI20530 (to D.M.K.), and RO1 GM63244 and RO1 AI39576 (to R.W.F)
Abbreviations: HSV-1; herpes simplex virus 1; TLR, Toll-like receptor; pfu, plaque-forming unit; moi, multiplicity of infection; HEK, human embryonic kidney; MCP-1, monocyte chemoattractant protein 1.
References
- 1.Corey, L. (2001) in Harrison's Principles of Internal Medicine, eds. Braunwald, E., Fauci, A. S., Kasper, D. L., Hauser, S. L., Longo, D. L. & Jameson, J. L. (McGraw–Hill, New York), pp. 1100–1106.
- 2.Whitley, R. J. (2001) in Fields' Virology, eds. Knipe, D. M., Howley, P. M., Griffin, D. E., Lamb, R. A., Martin, M. A., Roizman, B. & Straus, S. E. (Lippincott, Williams & Wilkins, Philadelphia), 4th. Ed., pp. 2461–2509.
- 3.Whitley, R., Arvin, A., Prober, C., Corey, L., Burchett, S., Plotkin, S., Starr, S., Jacobs, R., Powell, D., Nahmias, A., et al. (1991) N. Engl. J. Med. 324, 450–454. [DOI] [PubMed] [Google Scholar]
- 4.Takeda, K., Kaisho, T. & Akira, S. (2003) Annu. Rev. Immunol. 21, 335–376. [DOI] [PubMed] [Google Scholar]
- 5.Imler, J. L. & Zheng, L. (July 15, 2003) J. Leukocyte Biol., 10.1189/jlb.0403160.
- 6.Rock, F. L., Hardiman, G., Timans, J. C., Kastelein, R. A. & Bazan, J. F. (1998) Proc. Natl. Acad. Sci. USA 95, 588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhary, P. M., Ferguson, C., Nguyen, V., Nguyen, O., Massa, H. F., Eby, M., Jasmin, A., Trask, B. J., Hood, L. & Nelson, P. S. (1998) Blood 91, 4020–4027. [PubMed] [Google Scholar]
- 8.Medzhitov, R., Preston-Hurlburt, P. & Janeway, C. A., Jr. (1997) Nature 388, 394–397. [DOI] [PubMed] [Google Scholar]
- 9.Kaisho, T. & Akira, S. (2000) Crit. Rev. Immunol. 20, 393–405. [PubMed] [Google Scholar]
- 10.Imler, J. L. & Hoffmann, J. A. (2001) Trends Cell Biol. 11, 304–311. [DOI] [PubMed] [Google Scholar]
- 11.Bowie, A. & O'Neill, L. A. (2000) J. Leukocyte Biol. 67, 508–514. [DOI] [PubMed] [Google Scholar]
- 12.Akira, S., Takeda, K. & Kaisho, T. (2001) Nat. Immunol. 2, 675–680. [DOI] [PubMed] [Google Scholar]
- 13.O'Neill, L. (2000) Biochem. Soc. Trans. 28, 557–563. [DOI] [PubMed] [Google Scholar]
- 14.Poltorak, A., He, X., Smirnova, I., Liu, M. Y., Huffel, C. V., Du, X., Birdwell, D., Alejos, E., Silva, M., Galanos, C., et al. (1998) Science 282, 2085–2088. [DOI] [PubMed] [Google Scholar]
- 15.Hoshino, K., Takeuchi, O., Kawai, T., Sanjo, H., Ogawa, T., Takeda, Y., Takeda, K. & Akira, S. (1999) J. Immunol. 162, 3749–3752. [PubMed] [Google Scholar]
- 16.Takeuchi, O., Hoshino, K. & Akira, S. (2000) J. Immunol. 165, 5392–5396. [DOI] [PubMed] [Google Scholar]
- 17.Kurt-Jones, E. A., Popova, L., Kwinn, L., Haynes, L. M., Jones, L. P., Tripp, R. A., Walsh, E. E., Freeman, M. W., Golenbock, D. T., Anderson, L. J. & Finberg, R. W. (2000) Nat. Immunol. 1, 398–401. [DOI] [PubMed] [Google Scholar]
- 18.Haynes, L. M., Moore, D. D., Kurt-Jones, E. A., Finberg, R. W., Anderson, L. J. & Tripp, R. A. (2001) J. Virol. 75, 10730–10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Compton, T., Kurt-Jones, E. A., Boehme, K. W., Belko, J., Latz, E., Golenbock, D. T. & Finberg, R. W. (2003) J. Virol. 77, 4588–4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bieback, K., Lien, E., Klagge, I. M., Avota, E., Schneider-Schaulies, J., Duprex, W. P., Wagner, H., Kirschning, C. J., Ter Meulen, V. & Schneider-Schaulies, S. (2002) J. Virol. 76, 8729–8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. (2001) Nature 413, 732–738. [DOI] [PubMed] [Google Scholar]
- 22.Lund, J., Sato, A., Akira, S., Medzhitov, R. & Iwasaki, A. (2003) J. Exp. Med. 198, 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurt-Jones, E. A., Mandell, L., Whitney, C., Padgett, A., Gosselin, K., Newburger, P. E. & Finberg, R. W. (2002) Blood 100, 1860–1868. [PubMed] [Google Scholar]
- 24.Latz, E., Visintin, A., Lien, E., Fitzgerald, K. A., Monks, B. G., Kurt-Jones, E. A., Golenbock, D. T. & Espevik, T. (2002) J. Biol. Chem. 277, 47834–47843. [DOI] [PubMed] [Google Scholar]
- 25.Lien, E., Sellati, T. J., Yoshimura, A., Flo, T. H., Rawadi, G., Finberg, R. W., Carroll, J. D., Espevik, T., Ingalls, R. R., Radolf, J. D. & Golenbock, D. T. (1999) J. Biol. Chem. 274, 33419–33425. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi, O., Kawai, T., Muhlradt, P. F., Morr, M., Radolf, J. D., Zychlinsky, A., Takeda, K. & Akira, S. (2001) Int. Immunol. 13, 933–940. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi, O., Sato, S., Horiuchi, T., Hoshino, K., Takeda, K., Dong, Z., Modlin, R. L. & Akira, S. (2002) J. Immunol. 169, 10–14. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi, O., Hoshino, K., Kawai, T., Sanjo, H., Takada, H., Ogawa, T., Takeda, K. & Akira, S. (1999) Immunity 11, 443–451. [DOI] [PubMed] [Google Scholar]
- 29.Brockman, M. A. & Knipe, D. M. (2002) J. Virol. 76, 3678–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krug, A., Luker, G. D., Barchet, W., Leib, D. A., Akira, S. & Colonna, M. (October 16, 2003) Blood, 10.1182/blood-2003-08.2674.
- 31.Hajjar, A. M., O'Mahony, D. S., Ozinsky, A., Underhill, D. M., Aderem, A., Klebanoff, S. J. & Wilson, C. B. (2001) J. Immunol. 166, 15–19. [DOI] [PubMed] [Google Scholar]
- 32.Ozinsky, A., Underhill, D. M., Fontenot, J. D., Hajjar, A. M., Smith, K. D., Wilson, C. B., Schroeder, L. & Aderem, A. (2000) Proc. Natl. Acad. Sci. USA 97, 13766–13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyllie, D. H., Kiss-Toth, E., Visintin, A., Smith, S. C., Boussouf, S., Segal, D. M., Duff, G. W. & Dower, S. K. (2000) J. Immunol. 165, 7125–7132. [DOI] [PubMed] [Google Scholar]
- 34.Sandor, F., Latz, E., Re, F., Mandell, L., Repik, G., Golenbock, D. T., Espevik, T., Kurt-Jones, E. A. & Finberg, R. W. (2003) J. Cell Biol. 162, 1099–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Overall, J. C., Jr. (1998) in Textbook of Pediatric Infectious Diseases, eds. Feigin, R. D. & Cherry, J. D. (Saunders, Philadelphia), Vol. 1, pp. 856–891. [Google Scholar]
- 36.Zawatzky, R., Engler, H. & Kirchner, H. (1982) J. Gen. Virol. 60, 25–29. [DOI] [PubMed] [Google Scholar]
- 37.Karlsson, H., Hessle, C. & Rudin, A. (2002) Infect. Immun. 70, 6688–6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renshaw, M., Rockwell, J., Engleman, C., Gewirtz, A., Katz, J. & Sambhara, S. (2002) J. Immunol. 169, 4697–4701. [DOI] [PubMed] [Google Scholar]
- 39.Boivin, G., Coulombe, Z. & Rivest, S. (2002) Eur. J. Neurosci. 16, 29–43. [DOI] [PubMed] [Google Scholar]
- 40.Rivest, S. (2003) Brain Behav. Immun. 17, 13–19. [DOI] [PubMed] [Google Scholar]
- 41.Laflamme, N., Echchannaoui, H., Landmann, R. & Rivest, S. (2003) Eur. J. Immunol. 33, 1127–1138. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen, M. D., Julien, J. P. & Rivest, S. (2002) Nat. Rev. Neurosci. 3, 216–227. [DOI] [PubMed] [Google Scholar]
- 43.Oliveira, R. B., Ochoa, M. T., Sieling, P. A., Rea, T. H., Rambukkana, A., Sarno, E. N. & Modlin, R. L. (2003) Infect. Immun. 71, 1427–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lehnardt, S., Massillon, L., Follett, P., Jensen, F. E., Ratan, R., Rosenberg, P. A., Volpe, J. J. & Vartanian, T. (2003) Proc. Natl. Acad. Sci. USA 100, 8514–8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen, Y., Hallenbeck, J. M., Ruetzler, C., Bol, D., Thomas, K., Berman, N. E. & Vogel, S. N. (2003) J. Cereb. Blood Flow Metab. 23, 748–755. [DOI] [PubMed] [Google Scholar]