Abstract
Androgens control spermatogenesis, but germ cells themselves do not express a functional androgen receptor (AR). Androgen regulation is thought to be mediated by Sertoli and peritubular myoid cells, but their relative roles and the mechanisms involved remain largely unknown. Using Cre/loxP technology, we have generated mice with a ubiquitous knockout of the AR as well as mice with a selective AR knockout in Sertoli cells (SC) only. Mice with a floxed exon 2 of the AR gene were crossed with mice expressing Cre recombinase ubiquitously or selectively in SC (under control of the anti-Müllerian hormone gene promoter). AR knockout males displayed a complete androgen insensitivity phenotype. Testes were located abdominally, and germ cell development was severely disrupted. In contrast, SC AR knockout males showed normal testis descent and development of the male urogenital tract. Expression of the homeobox gene Pem, which is androgen-regulated in SC, was severely decreased. Testis weight was reduced to 28% of that in WT littermates. Stereological analysis indicated that the number of SC was unchanged, whereas numbers of spermatocytes, round spermatids, and elongated spermatids were reduced to 64%, 3%, and 0% respectively of WT. These changes were associated with increased germ cell apoptosis and grossly reduced expression of genes specific for late spermatocyte or spermatid development. It is concluded that cell-autonomous action of the AR in SC is an absolute requirement for androgen maintenance of complete spermatogenesis, and that spermatocyte/spermatid development/survival critically depends on androgens.
Testosterone and follicle-stimulating hormone (FSH) are the most important hormones controlling spermatogenesis. FSH is secreted by the pituitary and acts via specific G-coupled receptors located exclusively on Sertoli cells (SC). Testosterone, the main androgenic steroid, is produced intratesticularly by the Leydig cells (LC), and its production is regulated by luteinizing hormone (LH). FSH controls the proliferation of SC during the perinatal and/or pubertal period and, as a consequence, is a major determinant of adult spermatogenic capacity. Testosterone, however, is considered the crucial (for review, see refs. 1–3) hormone responsible for the initiation and maintenance of spermatogenesis. In fact, testosterone alone is able to restore spermatogenesis under experimental conditions where FSH is virtually absent, such as in hypogonadal mice genetically deficient in gonadotropin-releasing hormone (GnRH) (hpg mice) (4).
The target cells by which androgens control spermatogenesis are still debated. According to most studies, male germ cells are devoid of the androgen receptor (AR) (1–3, 5), an X chromosome-encoded member of the nuclear receptor family mediating most effects of androgens in androgen target tissues (6, 7). In line with these observations, male mice, chimeric for an androgen-resistant TfmX/Y (Tfm, testicular feminization) genotype (due to an inactivating mutation in the AR) and a normal (X/Y) genotype, are able to produce offspring from the TfmX/Y component (8). Similarly, transplantation of germ cells from TfmX/Y mice into seminiferous tubules of azoospermic mice expressing a functional AR results in complete and qualitatively normal donor-derived spermatogenesis (9).
Because germ cells seem not to require a functional AR, androgens must affect spermatogenesis indirectly via somatic testicular cells. In this case, SC are prime candidates. These cells interact directly with developing germ cells (10, 11) and express a functional AR with highest expression occurring at stages of the spermatogenic cycle when androgens are thought to act (1). Major changes in overall protein synthesis have been shown to be androgen-dependent at these stages (1, 12), but despite extensive investigations, by using either in vivo or in vitro approaches, limited changes in expression of individual proteins by SC in response to androgens have been shown (1, 12). Alternatively, the peritubular myoid cell (PT) might represent a major site of androgen action. These cells interact intimately with SC and spermatogonia in the basal tubular compartment (13–15), and evidence suggests that, under the influence of androgens, PT produce one or more paracrine factors, which modulate SC function (commonly referred to as PmodS; refs. 16–19).
To clarify the effects of androgens on spermatogenesis and to define more precisely the role of SC as a direct target of androgen action, we have used Cre/loxP technology to generate mice with a ubiquitous knockout of the AR (ARKO) as well as mice with a SC-selective knockout (SCARKO). Mice in which exon 2 of the AR was floxed were crossed with mice expressing Cre recombinase ubiquitously or selectively in SC (driven by an anti-Müllerian hormone gene promoter). Exon 2 encodes the crucial first Zn finger of the AR DNA-binding domain, and its deletion provokes a frame shift and premature termination of AR transcription (20, 21). We have demonstrated that, in contrast to ARKO males, SCARKOs have normal urogenital tracts and normally descended testes. Nonetheless, from puberty onward, SCARKO testes display a spermatogenic arrest at the late spermatocyte/spermatid stage. The data indicate that cell-autonomous action of the AR in SC is an absolute requirement for androgens to maintain complete spermatogenesis and define spermatocyte/spermatid development as a major site of SC-mediated androgen action to enable germ cell development.
Methods
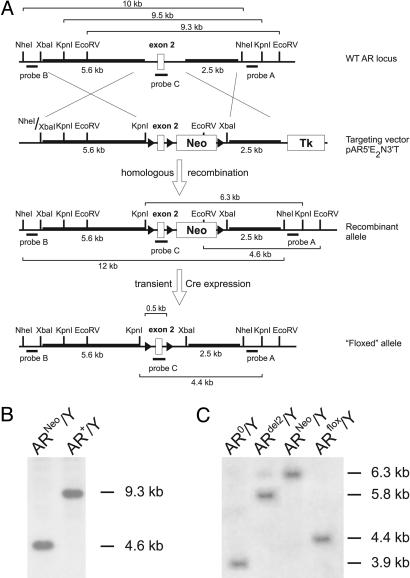
Construction of Targeting Vector to Flox AR Gene Exon 2. A 120-kb genomic HindIII-fragment of the mouse AR gene containing exons 1 and 2 was isolated from a mouse embryonic stem (ES) cell 129/SvJ BAC library (Genome Systems, St. Louis) after PCR identification. The identity of the fragment was confirmed by Southern blotting and sequencing. A targeting vector (pAR5′E2N3′T) was constructed containing: a 5.6-kb XbaI-XhoI fragment of AR intron 1 at the 5′ homology region, the AR exon 2 flanked by loxP sites (floxed), a floxed positive selection marker [the phosphoglycerate kinase-1 (PGK) neomycin (neo) expression cassette], a 2.5-kb genomic PvuII-fragment of the downstream region of AR exon 2, representing the 3′-homology region, and a negative selection marker PGK-thymidine kinase expression cassette (Fig. 1A). The selection cassettes and backbone were derived from a modified pNTlox2 vector, derived from the original pNT (22).
Fig. 1.
Generation of an AR allele with a floxed exon 2. (A) Schematic overview of the followed strategy. LoxP sites are indicated by triangles. Genomic fragments (5.6 and 2.5 kb) (bold) were used to allow homologous recombination of a region containing a floxed AR exon 2 and neo cassette in the targeting vector with the WT AR locus. Probes A, B, and C were used to screen for correct homologous recombination by Southern blotting. The neo cassette was excised by transient transfection of a Cre expression plasmid (pOG231) in a correctly recombined ES clone. (B) Southern blot analysis of EcoRV-digested DNA from ES cells transfected with the targeting vector by using probe A. The presence of a 4.6-kb fragment (ARneo/Y) vs. a WT band of 9.3 kb (AR+/Y) revealed correct homologous recombination. (C) Southern blot analysis of DNA isolated from pOG231 transfected recombined ES cells. DNA was digested with KpnI and hybridized with probe A. All possible recombinations are shown: complete excision (AR0/Y); excision of exon 2 only (ARdel2/Y); no excision (ARneo/Y); and the desired excision of the neo cassette only (ARflox/Y) showing a 4.4-kb fragment.
ES Cell Targeting. R1 ES cells [male AR+/Y; genetic background 129Sv × 129cX/Sv (23)] were electroporated with the linearized targeting vector as described (24) and subjected to positive and negative selection with G418 (0.2 mg/ml Geneticin, GIBCO) and gancyclovir (2 μM; Sigma–Aldrich). Correct homologous recombination at the 3′ side was verified by Southern blot analysis of EcoRV-digested genomic DNA with an external probe (probe A) (Fig. 1 A), yielding a 9.3-kb WT and 4.6-kb recombined band (Fig. 1B). Additional confirmatory diagnostic digests were performed by using KpnI and NheI digestion with external probe B and internal probe C, respectively (Fig. 1 A). The internal probe was used to verify for absence of additional random integration of the targeting vector. Correctly recombined clones (ARneo/Y) were expanded and transiently transfected with the Cre expression plasmid pOG231 (25) to excise the floxed neo cassette. Correct excision was verified by Southern blot analysis of KpnI-digested DNA with probe A, revealing a 4.4-kb band after the desired recombination (Fig. 1C). A correctly excised clone (ARflox/Y; one of 216 studied) was expanded in Thromb-X medium (Thromb-X embryonic stem cell medium) (26) to optimize subsequent germline transfer.
Generation of Chimera Founder Mice with a Floxed AR Exon 2. The targeted ARflox/Y ES cells were injected into blastocysts from Swiss–Webster females and implanted into pseudopregnant mothers to proceed to term. One female and seven male chimeric animals were identified by the presence of agouti coat color. Chimeric males were mated to Swiss females to test for germline transmission. Germline offspring were identified again by agouti coat and black eye color. Genotyping of the offspring was done by PCR on genomic DNA prepared from tail biopsies. Two chimeric males displaying 100% germline transmission were used as founders.
PCR Genotyping. PCR genotyping with an appropriate primer pair (AR exon 2 locus; Table 4, which is published as supporting information on the PNAS web site) was used to identify mice with a WT, floxed, or excised allele of the AR, revealing bands of 855, 952, and 404 bp, respectively. For ARKO animals, the genetic sex (i.e., presence of the Y chromosome) was confirmed by the presence of the Zfy gene (27). The presence of the Cre transgene was determined by use of appropriate primers (Table 4).
Histochemical Techniques. Urogenital systems from WT, ARKO, and SCARKO males (50 days old) were removed, fixed in Bouins fluid for 4 h, then transferred to 70% ethanol. Testes, epididymides, coagulating glands, and seminal vesicles were dissected, then processed into paraffin wax by using standard methods. Five-micrometer sections were stained with hematoxylin/eosin, and apoptotic germ cells were identified based on DNA fragmentation by using a nonradioactive labeling method as described and validated in detail previously (Apotag; ref. 28). Immunohistochemical demonstration of the AR was performed on dewaxed sections after heat-induced antigen retrieval for 5 min in 0.01 M citrate buffer, pH 6.0 (Sigma–Aldrich), utilizing a pressure cooker. A rabbit anti-AR antiserum (sc-816, Santa Cruz Biotechnology) at 1:200 was used in conjunction with a swine anti-rabbit biotinylated second antibody (E0353, DAKO). Bound antibodies were visualized by incubating the sections with R.T.U. VECTASTAIN Elite ABC-HRP reagent (PK-7100, Vector Laboratories) followed by color development with 3,3′-diaminobenzidine tetrahydrochloride chromogenic substrate (K3468, Liquid DAB+ kit, DAKO), monitored microscopically. Sections were counterstained with hematoxylin, and images were captured by using an Olympus Provis microscope (Olympus, London) equipped with a Kodak DCS330 camera (Eastman Kodak).
Determination of Testicular Cell Composition. Standard stereological methods involving the point counting of cell nuclei were used as described (29, 30), to determine the nuclear volume per testis of SC, germ cells (apoptotic and nonapoptotic), and the relative volumes of interstitium, seminiferous epithelium, and seminiferous tubule lumen. Briefly, cross sections of testes from five WT and three SCARKO mice (all 50 days old) were examined under oil immersion by using a Leitz 363 plan apo objective fitted to a Leitz Laborlux microscope and a 121-point eyepiece graticule. For each animal, 32–64 microscopic fields were counted, and values for percent nuclear volume were converted to absolute nuclear volumes per testis by reference to testis volume (=weight), because shrinkage was minimal. Cell nuclear volume can be equated to numbers of cells per testis, assuming no change in nuclear diameter of the target cell in the different groups. Data were used to determine for each animal the following: (i) the relative contribution of interstitium, seminiferous epithelium, and lumen to the testis volume; (ii) effects of the loss of AR in SC on the nuclear volumes of SC and germ cells per testis; and (iii) the germ cell apoptotic index based on the ratio of the nuclear volume of apoptotic/total germ cells per testis. Measurement of spermatogonial and spermatocyte nuclear diameter in both the groups in the present study confirmed no significant difference (data not shown). Because of the complex shape of the SC nucleus, average SC nuclear size was not determined, but we have shown previously that point-count measurement of SC nuclear volume per testis equates broadly to SC number determined by the dissector method (29, 30). It was therefore not possible to convert the present data to absolute numbers of germ cells per SC. Furthermore, because apoptotic germ cells cannot be identified with certainty, the apoptotic index reported was derived by expressing the relative nuclear volume per testis of apoptotic germ cells to the combined nuclear volumes per testis of all types of germ cells observed (29, 30).
RNA Analysis. Tissue samples (day 20 or 50) were removed and snap-frozen in liquid nitrogen. cDNA was synthesized from DNaseI-treated total RNA (RNeasy kit, Qiagen, Chatsworth, CA) by using Superscript II RNaseH– reverse transcriptase and random hexamer primers (Invitrogen). Primer pairs spanned an intron and were designed by using Primer Express (Applied Biosystems) (Table 4). For quantification of gene expression, the ABI Prism 7700 sequence detector PCR detection system (Applied Biosystems) was used with a two-step RT-quantitative-PCR protocol. Gene expression was corrected for well-to-well loading variation by expressing data as a ratio to 18S rRNA. All samples and standard curves were run in duplicate.
Hormone Measurements. Mice were anesthetized and exsanguinated by cardiac puncture. Sera were separated and stored at –20°C until assayed. LH and FSH were measured by doubleantibody radioimmunoassays by using reagents supplied by A. F. Parlow (Harbor–University of California, Los Angeles, Torrance, CA) and the National Institute of Diabetes and Digestive and Kidney Diseases National Hormone and Peptide Program. The standard preparations used were mLH-RP (lot no. AFP5306A) and mFSH-RP (lot no. AFP5308D). Tracers were prepared from rLH-I-10 and rFSH-I-9; antisera were anti-rLH-S-11 and anti-rFSH-S-11. Testosterone was measured by using the Testo-RIA-CT kit (BioSource International, Camarillo, CA). All samples from an individual experiment were run in a single assay, and the within-assay coefficients of variation for T, LH, and FSH were 4.7%, 11.0%, and 5.2%, respectively.
Statistical Analysis. Where indicated, statistical analysis was performed by using one-way ANOVA, supplemented with a Tukey–Kramer multiple comparison test by using ncss 2000 software (NCSS Statistical Analysis and Data Analysis Software, Kaysville, UT).
Results
Generation of Knockout Animals. Mice carrying on their X chromosome an AR gene with a floxed exon 2 were generated by homologous recombination in ES cells (Fig. 1) and blastocyst injection, as described in Methods. To produce ARKO mice, female mice (129/Swiss) heterozygous for the floxed AR allele (ARflox/AR+) were mated to male mice (C57BL/6) expressing the Crerecombinase ubiquitously under the control of the strong PGK promoter (PGK-Crem) (31). Male progeny heterozygous for the PGK-Crem allele and inheriting the ARflox allele from their mothers are expected to undergo exon 2 excision from early zygote stage on (31) and to code for a defective AR (AR0/Y).
To generate SCARKO mice, ARflox/AR+ females were crossed with male mice (C57BL/6) heterozygous or homozygous for the AMH-Cre transgene (32). Studies with reporter transgenic mice conditionally expressing LacZ have shown that the AMH-Cre transgene is expressed specifically in SC from 15 days postcoitum on, and that expression occurs in every SC. Accordingly, male progeny with an AMH-Cre+/– and ARflox/Y genotype should undergo excision of the AR exon 2 and should be unable to express functional AR protein in SC.
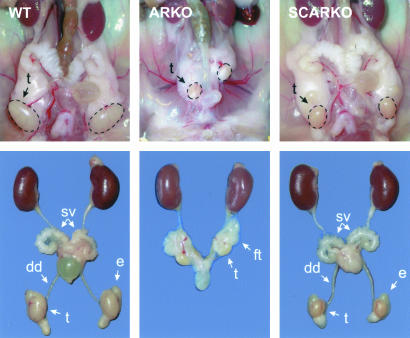
Phenotypic Differences Between ARKO and SCARKO Males. The phenotypic sex observed in litters containing ARKO males (75.2% female phenotypes among 206 pups) deviated markedly from the 1:1 male/female ratio expected for normal litters. PCR genotyping revealed the presence of a Y chromosome in 29.0% of the phenotypical females and confirmed the absence of AR gene exon 2 in the same animals, identifying them as ARKO males. In the same litters, 53.6% of the genetic females also carried a copy of the AR with a deleted exon 2. Growth curves of ARKO males followed those of female littermates (data not shown). Dissection of adult male ARKO mice confirmed a phenotype consistent with complete androgen insensitivity syndrome (6, 7). Testes were much smaller than in normal males (Table 1) and were located in the abdominal or inguinal region (Fig. 2). Structures derived from the Wolffian ducts (ductus deferens, epididymis, and seminal vesicles) or urogenital sinus (prostate) were absent. No uterus or fallopian tubes were observed. These data confirmed that introduction of loxP sites in the AR gene allowed excision of exon 2 and a functional knockout of the AR by ubiquitously expressed Cre driven by the strong PGK-1 promoter. Male mice carrying the ARflox/Y genotype (without AMH-Cre) had a male phenotype and showed normal development of the male urogenital system (not shown).
Table 1. Organ weights of reproductive organs and accessory glands in WT, ARKO, and SCARKO male mice aged 50 days.
| Testis, mg | Coagulating gland, mg | Seminal vesicle, mg | Epididymis, mg | |
|---|---|---|---|---|
| WT (n = 8) | 93.4 ± 3.8 | 13.7 ± 1.2 | 67.8 ± 3.7 | 26.4 ± 1.1 |
| ARKO (n = 4) | 7.5 ± 0.5* | - | - | - |
| SCARKO (n = 4) | 26.5 ± 2.7* | 13.5 ± 1.6 | 73.3 ± 8.5 | 15.7 ± 0.7* |
Values are mean ± SEM for the number of mice indicated (n). *, P < 0.01 vs. WT.
Fig. 2.
Dissection of urogenital tracts of WT, ARKO, and SCARKO male mice at the age of 50 days. dd, ductus deferens; sv, seminal vesicles; t, testis; e, epididymis; ft, fat tissue.
SCARKO litters contained the different genotypes, as expected for a Mendelian inheritance pattern. Male SCARKO mice showed normal external sexual development, and their growth curve was similar to that of WT male littermates (data not shown). On dissection, the testes were found to be located in exactly the same location as in normal WT males (Fig. 2). The size of the testes, however, was reduced to 28.4% of WT littermates at 50 days of age (Table 1). In contrast with the ARKO males, SCARKO males displayed normal development of epididymis, ductus deferens, coagulating gland, seminal vesicles, and prostate. On day 50, seminal vesicle and coagulating gland weights of SCARKO and control normal littermates were comparable. Epididymis weight was reduced by 30% (Table 1).
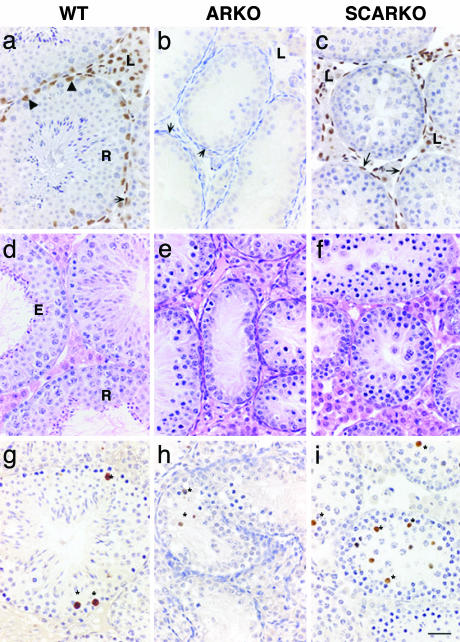
Selective Knockout of AR in SC in SCARKO Testes. Immunohistochemical analysis of the testes from WT animals revealed the expected pattern of expression of AR with immunopositive nuclei in LC, PT, and SC but not in germ cells (Fig. 3a). No AR staining was detected in cell nuclei within the testes from ARKO animals (Fig. 3b). Western blotting confirmed the complete absence of intact AR or AR fragments in whole testis protein extracts from ARKO mice, whereas a 110-kDa band corresponding to the full length AR was visualized in WT littermates (data not shown). In the testes of SCARKO animals, LC and PT nuclei clearly stained positive, whereas no immunopositive staining was detected in SC (Fig. 3c).
Fig. 3.
Comparison between the testes obtained from adult WT, ARKO, and SCARKO mice. In WT mice (a), AR are immunolocalized to nuclei of SC (arrowheads), PT (arrow), and LC (L); no immunopositive staining was detected in ARKO (b), and PT were multilayered (arrows). In SCARKO (c), LC (L) and PT (arrows) were immunopositive for AR, but no SC staining was detected. Hematoxylin/eosin staining of testes from day 50 WT (d), ARKO (e), and SCARKO (f) revealed clear differences in germ cell complement. Complete spermatogenesis including round (R) and elongate (E) spermatids was observed in WT, but spermatogenic arrest occurred in both AR mutants (e and f). Apotag-positive germ cells (*) were rare in WT (g) but were observed in many tubules in ARKO (h) and SCARKO (i). (Bar = 50 μm.)
Further evidence for the absence of a functional AR in SC from SCARKO animals was obtained from quantitative RT-PCR measurements of Pem, a SC-specific homeobox-gene that displays a 50-fold androgen-induced increase in expression during pubertal development (33). Measurement of Pem transcripts in testis RNA extracts of 20-day-old animals (to minimize differences in tissue composition due to differential development of spermatogenesis) revealed Pem values in SCARKO of <1% of those observed in WT (4 ± 1 vs. 516 ± 108 copies/108 copies 18S rRNA; mean ± SEM, n = 6).
SCARKO Males Display Incomplete Spermatogenesis. Hematoxylin/eosin staining confirmed normal spermatogenesis in WT mice with formation of a seminiferous tubule lumen surrounded by mature elongated spermatids (Fig. 3d). In ARKO testes, small tubules without a lumen containing reduced numbers of germ cells and surrounded by multilayered PT were observed (Fig. 3e). In SCARKO testes (Fig. 3f), germ cell maturation was markedly advanced compared with that of the ARKO, and PT did not appear multilayered. However, the germ cell complement was reduced compared with WT littermates, and spermatogenesis was incomplete (compare Fig. 3 f with d). Morphological analysis of SCARKO testes established that germ cell entry into meiosis appeared normal, but there was progressive loss of pachytene primary spermatocytes between stages VI and XII. Diplotene and secondary spermatocytes occurred, but in greatly reduced numbers, and the few round spermatids that were formed appeared abnormal and did not survive beyond stages VII— VIII. No elongated spermatids were detected. In both ARKO and SCARKO, reduced germ cell number was associated with increased apotag staining of germ cells compared with WT (Fig. 3 g–i), which was confirmed by counts revealing a 4- to 5-fold increase in the fraction of apoptotic germ cells/total germ cells comparing WT and SCARKO (0.009 ± 0.001 for WT vs. 0.041 ± 0.003 for SCARKO).
The absence of spermatid development in SCARKO mice was confirmed by quantitative PCR measurements showing failure of expression of both transition proteins 1 and 2 and protamines 1 and 2 (Fig. 4, which is published as supporting information on the PNAS web site). The expression of A-myb, a transcription regulator active in early meiotic prophase (34), tended to be 49% lower (although not statistically significant) in SCARKO testes, whereas the expression of pro-acrosin-binding protein, a gene activated in late stage primary spermatocytes (35), was reduced to 12% in SCARKO testes, consistent with our morphological observations.
Quantitative Analysis of Cellular Composition of WT and SCARKO Adult Testes. In ARKO males, the contribution of absence of androgen action to the overall disruption of spermatogenesis is difficult to interpret, given the known deleterious effects of the intraabdominal location of the testes. Accordingly, cellular counts were limited to WT and SCARKO testes. In SCARKO testes, seminiferous tubules were reduced in diameter: 219 ± 4 μminWT animals vs. 134 ± 4 μm in SCARKO animals (mean ± SEM; 18–30 tubules measured per testis cross section for five and three mice, respectively) (Fig. 3). Stereological analysis revealed a significantly lower proportion (volume percent) of seminiferous tubule lumen in SCARKO testes (5.48 ± 0.35 for SCARKO (n = 3) vs. 14.52 ± 0.99 for WT (n = 5), P < 0.05), indicative of fluid-secretory dysfunction by SC, which is normally androgen-dependent (1). The relative proportion of seminiferous epithelium per testis was comparable in SCARKO and WT testes. Quantitative analysis of cell composition (Table 2) revealed no significant differences in SC nuclear volume per testis between WT and SCARKO. Therefore, data for spermatocytes and spermatogonia expressed as nuclear volumes per testis reflect changes in the ability of SC to support germ cell development rather than a difference in SC number. Nuclear volume per testis of spermatogonia was unchanged in SCARKO vs. WT testes. In contrast, spermatocyte nuclear volume per testis was 36% lower in SCARKO testes, and this decrement increased to 97% for round spermatids (Table 2). No elongated spermatids were found in SCARKOs.
Table 2. Cellular composition of the seminiferous tubules of WT and SCARKO male mice at 50 days of age.
| Sertoli cells | Spermatogonia | Spermatocytes | Round spermatids | Elongated spermatids | |
|---|---|---|---|---|---|
| WT mm3 (n = 5) | 1.29 ± 0.08 | 0.81 ± 0.07 | 8.66 ± 0.37 | 5.75 ± 0.54 | 4.65 ± 0.40 |
| SCARKO, mm3 (n = 3) | 1.13 ± 0.18 | 0.74 ± 0.13 | 5.51 ± 0.13* | 0.19 ± 0.05* | 0.00 ± 0.00* |
| SCARKO/WT, % | 88 | 91 | 64 | 3 | 0 |
Data are expressed as nuclear volume per testis. Values are mean ± SEM for the number of animals indicated (n). SCARKO nuclear volume as percent of the WT is also indicated. *, P < 0.05 vs. WT.
Hormonal Profile. Serum levels of testosterone and LH revealed no significant difference between WT and SCARKO animals and were reflected in similar organ weights of androgen target tissues (Table 1). In accordance with the observed spermatogenic arrest, FSH levels were 34% higher in SCARKO males (Table 3).
Table 3. Serum levels of gonadotropins and testosterone in day 50 WT and SCARKO male animals.
| Serum testosterone, ng/ml | LH, ng/ml | FSH, ng/ml | |
|---|---|---|---|
| WT | 0.56 ± 0.25 (14) | 0.79 ± 0.21 (12) | 21.09 ± 1.35 (13) |
| SCARKO | 0.81 ± 0.43 (9) | 0.74 ± 0.12 (7) | 28.28 ± 2.28* (7) |
Values are mean ± SEM of the number of samples indicated (n). *, P < 0.05 vs. WT.
Discussion
The data described confirm that we have succeeded in the development of SCARKO mice. In contrast to ARKO mice, such animals have a normally developed male urogenital tract and normally descended testes. As a consequence, SCARKOs present a previously undescribed paradigm for the study of androgen action in the testis. At least three conclusions can be drawn from our present studies with this model.
First, selective inactivation of the AR in SC does not interfere with testicular descent and allows normal development of the non-SC somatic components of the testis. In fact, although more detailed studies may be needed to exclude minor alterations, the available data suggest that, consistent with the normal serum levels of testosterone and LH, development and function of LC in SCARKO males are normal. Similarly, PT development appears grossly normal in SCARKOs. In contrast, ARKO testes were incompletely descended and displayed more pronounced, but variable, impairment of spermatogenesis, consistent with previous studies of testes from Tfm mice and rats (8, 36). According to our preliminary data, ARKO males also show changes to the somatic cells of the testis, SC numbers being reduced, and PT forming multilayers, changes also reported in Tfm mice (37, 38). The degree of impairment of spermatogenesis in ARKO males is consistent with that found in other cases of incomplete testis descent (39), but we cannot exclude that impaired androgen action on non-SC components of the testis also contributes to the phenotype.
Second, cell-autonomous activation of the AR is an absolute requirement for the ability of SC to support complete spermatogenesis. SCARKO males have essentially normal numbers of SC (based on measurement of nuclear volume per testis), but tubular diameters are markedly reduced, and incomplete spermatogenesis was observed, suggesting defective SC function. These findings show unambiguously that direct actions of androgens on SC, mediated by the classical AR, play a pivotal role in spermatocyte, and possibly spermatid, development. During the last 10 years, studies from several laboratories have suggested that PT might be a major, and maybe even the main, site of androgen action relevant to spermatogenesis (16–18). The present data do not exclude that part of the effects of androgens on SC may be mediated by indirect pathways (such as PmodS), but they stress the important and independent role of SC as a direct androgen target.
Third, androgen action on SC is required to allow germ cells to complete meiosis. In SCARKOs, spermatocyte and spermatid numbers are reduced to ≈64 and 3% of those in WT littermates. These findings are reminiscent of, although not completely identical to, those described in a rat model in which testicular androgen concentration was reduced by administration of testosterone and estradiol (3, 40). In this model, some decrease in germ cells is already observed at the spermatogonial level (≈80% of control), whereas round spermatids appear slightly less affected than in SCARKOs (suppressed to 20% at stages I–VII and to 5% at stage VIII). In both models, however, elongated spermatids are absent. The similarity of the spermatogenic defects observed in a model based on reduction of androgen levels in the testis and a model based on selective inactivation of the AR in SC underlines the pivotal role of SC and the classical AR in the androgenic control of spermatogenesis. The more pronounced reduction in round spermatids in SCARKO animals (apart from methodological and species differences) may be due both to the fact that the AR is already inactivated from an early age and (as suggested by the Pem data) to a more complete elimination of androgen action than in a model based on administration of androgens. The mechanism by which inactivation of the AR in SC disturbs progression of germ cells through meiosis remains to be investigated. The present data showing a 5-fold increase in germ cell apoptosis in SCARKOs may indicate disturbed SC–germ cell interactions. Defects in meiotic progression have been identified in a number of other studies and models exploring the effects of androgens on spermatogenesis (3, 41–44). Well-documented additional effects of androgens on spermatogenesis related to transformation of round spermatids into elongated spermatids and spermiation (for an overview, see ref. 3) cannot be studied in SCARKOs due to the absence of these cells.
As a model for the study of androgen action in the testis, SCARKO mice display a number of advantages and peculiarities. (i) Because the AMH-Cre transgene is active from 15 days postcoitum onward, inactivation of the AR gene occurs well before its normal expression in SC in the neonatal period (45). Accordingly, the observed consequences reflect mainly lack of androgen action during initiation (and maintenance) of spermatogenesis. (ii) The SCARKO model selectively blocks androgen action in SC and only those effects mediated by the classical AR. Potential indirect effects related to PModS or other paracrine mediators produced by other testicular cells may be preserved. Androgen effects mediated by the estrogen receptor or by alternative mechanisms [such as androgen-induced changes in SC Ca2+-concentration (46)] may also still be present. (iii) The immunohistochemical data, the Pem measurements, and the complete spermatogenic arrest all suggest that androgen ablation may be more complete than in other models based on administration of androgens (with or without estrogens), GnRH agonists, or antagonists. (iv) In contrast with many other methods [hypophysectomy, destruction of LC by EDS, gonadotropin-releasing hormone (GnRH) (ant)agonist administration], there are no direct effects on the production of other hormones or paracrine factors (FSH, putative paracrine factors produced by LC,...) that may affect spermatogenesis. Secondary effects, for instance as a consequence of the rise in FSH, may still be present.
This study has focused on androgens and spermatogenesis. It should be noted, however, that the successful use of AMH-Cre mice in the production of SCARKOs paves the way for study of the role of the AR also in granulosa cells, the only cells to which Cre is targeted by the AMH-Cre construct in female mice.
Conclusion
Despite normal testicular descent, SCARKOs display a spermatogenic arrest preventing the completion of meiosis and formation of round spermatids. This proves unambiguously that the initiation and maintenance of spermatogenesis depend absolutely on AR activation in SC. SCARKOs form a unique tool for further analysis of the molecular mechanisms of androgen action in the testis and in particular in SC.
Supplementary Material
Acknowledgments
We thank Hilde Geeraerts and Ludo Deboel for skillful technical assistance and Arantza Esnal for excellent histology. This work was supported by grants from a Concerted Research Action (Research Fund Catholic University of Leuven) and from the Fund for Scientific Research–Flanders (Belgium).
Abbreviations: FSH, follicle-stimulating hormone; AR, androgen receptor; SC, Sertoli cell; LC, Leydig cell; PT, peritubular myoid cell; ARKO, AR knockout mouse; SCARKO, SC-selective AR knockout mouse; Tfm, testicular feminization; neo, neomycin; ES, embryonic stem; PGK-1, phosphoglycerate kinase-1.
References
- 1.Sharpe, R. M. (1994) in The Physiology of Reproduction, eds. Knobil, E. & Neill, J. D. (Raven, New York), pp. 1363–2434.
- 2.Weinbauer, G. F. & Nieschlag, E. (1998) in Testosterone: Action–Deficiency, Substitution, eds. Nieschlag, E. & Behre, H. M. (Springer, Berlin), pp. 143–168.
- 3.McLachlan, R. I., O'Donnell, L., Meachem, S. J., Stanton, P. G., de Kretser, D. M., Pratis, K. & Robertson, D. M. (2002) Recent Prog. Horm. Res. 57, 149–179. [DOI] [PubMed] [Google Scholar]
- 4.Singh, J., O'Neill, C. & Handelsman, D. J. (1995) Endocrinology 136, 5311–5321. [DOI] [PubMed] [Google Scholar]
- 5.Bremner, W. J., Millar, M. R., Sharpe, R. M. & Saunders, P. T. (1994) Endocrinology 135, 1227–1234. [DOI] [PubMed] [Google Scholar]
- 6.Wilson, J. D. (1992) Biol. Reprod. 46, 168–173. [DOI] [PubMed] [Google Scholar]
- 7.Quigley, C. A., De Bellis, A., Marschke, K. B., el-Awady, M. K., Wilson, E. M. & French, F. S. (1995) Endocr. Rev. 16, 271–321. [DOI] [PubMed] [Google Scholar]
- 8.Lyon, M. F., Glenister, P. H. & Lamoreux, M. L. (1975) Nature 258, 620–622. [DOI] [PubMed] [Google Scholar]
- 9.Johnston, D. S., Russell, L. D., Friel, P. J. & Griswold, M. D. (2001) Endocrinology 142, 2405–2408. [DOI] [PubMed] [Google Scholar]
- 10.Jegou, B., Cudicini, C., Gomez, E. & Stephan, J. P. (1995) Reprod. Fertil. Dev. 7, 723–730. [DOI] [PubMed] [Google Scholar]
- 11.Griswold, M. D. (1998) Semin. Cell Dev. Biol. 9, 411–416. [DOI] [PubMed] [Google Scholar]
- 12.McKinnell, C. & Sharpe, R. M. (1995) J. Androl. 16, 499–509. [PubMed] [Google Scholar]
- 13.Verhoeven, G. (1992) Baillieres Clin. Endocrinol. Metab. 6, 313–333. [DOI] [PubMed] [Google Scholar]
- 14.Dym, M. (1994) Endocr. Rev. 15, 102–115. [DOI] [PubMed] [Google Scholar]
- 15.Skinner, M. K. & Fritz, I. B. (1985) Proc. Natl. Acad. Sci. USA 82, 114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skinner, M. K., Fetterolf, P. M. & Anthony, C. T. (1988) J. Biol. Chem. 263, 2884–2890. [PubMed] [Google Scholar]
- 17.Verhoeven, G. & Cailleau, J. (1988) Endocrinology 123, 2100–2110. [DOI] [PubMed] [Google Scholar]
- 18.Swinnen, K., Cailleau, J., Heyns, W. & Verhoeven, G. (1990) Endocrinology 126, 142–150. [DOI] [PubMed] [Google Scholar]
- 19.Verhoeven, G., Hoeben, E. & De Gendt, K. (2000) Andrologia 32, 42–45. [PubMed] [Google Scholar]
- 20.Hellwinkel, O. J.-C., Bull, K., Holterhus, P.-M., Homburg, N., Struve, D. & Hiort, O. (1999) J. Steroid. Biochem. Mol. Biol. 68, 1–9. [DOI] [PubMed] [Google Scholar]
- 21.Yeh, S., Tsai, M.-Y., Xu, Q., Mu, X.-M., Lardy, H., Huang, K.-E., Lin, H., Yeh, S.-D., Altuwaijiri, S., Zhou, X. et al. (2002) Proc. Natl. Acad. Sci. USA 99, 13498–13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tybulewicz, V. L., Crawford, C. E., Jackson, P. K., Bronson, R. T. & Mulligan, R. C. (1991) Cell 65, 1153–1163. [DOI] [PubMed] [Google Scholar]
- 23.Nagy, A., Rossant, J., Nagy, R., Abramow-Newerly, W. & Roder, J. C. (1993) Proc. Natl. Acad. Sci. USA 90, 8424–8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dewerchin, M., Liang, Z., Moons, L., Carmeliet, P., Castellino, F. J., Collen, D. & Rosen, E. D. (2000) Thromb. Haemostasis 83, 185–190. [PubMed] [Google Scholar]
- 25.O'Gorman, S., Dagenais, N. A., Qian, M. & Marchuk, Y. (1997) Proc. Natl. Acad. Sci. USA 94, 14602–14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoonjans, L., Kreemers, V., Danloy, S., Moreadith, R. W., Laroche, Y. & Collen, D. (2003) Stem Cells 21, 90–97. [DOI] [PubMed] [Google Scholar]
- 27.Kunieda, T., Xian, M., Kobayashi, E., Imamichi, T., Moriwaki, K. & Toyoda, Y. (1992) Biol. Reprod. 46, 692–697. [DOI] [PubMed] [Google Scholar]
- 28.Sharpe, R. M., Atanassova, N., McKinnell, C., Parte, P., Turner, K. J., Kerr, J. B., Groome, N. P., McPherson, S., Millar, M. R. & Saunders, P. T. (1998) Biol. Reprod. 59, 1084–1094. [DOI] [PubMed] [Google Scholar]
- 29.Atanassova, N., McKinnell, C., Walker, M., Turner, K. J., Fisher, J. S., Morley, M., Millar, M. R., Groome, N. P. & Sharpe, R. M. (1999) Endocrinology 140, 5364–5373. [DOI] [PubMed] [Google Scholar]
- 30.Atanassova, N., McKinnell, C., Turner, K. J., Walker, M., Fisher, J. S., Morley, M., Millar, M. R., Groome, N. P. & Sharpe, R. M. (2000) Endocrinology 141, 3898–3907. [DOI] [PubMed] [Google Scholar]
- 31.Lallemand, Y., Luria, V., Haffner-Krausz, R. & Lonai, P. (1998) Transgenic Res. 7, 105–112. [DOI] [PubMed] [Google Scholar]
- 32.Lécureuil, C., Fontaine, I., Crepieux, P. & Guillou, F. (2002) Genesis 33, 114–118. [DOI] [PubMed] [Google Scholar]
- 33.Lindsey, J. S. & Wilkinson, M. F. (1996) Dev. Biol. 179, 471–484. [DOI] [PubMed] [Google Scholar]
- 34.Toscani, A., Mettus, R. V., Coupland, R., Simpkins, H., Litvin, J., Orth, J., Hatton, K. S. & Reddy, E. P. (1997) Nature 386, 713–717. [DOI] [PubMed] [Google Scholar]
- 35.Baba, T., Niida, Y., Michikawa, Y., Kashiwabara, S., Kodaira, K., Takenaka, M., Kohno, N., Gerton, G. L. & Arai, Y. (1994) J. Biol. Chem. 269, 10133–10140. [PubMed] [Google Scholar]
- 36.Vanha-Perttula, T., Bardin, C. W., Allison, J. E., Gunbreck, L. G. & Stanley, A. J. (1970) Endocrinology 87, 611–619. [DOI] [PubMed] [Google Scholar]
- 37.Johnston, H., Baker, P. J., Abel, M., Charlton, H. M., Jackson, G., Fleming, L., Rajendra Kumar, T. & O'Shaughnessy, P. J. (2004) Endocrinology 145, 318–329. [DOI] [PubMed] [Google Scholar]
- 38.Clark, A. M., Garland, K. K. & Russell, L. D. (2000) Biol. Reprod. 63, 1825–1838. [DOI] [PubMed] [Google Scholar]
- 39.Kon, Y. & Endoh, D. (2001) Mol. Reprod. Dev. 58, 216–222. [DOI] [PubMed] [Google Scholar]
- 40.O'Donnell, L., McLachlan, R. I., Wreford, N. G. & Robertson, D. M. (1994) Endocrinology 135, 2608–2614. [DOI] [PubMed] [Google Scholar]
- 41.Russell, L. D. & Clermont, Y. (1977) Anat. Rec. 187, 347–366. [DOI] [PubMed] [Google Scholar]
- 42.Kerr, J. B., Millar, M., Maddocks, S. & Sharpe, R. M. (1993) Anat. Rec. 235, 547–559. [DOI] [PubMed] [Google Scholar]
- 43.Ganguly, A., Misro, M. M. & Das, R. P. (1994) Arch. Androl. 32, 111–120. [DOI] [PubMed] [Google Scholar]
- 44.Jeyakumar, M., Suresh, R., Krishnamurthy, H. N. & Moudgal, N. R. (1995) J. Endocrinol. 147, 111–120. [DOI] [PubMed] [Google Scholar]
- 45.Sharpe, R. M., McKinnell, C., Kivlin, C. & Fisher, J. S. (2003) Reproduction 125, 769–784. [DOI] [PubMed] [Google Scholar]
- 46.Gorczynska, E. & Handelsman, D. J. (1995) Endocrinology 136, 2052–2059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.