Abstract
Most flavonoids exist as sugar conjugates. Naturally occurring flavonoid sugar conjugates include glucose, galactose, glucuronide, rhamnose, xylose, and arabinose. These flavonoid glycosides have diverse physiological activities, depending on the type of sugar attached. To synthesize an unnatural flavonoid glycoside, Actinobacillus actinomycetemcomitans gene tll (encoding dTDP-6-deoxy-l-lyxo-4-hexulose reductase, which converts the endogenous nucleotide sugar dTDP-4-dehydro-6-deoxy-l-mannose to dTDP-6-deoxytalose) was introduced into Escherichia coli. In addition, nucleotide-sugar dependent glycosyltransferases (UGTs) were screened to find a UGT that could use dTDP-6-deoxytalose. Supplementation of this engineered strain of E. coli with quercetin resulted in the production of quercetin-3-O-(6-deoxytalose). To increase the production of quercetin 3-O-(6-deoxytalose) by increasing the supplement of dTDP-6-deoxytalose in E. coli, we engineered nucleotide biosynthetic genes of E. coli, such as galU (UTP-glucose 1-phosphate uridyltransferase), rffA (dTDP-4-oxo-6-deoxy-d-glucose transaminase), and/or rfbD (dTDP-4-dehydrorahmnose reductase). The engineered E. coli strain produced approximately 98 mg of quercetin 3-O-(6-deoxytalose)/liter, which is 7-fold more than that produced by the wild-type strain, and the by-products, quercetin 3-O-glucose and quercetin 3-O-rhamnose, were also significantly reduced.
INTRODUCTION
Many secondary metabolites found in nature exist as glycones (26). Subtle alteration of the sugars in natural products could change their molecular and cellular specificity, leading to changes in pharmacological properties (16). Glycones are biologically synthesized using nucleotide sugars as a sugar donor (25). Because the number of commercially available nucleotide sugars is limited, the creation of diverse glycosylated secondary metabolites is challenging. However, the unusual nucleotide sugars found in some bacteria could be a valuable resource for extending the diversity of glycones.
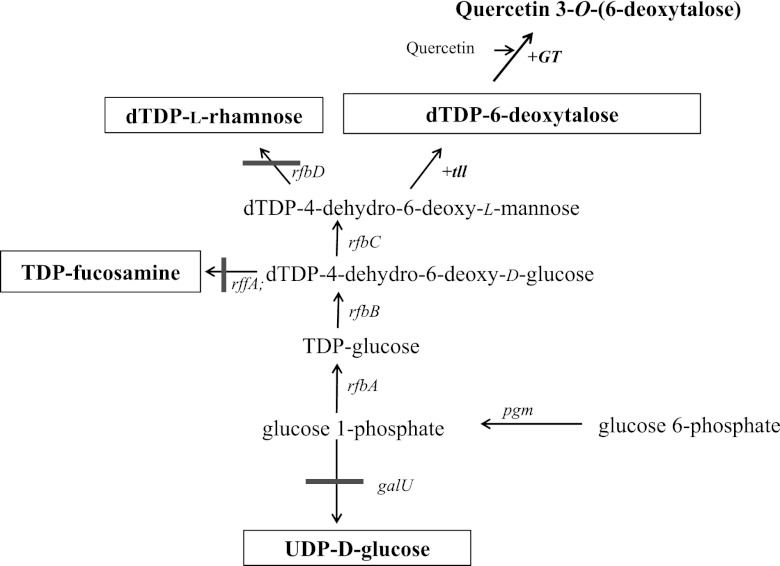
The outer membrane of Gram-negative bacteria is composed of lipopolysaccharide(s) (LPS). LPS acts as an endotoxin and triggers strong immune responses in animals. O antigen is a repetitive glycan polymer that is part of LPS (19). The building blocks of O antigen in Escherichia coli are UDP-d-galacto-1,4-furanose, dTDP-α-l-rhamnose, and UDP-α-N-acetyl-d-glucosamine. The genes and biosynthetic pathway of O-antigen are well defined and widespread in Gram-negative bacteria. dTDP-sugars, derived from glucose 1-phosphate, are involved in this pathway. In E. coli, biosynthesis of dTDP-l-rhamnose from glucose 1-phosphate involves in four genes; rfbA (dTDP-glucose pyrophosphorylase), rfbB (dTDP-4-glucose 4,6-dehydratase), rfbC (dTDP-4-dehydrorhamnose 3,5-epimerase), and rfbD (dTDP-4-dehydrorhamnose reductase). However, in some bacteria, other sugars are present. For example, in certain serotypes of Actinobacillus actinomycetemcomitans, O antigen contains d-fucose and 6-deoxytalose (2, 23). In A. actinomycetemcomitans, tll encodes dTDP-6-deoxy-l-lyxo-4-hexulose reductase for the biosynthesis of dTDP-6-deoxytalose and fcd encodes dTDP-6-deoxy-d-xylo-4-hexulose reductase for the biosynthesis of dTDP-d-fucose (15). Neither gene is found in E. coli. Although A. actinomycetemcomitans can synthesize unique types of nucleotide sugars, common intermediates are found in the biosynthesis of dTDP-6-deoxytalose and dTDP-d-fucose (Fig. 1). Therefore, engineering this nucleotide biosynthesis pathway in E. coli could lead to production of dTDP-6-deoxytalose or dTDP-d-fucose, which could serve as substrates for the production of glycones. In particular, dTDP-6-deoxy-l-lyxo-4-hexulose reductase encoded by tll uses dTDP-4-dehydro-6-deoxy-l-mannose, which is also a common substrate for dTDP-4-dehydrorhamnose reductase (Fig. 1). Therefore, the expression of tll into E. coli would produce dTDP-6-deoxytalose.
Fig 1.
Schematic diagram of the nucleotide pathway and the production of quercetin 3-O-(6-deoxytalose) in E. coli. galU, UTP-glucose 1-phosphate uridyltransferase; rffA, dTDP-4-oxo-6-deoxy-d-glucose transaminase; rfbA, dTDP-glucose pyrophosphorylase; rfbB, dTDP-glucose 4,6-dehydratase; rfbC, (dTDP-4-dehydrorhamnose 3,5-epimerase; rfbD, dTDP-4-dehydrorahmnose reductase; tll, dTDP-6-deoxy-l-lyxo-4-hexulose reductase; GT, glycosyltransferase.
The class of secondary metabolites known as flavonoids is synthesized mainly in plants, and most flavonoids exist as glycones. More than 350 glycones of quercetin have been found in various plants (4). However, arabinose, galactose, glucose, glucuronic acid, rhamnose, and xylose are the major sugars that are attached to flavonoids, because nucleotide sugar conjugates of these sugars are commonly found in plants (22). Attachment of sugars to flavonoids is catalyzed by UDP-dependent glycosyltransferase (UGT) (3). UGTs have specificity for sugar donors (nucleotide sugars) and for sugar acceptors. The PSPG (plant secondary product GT) motif at the C terminus of UGT is known to be critical for the recognition of the sugar donor (18). However, the basis sugar-donor selectivity is not yet known. Therefore, it is not easy to rationally design the attachment of a novel sugar to a flavonoid.
Glycosylation of flavonoids using E. coli harboring UGT results in the formation of flavonoid glucose because most UGTs have specificity for dUDP-glucose, and E. coli has endogenous dUDP-glucose (9, 13). However, a recent study by our group showed that the nucleotide sugar specificity of UGT could be changed when the preferred nucleotide sugar is not available (7). This suggests that unnatural flavonoid glycosides could be synthesized through modification of the nucleotide sugar metabolic pathway in the host and by rational selection of the appropriate UGT. Nucleotide sugars serve as the sugar donor for UGT. The nucleotide sugar biosynthesis pathway could be engineered to synthesize a nucleotide that is not normally present in E. coli. We wanted to synthesize dTDP-6-deoxy-l-talose. Most steps of dTDP-6-deoxy-l-talose biosynthesis are present in the dTDP-l-rhamnose biosynthetic pathway in E. coli. dTDP-4-dehydro-6-deoxy-l-mannose is converted to dTDP-l-rhamnose by rfbD at the final step of dTDP-l-rhamnose biosynthesis; however, it is converted to dTDP-6-deoxytalose by tll (Fig. 1).
The biological activities of flavonoid glycosides are more often modulated by the sugar than by the flavonoid (5, 24). Therefore, we want to synthesize a novel flavonoid glycoside, which might have a novel biological activity. Here, we show that by introducing a new gene for the biosynthesis of a nucleotide sugar, and screening for a UGT that could utilize the novel nucleotide sugar, an unnatural flavonoid glycoside, quecetin 3-O-(6-deoxytalose) can be synthesized. In addition, through engineering the nucleotide biosynthetic pathway of E. coli, the productivity of an unnatural flavonoid glycoside can be increased.
MATERIALS AND METHODS
DNA manipulation.
The tll gene was synthesized based on the sequence published by Nakano et al. (15). An EcoRI site was added to the 5′ end of the gene, and a NotI site was added to the 3′ end of the gene to facilitate cloning. To optimize the expression of tll in E. coli, codon optimization based on E. coli codon usage was performed prior to synthesis. The synthesized tll gene was verified by DNA sequencing. To express tll in E. coli, EcoRI/NotI-digested tll DNA was subcloned into the corresponding EcoRI/NotI site of the E. coli expression vectors pACYCDuet, pCDFDuet, and pETDuet. The resulting constructs were named pA-tll, pC-tll, and pE-tll, respectively (Table 1).
Table 1.
Plasmids and strains used in this study
| Plasmid or strain | Relevant properties or genetic markera | Source or reference |
|---|---|---|
| Plasmids | ||
| pACYCDuet | P15A ori; Cmr | Novagen |
| pCDFDuet | CloDE13 ori; Sper | Novagen |
| pETDuet | f1 ori; Ampr | Novagen |
| pGEX 5X-1 | pRR322 ori; Ampr | GE Healthcare Life Science |
| pG-D1 | pGEX 5X-2 + AtUGT78D1 from A. thaliana | This study |
| pA-D1 | pACYCDuet + AtUGT78D1 from A. thaliana | |
| pA-tll | pACYCDuet + tll from A. actinomycetemcomitans | This study |
| pC-tll | pCDFDuet + tll from A. actinomycetemcomitans | This study |
| pE-tll | pETDuet + tll from A. actinomycetemcomitans | This study |
| pA-tll-D1 | pACYCDuet + tll from A. actinomycetemcomitans + AtUGT78D1 from A. thaliana | This study |
| pC-tll-D1 | pCDFDuet + tll from A. actinomycetemcomitans + AtUGT78D1 from A. thaliana | This study |
| pE-tll-D1 | pETDuet + tll from A. actinomycetemcomitans + AtUGT78D1 from A. thaliana | This study |
| E. coli strains | ||
| BL21 | F−ompT hsdSB(rB− mB−) gal dcm lon (DE3) | Novagen |
| BrfbD | BL21(DE3) ΔrfbD-FRT-Kanr | This study |
| BrffA | BL21(DE3) ΔrffA-FRT-Kanr | This study |
| BgalU-rfbD | BL21(DE3) ΔgalU::FRT ΔrfbD-FRT-Kanr | This study |
| BgalU-rfbD-rffA | BL21(DE3) ΔgalU::FRT ΔrfbD::FRT ΔrffA-FRT-Kanr | This study |
| B11 | BL21(DE3) ΔgalU::FRT ΔrfbD::FRT ΔrffA-FRT-Kanr harboring pA-tll-D1 | This study |
| B12 | BL21(DE3) ΔgalU::FRT ΔrfbD::FRT ΔrffA-FRT-Kanr harboring pE-tll-D1 | This study |
| B13 | BL21(DE3) ΔgalU::FRT ΔrfbD::FRT ΔrffA-FRT-Kanr harboring pC-tll and pA-D1 | This study |
| B14 | BL21(DE3) ΔgalU::FRT ΔrfbD::FRT ΔrffA::FRT-Kanr-FRT harboring pE-tll and pA-D1 | This study |
Cmr, chloramphenicol resistance; Ampr, ampicillin resistance; Sper, spectinomycin resistance; Kanr, kanamycin resistance.
UGT genes from various sources were cloned into pGEX vector as described previously (8–10) and transformed into E. coli BL21(DE3), which was already transformed with pA-tll. As control, each UGT gene was transformed into E. coli BL21(DE3) containing empty pACYCDuet. Overnight cultures of each transformant were inoculated in 2 ml of Luria-Bertani (LB) medium containing 50 μg of ampicillin and chloramphenicol/ml. Induction, biotransformation, and analysis of reaction product were performed as described below.
AtUGT78D1 from Arabidopsis thaliana was cloned by reverse transcription-PCR. Briefly, total RNA was isolated from 2-week-old A. thaliana whole plants. cDNA was synthesized as described in Kim et al. (6). PCR was carried out using Hotstart DNA polymerase (Qiagen, Valencia, CA). Two primers—5′-ATGAATTCATGACCAAATTCTCCGA-3′ and 5′-CATGCGGCCGCTAAACTTTCACAATTTCGT-3′—were designed based on the published sequence (At1g30530). For the expression in E. coli, AtUGT78D1 was subcloned into pGEX 5X-1. The resulting construct was named pG-D1. The AtUGT78D1 gene was subcloned into the second cloning site (NdeI/EcoRV) of pA-tll, pC-tll, and pE-tll, and the resulting constructs were named pA-tll-D1, pC-tll-D1, and pE-tll-D1, respectively (Table 1).
Deletion of the rfbD (dTDP-4-dehydrorhamnose reductase), galU (UTP-glucose 1-phosphate uridyltransferase), and rffA (dTDP-4-oxo-6-deoxy-d-glucose transaminase) genes from E. coli BL21(DE3) was done using the Quick & Easy conditional knockout kit (Gene Bridges, Heidelberg, Germany). The primers used were listed in Table 2. Electroporation was performed with the Bio-Rad MicroPulser electroporation apparatus (Bio-Rad, Hercules, CA). The ΔgalU ΔrfbD double mutant (strain BgalU-rfbD in Table 1) and the ΔgalU ΔrfbD ΔrffA triple mutant (strain BgalU-rfbD-rffA in Table 1) were created by deleting the selection marker using plasmid 780FLPe.
Table 2.
Primers used for gene deletions in this study
| Gene | Primer | Sequence (5′–3′) |
|---|---|---|
| rfbD | rfbD-forward | TGGCATCATGAGCGAGATGCAAAAATTTGTTAAATTGCCGTAGTCGTAAAAATTAACCCTCACTAAAGGGCG |
| rfbD-reverse | GGTGCTTATCAGTCGTGGATTGAACAGAACTATGAGGGCCGCCAGTAATGTAATACGACTCACTATAGGGCTC | |
| rffA | rffA-forward | TCCACAAGGACGCTTTTGCCAACGACATATCAGGAAAAGTAGTTCAACAATGGACAGCAAGCGAACCGGAATTGC |
| rffA-reverse | TGGTGCGAATGTAGAAAGCACCGCGTACTGGTTATACAGGTGATCACATGTCAGAAGAACTCGTCAAGAAGGCG | |
| glaU | glaU-forward | ATGGCTGCCATTAATACGAAAGTCAAAAAAGCCGTTATCCCCGTTGCGGGAATTAACCCTCACTAAAGGGCG |
| galU-reverse | AGTCATGGCTCTTCCCTTTCATATGATAGGCTTCAACCGTTTCTTTTTCGTAATACGACTCACTATAGGGCTC |
Molecular modeling of AtUGT78D1.
Homology modeling software PRIME incorporated into the Schrodinger modeling software suite was used to generate a structure of the AtUGT78D1 that was homologous to the crystallographic structure of the flavonoid 3-O-glucosyltransferase (PDB 2C1X) (17, 20). The optimal model was selected based on bond angle stereochemistry using PROCHECK (11). UDP (uridine-5′-diphosphate)-glucose was taken from the template (2C1X) and modeled into the corresponding binding site in the homology structure of AtUGT78D1, and then the glucose moiety was modified to 6-deoxy-l-talose or l-rhamnose, respectively. After refinement of the loop structures, the model was subjected to energy minimization and molecular-dynamics (MD) simulations in order to obtain a stable, low-energy conformation. Energy minimization was performed using a conjugate gradient minimize (0.05 convergence criteria), the OPLS-AA force field, and GB/SA continuum water model. MD simulations were performed by pre-equilibration for 100 ps and simulation for 1 ns at 300K with a 1-fs time step and SHAKE applied to all hydrogen bonds. With the optimized model structures, docking of quercetin was carried out. We used the protein preparation utilities in Maestro to assign the charge state of ionizable residues, add hydrogens, and carry out energy minimization. The ligand quercetin was then docked into the homology models using the GLIDE (Schrodinger) module incorporated in Maestro. The default setting of the extreme precision mode of GLIDE was used for the docking, and up to 10 poses were saved for analysis.
Production of quercetin 3-O-(6-deoxytalose) in E. coli.
In order to compare the production of quercetin 3-O-(6-deoxytalose) in different E. coli strains, both pA-tll and pG-D1 were transformed into strains BL21, BrfbD, BrffA, BgalU-rfbD, and BgalU-rfbD-rffA (Table 1). An overnight culture of each transformant was inoculated into LB medium containing the appropriate antibiotics (50 μg/ml). Cells were grown at 37°C until the optical density at 600 nm (OD600) reached 0.8, and then 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added to induce protein expression. Cells were then incubated at 18°C for 18 h. The cells were harvested and resuspended in M9 containing 2% glucose. At this step, the cell density was adjusted to OD600 of 3. Quercetin (100 μM) was added to the reaction mixture, which was then incubated at 30°C for 4 h. The reaction product was extracted twice with ethyl acetate, and the organic layer was dried by evaporation. Analysis of reaction product using high-performance liquid chromatography (HPLC) was performed as described by Kim et al. (7). The production of quercetin 3-O-(6-deoxytalose) was calculated using quercetin 3-O-glucose as a standard molecule because quercetin 3-O-(6-deoxytalose) is not commercially available.
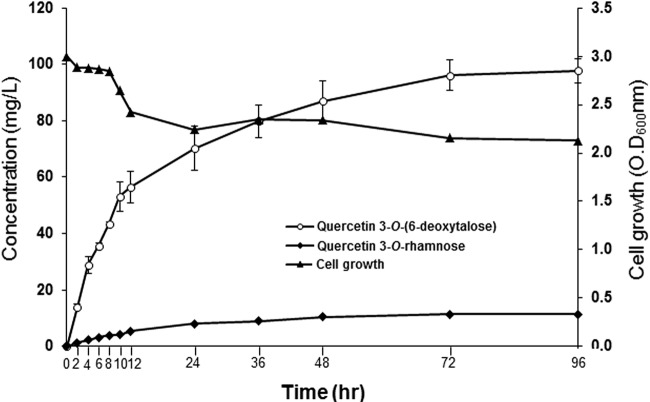
To select the best vector combination for the production of quercetin 3-O-(6-deoxytalose), vector combinations were transformed into BgalU-rffA-rfbD. The resulting E. coli strains (B11 to B14 [Table 1]) were induced, and biotransformation was performed as described above. To determine the production of quercetin 3-O-(6-deoxytalose) using E. coli B12, the cells were induced as described above. The reaction volume was 10 ml. Initially, 100 μM quercetin was added, and then 50 μM was added at 8, 12, and 36 h. Therefore, a total of 250 μM (75.5 mg/liter) of quercetin was added. The reaction product (300 μl) was collected at 2, 4, 6, 8, 10, 12, 24, 36, 48, 72, and 96 h. Three independent experiments were performed.
Nuclear magnetic resonance (NMR) experiments were performed on a Bruker Avance 400 (Karlsruhe, Germany). The fraction containing the reaction product was collected from several HPLC runs. The collected sample was dried by evaporation and was dissolved in 150 μl of dimethyl sulfoxide (DMSO)-d6 and transferred into 2.5-mm NMR tube. The NMR data for quercetin-3-O-(6-deoxytalose) were as follows: 1HNMR (DMSO-d6, 400 MHz): δ 6.22 (d, J = 1.9 Hz, H-6), 6.41 (d, J = 1.9 Hz, H-8), 7.31 (d, J = 2.0 Hz, H-2′), 6.90 (d, J = 8.3 Hz, H-5′), 7.24 (dd, J = 8.3, J = 2.0 Hz, H-6′), 5.39 (d, J = 1.1 Hz, H-1″), 3.90 (m, H-2″), 3.66 (t, J = 2.9 Hz, H-3″), 3.32 (m, H-4″), 3.45 (q, J = 6.4 Hz, H-5″), 0.79 (d, J = 6.4 Hz, H-6″), 12.62 (5-OH); 13CNMR (DMSO-d6, 100 MHZ): δ 157.4 (C-2), 134.1 (C-3), 177.7 (C-4), 161.1 (C-5), 98.8 (C-6), 164.5 (C-7), 93.8 (C-8), 156.6 (C-9), 104.1 (C-10), 120.8 (C-1′), 115.9 (C-2′), 145.3 (C-3′), 148.6 (C-4′), 115.6 (C-5′), 121.0 (C-6′), 102.2 (C-1″), 69.9 (C-2″), 65.2 (C-3″), 72.3 (C-4″), 68.9 (C-5″), and 16.5 (C-6″). NMR data are provided in Fig. S1 in the supplemental material.
RESULTS
Production of quercetin 3-O-(6-deoxytalose) in E. coli.
To synthesize a novel flavonoid glycoside in E. coli, at least two things need to be provided: a novel nucleotide sugar and UGT. To synthesize a nucleotide sugar donor, dTDP-6-deoxytalose in E. coli, the tll gene was overexpressed in the E. coli strain BL21(DE3). As a control, empty pACYCDuet vector was also transformed. The production of dTDP-6-deoxytalose in E. coli was examined, but we did not detect the production of dTDP-6-deoxytalose. It seems that the amount of dTDP-6-deoxytalose was tiny so that it could not be detected. This has also been reported that for another endogenous nucleotide sugar, dTDP-l-rhamnose, which is produced from the same substrate as dTDP-6-deoxytalose but was also not detected because only a tiny amount of dTDP-l-rhamnose is present in E. coli (14).
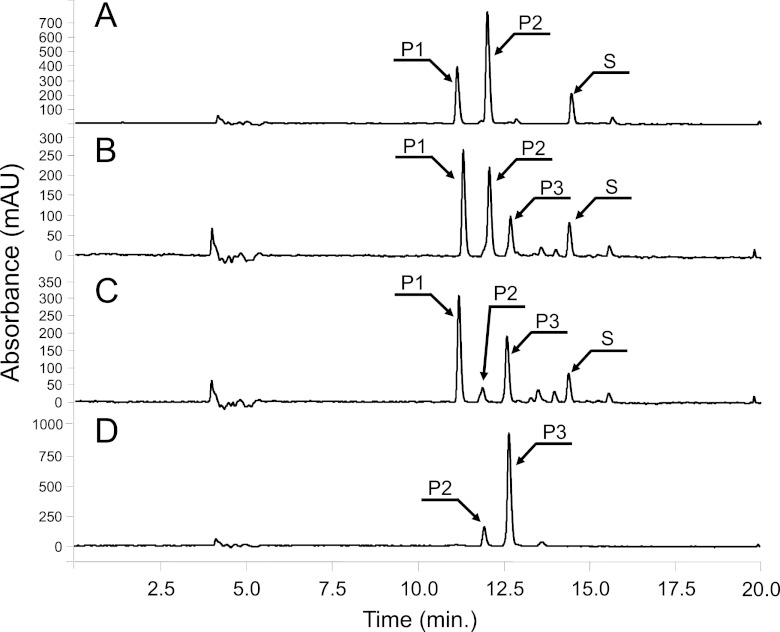
We also monitored production of a new flavonol glycoside. Because UGT has nucleotide sugar specificity, 50 UGTs from Oryza sativa, Glycine max, Bacillus subtilis, and Arabidopsis thaliana were screened to find a UGT that could use quercetin as a sugar acceptor and dTDP-6-deoxytalose as a sugar donor. Each UGT gene used had previously been subcloned into the E. coli expression vector pGEX (8–10). Each UGT gene was transformed into BL21(DE3) containing pA-tll or the pACYCDuet vector, and the biotransformation of quercetin by each transformant was evaluated. Among the 50 transformants harboring different UGTs, one transformant expressing AtUGT78D1, yielded a new peak that was not present in the transformant not expressing tll (Fig. 2A and B). The molecular mass of the new peak was at 448.1 Da, indicating that a 164.1-Da sugar was attached to quercetin (302 Da). Using NMR, the structure of this new peak was determined to be quercetin 3-O-(6-deoxytalose). The peak at 12.0 min observed in Fig. 2A was quercetin 3-O-rhamnose, and the peak at 11.1 min was quercetin 3-O-glucose. That is, quercetin 3-O-rhamnose and quercetin 3-O-glucose were produced also by coexpression of AtUGT78D1. Ideally, the production of these two compounds should be reduced. Therefore, we engineered the nucleotide-sugar biosynthesis pathway of E. coli.
Fig 2.
HPLC profiling of reaction product from different E. coli strains. (A) BL21 harboring pG-D1; (B) BL21 harboring pG-D1 and pA-tll; (C) BrfbD harboring pG-D1 and pA-tll; (D) BgalU-rfbD-rffA harboring pG-D1 and pA-tll. S, quercetin; P1, quercetin 3-O-glucose; P2, quercetin 3-O-rhamnose; P3, quercetin 3-O-(6-deoxytalose).
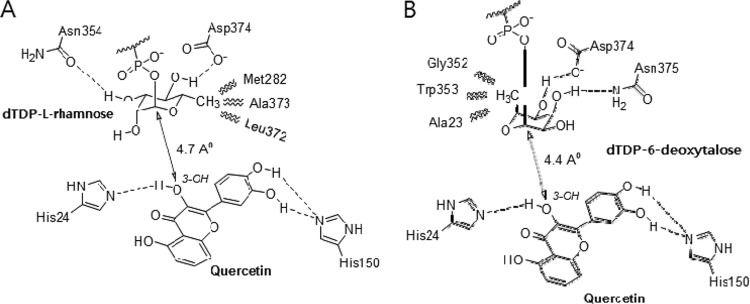
AtUGT78D1 was previously characterized as a flavonol 3-O-rhamnosyltransferase (4). Because dTDP-6-deoxytalose is not commercially available, molecular docking of dTDP-rhamnose or dTDP-6-deoxytalose to AtUGT78D1 was performed. Both dTDP-6-deoxytalose and quercetin were docked to AtUGT78D1, and the docking structure was compared to that of dTDP-l-rhamnose and quercetin (Fig. 3). Quercetin forms a hydrogen bond with His150, and the 3-hydroxy group of quercetin is located close to His24, which serves as a base for deprotonation from the 3-hydroxy group. dTDP-6-deoxytalose was fitted into AtUGT78D1 by forming hydrogen bonds with Asp374 and Asn375. Like dTDP-6-deoxytalose, dTDP-l-rhamnose formed hydrogen bonds between the 3-hydroxy group of rhamnose and Asn354 of AtUGT78D1 and between the 4-hydroxy group of rhamnose and Asp374 of AtUGT78D1. The key reason why AtUGT78D1 showed higher affinity for dTDP-l-rhamnose than dTDP-6-deoxytalose is that the methyl group fits well in the substrate-binding pocket of AtUGTD1. A methyl group of 6-deoxytalose could be involved in hydrophobic interactions with Ala23, Trp353, and Gly352; however, these interactions were not effective because the distance between the methyl group and these amino acids were not close enough. In contrast, the methyl group of rhamnose went into the hydrophobic pocket formed by Met282, Ala373, and Leu372. Therefore, although overall, dTDP-l-rhamnose bound to AtUGT78D1 better than dTDP-6-deoxytalose, AtUGT78D1 could use dTDP-6-deoxytalose as a sugar donor.
Fig 3.
Docking of AtUGT78D1 with dTDP-l-rhamnose (A) or dTDP-6-deoxytalose (B).
Engineering E. coli to increase the production of quercetin 3-O-(6-deoxytalose).
Glucose 1-phosphate is a central molecule in the nucleotide sugar biosynthetic pathway of E. coli. Glucose 1-phosphate leads to the synthesis of dUDP-glucose and dTDP-glucose. Eventually, dUDP-glucose is converted to dUDP-galactose and to dUDP-l-4-deoxy-4-formamido-l-arabinose via dUDP-glucuronic acid. In addition, dTDP-glucose is converted into dTDP-fucosamine and dTDP-rhamnose (21).
To increase the production of quercetin 3-O-(6-deoxytalose) through the increasing the level of substrate for the production of dTDP-6-dexoytalose, it would be necessary to modulate the nucleotide sugar biosynthesis pathway. Three genes were selected; the first was galU (UTP-glucose 1-phosphate uridyltransferase), which is at the branch point between dUDP-glucose and dTDP-glucose. The second was rffA (dTDP-4-oxo-6-deoxy-d-glucose transaminase), which leads to the synthesis of dTDP-fucosamine from dTDP-4-dehydro-6-deoxy-d-glucose. Finally, the third gene was rfbD (dTDP-4-dehydrorhamnose reductase), which competes for dTDP-4-dehydro-6-deoxy-l-mannose with tll. Combinations of these three genes were deleted (Fig. 3) and, as a result, four mutants, BrfbD, BrffA, BgalU-rfbD, and BgalU-rfbD-rffA, were made (Table 1). pG-D1 and pA-tll were transformed into each mutant. The HPLC profiles of the reaction products from each transformant differed (Fig. 2). After a 4 h of incubation, BL21 produced 18 mg of quercetin 3-O-glucose, 14 mg of quercetin 3-O-rhamnose, and 3.5 mg of quercetin 3-O-(6-deoxytalose)/liter. BrffA produced a higher concentration of quercetin 3-O-(6-deoxytalose) (6.5 mg/liter) than did wild type but still produced almost the same concentration of quercetin 3-O-glucose and quercetin 3-O-rhamnose as BL21. In contrast, BrfbD produced a higher concentration of quercetin 3-O-(6-deoxytalose) (12 mg/liter). In this strain, production of quercetin 3-O-rhamnose was significantly reduced. However, a high amount of quercetin 3-O-glucose was still produced. The BgalU-rfbD double mutant produced ∼5-fold higher levels of quercetin 3-O-(6-deoxytalose) (17 mg/liter) than the wild-type BL21. In addition, the production of quercetin 3-O-glucose and quercetin 3-O-rhamnose was reduced in this mutant. More specifically, only a negligible amount of quercetin 3-O-glucose was detected, which seemed to result from deletion of galU. The triple mutant, BgalU-rfbD-rffA, produced the highest amount of quercetin 3-O-(6-deoxytalose) (23 mg/liter), and the smallest amount of by-products among the tested strains. The production of quercetin 3-O-(6-deoxytalose) production in this strain was >6.5-fold higher than that in wild-type BL21. Taken together, BgalU-rffA-rfbD not only produced higher concentration of quercetin 3-O-(6-deoxytalose) but also produced fewer by-products such as quercetin 3-O-glucose and quercetin 3-O-rhamnose. The relative amount of reaction products produced by the different E. coli strains is summarized in Fig. 4.
Fig 4.
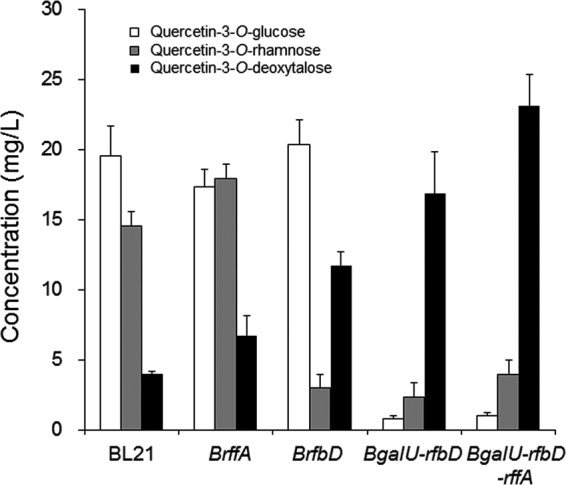
Relative production of three quercetin 3-O-glycoses in different mutant strains harboring pG-D1 and pA-tll. Three independent experiments were carried out. Error bars represent the standard deviation.
Production of quercetin 3-O-(6-deoxytalose).
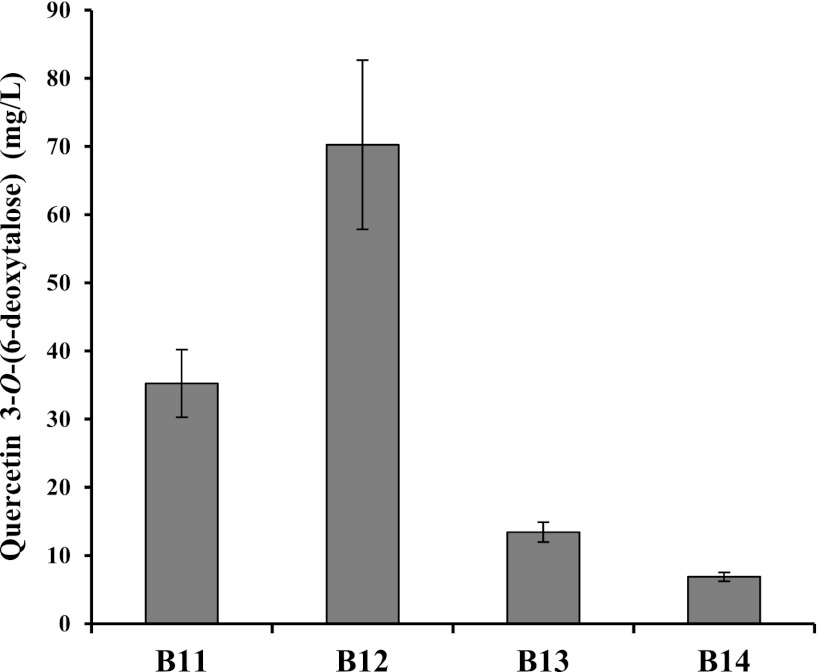
The production of quercetin 3-O-(6-deoxytalose) was optimized. Different copies and combinations of E. coli cloning vectors were tested to determine which plasmid was best for the production of quercetin 3-O-(6-deoxytalose). The tll and AtUGT78D1 genes were both cloned into pACYCDuet, and pETDuet, which have different copy numbers in E. coli. The resulting constructs, pA-tll-D1 and pE-tll-D1, were transformed into BgalU-rfbD-rffA to make strains B11 and B12, respectively. tll was subcloned into plasmids having higher copy number in E. coli vectors pCDFDuet and pETDuet than the plasmid expressing AtUGT78D1, which was pACYCDuet. Both plasmids containing tll and AtUGT78D1 were transformed into BgalU-rfbD-rffA to create strains B13 and B14. Using these four strains, production of quercetin 3-O-(6-deoxytalose) was examined. B12 produced ∼70 mg of quercetin 3-O-(6-deoxytalose)/liter, B11 produced 35 mg/liter, B13 produced 13 mg/liter, and B14 produced 7 mg/liter (Fig. 5). The copy number of construct was not related with the productivity of quercetin 3-O-(6-deoxytalose). It seemed that the highest copy number gave a higher metabolic load to E. coli. On the other hand, a lower copy number of the construct did not produce enough proteins to convert quercetin into quercetin 3-O-(6-deoxytalose). Therefore, the best construct was selected to maximize the production of quercetin 3-O-(6-deoxytalose). Using strain B12, the production of quercetin 3-O-(6-deoxytalose) was monitored. A final concentration of 250 μM quercetin (75.5 mg/liter) was added. Conversion of quercetin into quercetin 3-O-(6-deoxytalose) dramatically increased until 24 h. After 96 h, ∼98 mg of quercetin 3-O-(6-deoxytalose)/liter was produced (Fig. 6).
Fig 5.
Production of quercetin 3-O-(6-deoxytalose) by BgalU-rfbD-rffA harboring different plasmid combinations. Three independent experiments were carried out. Error bars represent the standard deviation.
Fig 6.
Production of quercetin 3-O-(6-deoxytalose) by biotransformation using E. coli B12. Initially, 100 μM quercetin was added, and then 50 μM was added at 8, 12, and 36 h. Therefore, the total concentration of quercetin was 250 μM (75.5 mg/liter) of quercetin. Reaction product samples (500 μl) were collected at 2, 4, 6, 8, 10, 12, 24, 36, 48, 72, and 96 h. Three independent experiments were carried out. Error bars represent the standard deviation.
DISCUSSION
By engineering the nucleotide biosynthetic pathway in E. coli, the production of quercetin 3-O-(6-deoxytalose) in BgalU-rfbD-rffA increased ∼7-fold compared to the wild-type strain. In addition, the production of the by-products was also significantly reduced. This demonstrates that it is possible to increase the levels of a particular reaction product by metabolic engineering of the nucleotide sugar pathway. We also optimized the plasmid vectors to maximize the production of quercetin 3-O-(6-deoxytalose). The production of 3-O-(6-deoxytalose) differed remarkably (∼10-fold) depending on the vectors used. After selection of optimal combination of plasmid vector and E. coli strain, ∼98 mg of 3-O-(6-deoxytalose)/liter was synthesized.
The biological activity of quercetin 3-O-(6-deoxytalose) remains elusive. However, different quercetin 3-O-glycoses display different biological activities. For example, quercetin 3-O-glucose inhibits adipocyte differentiation (12), which might control obesity, whereas quercetin 3-O-galactose contains neuroprotective effects (28). Therefore, the quercetin 3-O-(6-deoxytalose) synthesized here might contain a novel biological activity.
UGTs from A. thaliana, G. max, O sativa, and B. cereus were used to find a UGT able to use dTDP-6-deoxytalose as sugar donor. Of the 50 UGTs tested, only one UGT, AtUGT78D1 was able to use dTDP-6-deoxytalose. Most UGTs use UDP-glucose as a sugar donor, and a few use other nucleotide sugars. UGTs from plants are known to generally have a narrow sugar-donor range (18). Changing the sugar selectivity of UGTs is not easy. Although the molecular structures of several plant UGTs have been determined, there has been no report of the domain or motif critical for recognizing the sugar donor selectivity. In silico screening of UGTs might be alternative approach to find an appropriate UGT. However, the number of UGTs available for in silico screening is limited. An alternate approach, site-directed mutagenesis is also time-consuming.
In vitro and in vivo functional study of AtUGT78D1 showed that it uses dUDP-rhamnose as a sugar donor and quercetin as a sugar acceptor. Our results also show that AtUGT78D1 can also use dTDP-6-deoxytalose, indicating that this enzyme is promiscuous. dTDP-6-deoxytalose is not present in plant. Promiscuous enzyme activities are considered an adventitious activity without a physiological role (1). Therefore, although plants do not utilize dTDP-6-deoxytalose, this adventitious activity can be used to produce novel compounds in a heterologous expression system.
Talosin A from Kitasatospora kifunensis is genistein 7-O-(6-deoxytalose) (27). Although talosin A displays antifungal activity, its production is low (∼3.2 mg/liter after 7 days). The E. coli strain we created in the present study could be a host for the production of talosin A. However, the E. coli B12 strain does not produce talosin A after biotransformation of genistein. Therefore, screening for a UGT that recognizes genistein as well as TDP-6-deoxytalose is needed. Ultimately, the BgalU-rffA-rfbD string harboring tll developed here could serve as a host to attach 6-deoxytalose to other small molecules.
We tried to compare the nucleotide sugar profiles in the wild type as well as in the mutant strains; however, we could not detect any differences. Nucleotide sugars, such as UDP-glucose, would be in a static state because they serve as the substrates for biosynthesis of various other nucleotide sugars. Therefore, the quantification of the nucleotide sugars in each cell is difficult. Although we could not detect nucleotide sugar difference in E. coli strains, it is likely that the pool of each nucleotide sugar significantly changes in each E. coli mutant since the profile of quercetin reaction products in each mutant was different from one another (Fig. 1). Although UGTs from plants show sugar donor specificity, it is apparent that UGTs can utilize other nucleotide sugars when the appropriate proper sugar donor is not available (7).
It was assumed that the rfbD mutant did not produce quercetin 3-O-rhamnose. However, a small amount of quercetin 3-O-rhamnose was still produced in strain BrfbD. Biotransformation of quercetin using BrfbD harboring only pG-D1 did not produce quercetin 3-O-rhamnose. Therefore, it is likely that using dTDP-4-dehydro-6-deoxy-l-mannose as a substrate, tll produced dTDP-6-deoxytalose as a major product and dTDP-l-rhamnose as a minor product. The resulting nucleotide sugars served as sugar donors for AtUGT78D1 in production of quercetin 3-O-(6-deoxytalose) and quercetin 3-O-rhamnose.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a grant from the Next-Generation BioGreen 21 Program (SSAC, grant PJ007975), Rural Development Administration, Republic of Korea, and also partially by the Priority Research Centers Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (2009-0093824).
Footnotes
Published ahead of print 6 April 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Allewell NM. 2012. Thematic minireview series on enzyme evolution in the post-genomic era. J. Biol. Chem. 287:1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amano K, Nishihara T, Shibuya N, Noguchi T, Koga T. 1989. Immunochemical and structural characterization of a serotype-specific polysaccharide antigen from Actinobacillus actinomycetemcomitans Y4 (serotype b). Infect. Immun. 57:2942–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bowles D, Isayenkova J, Lim Poppenberger E-KB. 2005. Glycosyltransferases: managers of small molecules. Cur. Opin. Plant Biol. 8:254–263 [DOI] [PubMed] [Google Scholar]
- 4. Jones P, Messner B, Nakajima J, Schäffner AR, Saito K. 2003. UGT73C6 and UGT78D1, glycosyltransferases involved in flavonol glycoside biosynthesis in Arabidopsis thaliana. J. Biol. Chem. 278:43910–43918 [DOI] [PubMed] [Google Scholar]
- 5. Jüergenliemk G, et al. 2003. In vitro studies indicate that miquelianin (quercetin 3-O-beta-d-glucuronopyranoside) is able to reach the CNS from the small intestine. Planta Med. 69:1013–1017 [DOI] [PubMed] [Google Scholar]
- 6. Kim B-G, et al. 2003. Cloning and expression of the isoflavone synthase gene (IFS-Tp) from Trifolium pratense. Mol. Cells 15:301–306 [PubMed] [Google Scholar]
- 7. Kim B-G, Sung SH, Ahn J-H. 2012. Biological synthesis of quercetin 3-O-N-acetylglucosamine conjugate using engineered Escherichia coli expressing UGT78D2. Appl. Microbiol. Biotechnol. 93:2447–2453 [DOI] [PubMed] [Google Scholar]
- 8. Kim JH, et al. 2006. Characterization of flavonoid 7-O-glucosyltransferase from Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 70:1471–1477 [DOI] [PubMed] [Google Scholar]
- 9. Ko JH, Kim B-G, Ahn J-H. 2006. Glycosylation of flavonoids with a glycosyltransferase from Bacillus cereus. FEMS Microbiol. Lett. 258:263–268 [DOI] [PubMed] [Google Scholar]
- 10. Ko JH, et al. 2008. Four glucosyltransferases from rice: cDNA cloning, expression, and characterization. J. Plant Physiol. 165:435–444 [DOI] [PubMed] [Google Scholar]
- 11. Laskowski RA, MacArthur MW, Moss DS, Thornton JM. 1993. PROCHECK: a program to check the stereochemistry of protein structure. J. Appl. Crystallogr. 26:283–291 [Google Scholar]
- 12. Lee SH, et al. 2011. Persicaria hydropiper (L.) spach and its flavonoid components, isoquercitrin and isorhamnetin, activate the Wnt/β-catenin pathway and inhibit adipocyte differentiation of 3T3-L1 cells. Phytother. Res. 2:1629–1635 [DOI] [PubMed] [Google Scholar]
- 13. Lim E-K, Ashford DA, Hou B, Jackson RG, Bowles DJ. 2004. Arabidopsis glycosyltransferases as biocatalysts in fermentation for regioselective synthesis of diverse quercetin glucosides. Biotechnol. Bioeng. 87:623–631 [DOI] [PubMed] [Google Scholar]
- 14. Lim E-K, Ashford DA, Bowles DJ. 2006. The synthesis of small-molecule rhamnosides through the rational design of a whole-cell biocatalysis system. Chembiochem 7:1181–1185 [DOI] [PubMed] [Google Scholar]
- 15. Nakano Y, et al. 2000. Thymidine diphophate-6-deoxy-l-lyxo-4-hexose reductase synthesizing dTDP-6-deoxy-l-talose from Actinobacillus actinomycetemcomitans. J. Biol. Chem. 276:6806–6812 [DOI] [PubMed] [Google Scholar]
- 16. Newman DJ, Cragg GM. 2007. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 70:461–477 [DOI] [PubMed] [Google Scholar]
- 17. Offen W, et al. 2006. Structure of a flavonoid glucosyltransferase reveals the basis for plant natural product modification. EMBO J. 25:1396–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Osmani SA, Bak S, Moeller BL. 2009. Substrate specificity of plant UDP-dependent glycosyltransferase predicted from crystal structures and homology modeling. Phytochemistry 70:325–347 [DOI] [PubMed] [Google Scholar]
- 19. Raetz C, Whitfield C. 2002. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71:635–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sali A, Overington JP, Johnson MS, Blundell TL. 1990. From comparisons of protein sequences and structures to protein modeling and design. Trends Biochem. Sci. 15:235–240 [DOI] [PubMed] [Google Scholar]
- 21. Samuel G, Reeves P. 2003. Biosynthesis of O-antigens: genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly. Carbohydr. Res. 338:2503–2519 [DOI] [PubMed] [Google Scholar]
- 22. Seifert GJ. 2004. Nucleotide sugar interconversions and cell wall biosynthesis: how to bring the inside to the outside. Cur. Opin. Plant Biol. 7:277–284 [DOI] [PubMed] [Google Scholar]
- 23. Shibuya N, et al. 1991. 6-Deoxy-d-talan and 6-deoxy-l-talan: novel serotype-specific polysaccharide antigens from Actinobacillus actinomycetemcomitans. J. Biol. Chem. 266:16318–16323 [PubMed] [Google Scholar]
- 24. Soundararajan R, et al. 2008. Quercetin 3-glucoside protects neuroblastoma (SH-SY5Y) cells in vitro against oxidative damage by inducing sterol regulatory element-binding protein-2-mediated cholesterol biosynthesis. J. Biol. Chem. 283:2231–2245 [DOI] [PubMed] [Google Scholar]
- 25. Williams GJ, Gantt RW, Thorson JS. 2008. The impact of enzyme engineering upon natural product glycodiversification. Curr. Opin. Chem. Biol. 12:556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang J, Hoffmeister D, liu Fu LX, Thorson JS. 2004. Natural product glycorandomization. Bioorg. Med. Chem. 12:1577–1584 [DOI] [PubMed] [Google Scholar]
- 27. Yoon TM, Kim JW, Kim JG, Kim WG, Suh JW. 2006. Talosins A and B: new isoflavonol glycosides with potent antifungal activity from Kitasatospora kifunensis MJM341. J. Antibiot. 59:633–639 [DOI] [PubMed] [Google Scholar]
- 28. Zeng KW, et al. 2011. Hyperoside protects primary rat cortical neurons from neurotoxicity induced by amyloid β-protein via the PI3K/Akt/Bad/Bcl(XL)-regulated mitochondrial apoptotic pathway. Eur. J. Pharmacol. 672:45–55 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.