Abstract
Candida albicans is one of the main species able to form a biofilm on almost any surface, causing both skin and superficial mucosal infections. The worldwide increase in antifungal resistance has led to a decrease in the efficacy of standard therapies, prolonging treatment time and increasing health care costs. Therefore, the aim of this work was to demonstrate the applicability of atmospheric plasma at room temperature for inactivating C. albicans growing in biofilms without thermally damaging heat-sensitive materials. This so-called cold atmospheric plasma is produced by applying high voltage to accelerate electrons, which ionize the surrounding air, leading to the production of charged particles, reactive species, and photons. A newly developed plasma device was used, which exhibits a large plasma-generating surface area of 9 by 13 cm (117 cm2). Different time points were selected to achieve an optimum inactivation efficacy range of ≥3 log10 to 5 log10 reduction in CFU per milliliter, and the results were compared with those of 70% ethanol. The results obtained show that contact-free antifungal inactivation of Candida biofilms by cold atmospheric plasma is a promising tool for disinfection of surfaces (and items) in both health care settings and the food industry, where ethanol disinfection should be avoided.
INTRODUCTION
Candida albicans is one of the main fungal species with the ability to grow as a biofilm on almost all surfaces, such as medical devices, as well as human epithelial surfaces (31, 32). In the last decade, Candida growing in biofilms has shown increased levels of resistance to a wide spectrum of conventional antifungal drugs used in clinical practice, such as amphotericin B and fluconazole (15). Chandra and colleagues demonstrated that C. albicans biofilms can be up to 20 times more resistant to amphotericin B and more than 100 times more resistant to fluconazole than their planktonic counterparts (4, 5). Environmental contamination by microbes that originate in dust and soil is a major problem where the maintenance of a high level of hygiene is indispensable, such as in clinics or in food-processing facilities. Several studies demonstrated that conventional methods to inactivate free-floating microorganisms with antimicrobial agents or disinfection solutions are often less effective against pathogens within a biofilm (6, 9, 13). Furthermore, Vazquez et al. demonstrated the possibility of exogenous nosocomial colonization by Candida, including possible acquisition from the hospital environment (33). Transmission may be by indirect contact, since identical strains of Candida were recovered from patients who were not in direct contact but were temporally associated via a transmission route of indirect contact between the patients. Adherence of Candida species to host tissues and nonbiological materials is not a problem (28). Mucosal cells, fibrin-platelet matrices, vascular endothelial cells, or plastic materials can be colonized by Candida. Radford et al. showed that rough surfaces on, e.g., denture base materials promote the adhesion of C. albicans (26).
Therefore, research efforts have led to the development of new antimicrobial strategies, especially for killing of microorganisms growing in biofilms (10, 11, 16). Another new approach in combating organisms growing in biofilms is cold atmospheric plasma, which has demonstrated its bactericidal, virucidal, and fungicidal properties due to the generation of reactive species, charged molecules, and photons (20, 29, 34). Numerous studies have demonstrated the effectiveness of gas discharge or cold atmospheric plasma in killing only planktonic microorganisms (14, 17, 18, 29). The killing efficacies were dependent on the plasma exposure time (10 s to 10 min), the material the microorganisms were located on, and the cell density. Only recently, the bactericidal effect of a nonthermal argon plasma was shown in vitro and also in biofilms (8). Furthermore, Koban et al. showed inactivation of Candida biofilms using different plasma devices (19). An atmospheric plasma jet pen showed only minimal antifungal effects on Candida biofilms, whereas an argon dielectric barrier discharge (DBD) plasma device showed a reduction in the number of CFU/ml of up to 5 log10. The disadvantage of these dielectric barrier discharge plasmas is the fact that the surface of the growing Candida biofilm acts as the counterelectrode for plasma generation. This means that the electric current flows through the biofilm, which increases the electric field strength of the plasma, which in turn increases the bacterial killing efficacy of the biofilm. This is critical for in vivo applications. In the study presented here, we used surface microdischarge (SMD) plasma technology to generate plasma in ambient air (25) to inactivate C. albicans biofilm via a contact-free disinfection procedure. The plasma is produced indirectly and transported to the biofilm on the surface via diffusion. The surface does not serve as a counterelectrode, so complications with electric currents are avoided and “safe” applications in vivo are possible. The SMD plasma device operates at approximately room temperature (RT) and produces so-called electrical microdischarges. This plasma discharge is generated by applying high voltage to accelerate electrons to ionize the surrounding air molecules. Due to the high electric field (voltage), the electrons have high energies and therefore produce not only ions, but also chemically reactive atoms/molecules (O3, OH, O, NO, etc.). In addition to these particles, light—including UV—is emitted.
Our study on contact-free inactivation of C. albicans biofilms with cold atmospheric pressure plasma indicates efficient disinfection of inanimate surfaces where liquid disinfectants fail because protection from corrosive material is mandatory.
MATERIALS AND METHODS
SMD plasma device.
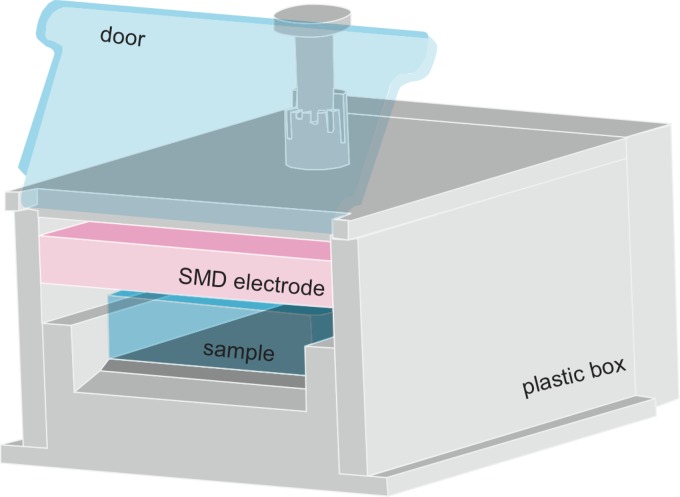
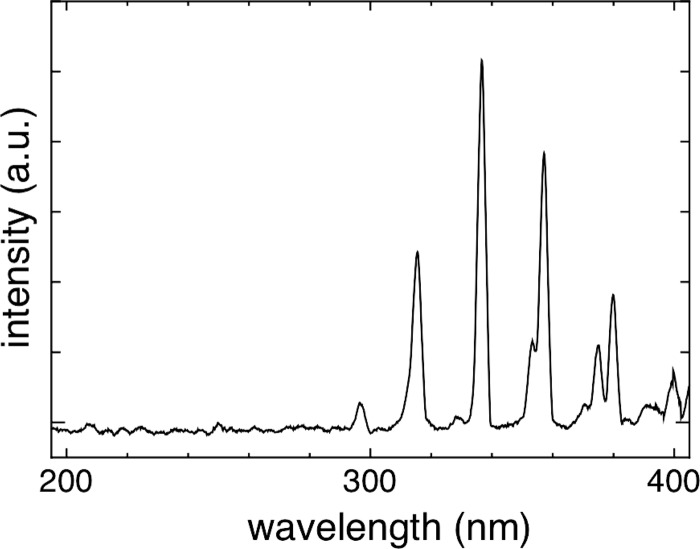
The SMD plasma device is incorporated into a box made of plastic (Teflon and polyoxymethylene). The electrode for producing the plasma is located inside the box, as shown in Fig. 1. On one side of the box, a door is installed so that the plasma gas produced is confined inside (Fig. 1). The plasma device is designed for the efficient treatment of a 96-well plate; therefore, the maximum area that can be treated is large (9 by 13 cm). The electrode, which produces the plasma, is located above the respective samples to be treated, and the distance between the electrode and the sample is adjustable. In this study, the distance was set at approximately 6 mm. The electrode for plasma production consists of a 0.5-mm-thick Teflon plate sandwiched between a brass planar plate and a stainless steel mesh grid (line width, 2 mm; opening, 10 mm; height, 1.5 mm). By applying a high sinusoidal voltage of 9 kVpp (i.e., voltage from peak to peak) with a frequency of 1 kHz between the brass plate and the mesh grid, the plasma is produced homogeneously in ambient air by many microdischarges (25). The plasma discharge is sustained through ionization processes, with electrons accelerated by applying the high voltage. These electrons produce electron/ion pairs. In addition to these pairs, chemically reactive species (O3, O, NO, etc.) are produced by approximately 600 chemical reactions driven by the electrons (dissociation of molecules, recombination, etc.). Furthermore, due to activated molecules/atoms, light emission can also be detected. The UV light emitted by plasma is mainly observed from the N2 positive system at a wavelength between 280 and 420 nm (the spectrum is shown in Fig. 2). Furthermore, peaks in the UVC region of the spectrum resulting from the NO γ system can be detected. The UV power density measured with a power meter (UV-Power Meter C8026; Hamamatsu, Japan) equaled 25 nW/cm2. This value is far below International Commission Non-Ionizing Radiation Protection (ICNIRP) safety levels. The measurement of ozone was performed by using UV absorption spectroscopy, and the NO2 concentration was measured using a gas detector (Multiwarn II; Dräger AG, Germany) (Table 1). The power consumption for the plasma discharge was approximately 0.02 W/cm2 as measured by the Lissajous method (19a) using 1-μF capacitance.
Fig 1.
Sketch of the plasma device. The device contains one SMD electrode, and the sample to be treated is placed below the electrode. In this study, the distance between the electrode and the sample was fixed at 6 mm.
Fig 2.
Spectrum of the plasma produced by the SMD device. The spectrum of the produced SMD plasma was measured in front of the electrode at a distance of 6 mm. The main UV components are in the wavelength range between 280 and 400 nm and are produced from nitrogen molecules excited by electron impact. a.u., arbitrary units.
Table 1.
Plasma components relevant to biomedical applications
| Charged particle/element | Electron, ion, or radiation | Density of charged particles at surface of electrode (∼1011 cm−3) or description |
|---|---|---|
| Reactive species | O3 | ∼500 ppm |
| NO | <1 ppm | |
| NO2 | ∼3 ppm | |
| O, OH | Present according to the literature | |
| Heat | Max 4°C above ambient temp | |
| Photons | UV, visible | UV power, ∼25 nW/cm2 (mainly UVA) |
| Static electric field | Max 106 V/m | |
| Electrical current through samples | Negligibly small; <100 μA |
Microorganism.
In order to obtain well-isolated discrete colonies, C. albicans was streaked on a Sabouraud dextrose agar plate and cultured at 37°C. A single colony of C. albicans (ATCC MYA-273) was picked up using a sterile inoculation loop and suspended in 5 ml of Sabouraud dextrose broth (SDB) (Sigma Chemical Co., St. Louis, MO). The suspension was cultured overnight at 37°C on a shaker platform (200 rpm). When the cultures reached the stationary phase of growth, the cells were harvested by centrifugation (1,800 relative centrifugal force [RCF]; 5 min) and washed once with Dulbecco's phosphate-buffered saline without Ca2+ and Mg2+ (PBS) (PAA Laboratories GmbH, Austria).
Biofilm formation.
The Candida cell density was determined using a microscope counting chamber. For biofilm formation, C. albicans cells were diluted to 106 cells ml−1 in 25% fetal bovine serum (FBS), and 2.5 ml was added to sterile flat-bottom 6-well polystyrene plates for cell culture and incubated at 37°C for 24 h without shaking.
Cold atmospheric plasma treatment.
In the first step, a proof of concept study was adopted from Shimizu et al. (29). Suspensions of free-floating Candida cells with a density of 2 × 106/ml were prepared in PBS; 3 serially diluted 20-μl samples were dropped out on Sabouraud dextrose agar plates and kept at RT under laminar flow for 30 min to dry the surface. After treatment with plasma, the agar plates were incubated at 37°C for 24 h, and surviving Candida colonies were counted.
Second, 24 h after biofilm generation, the samples were washed twice with PBS to remove free-floating Candida cells. The 6-well plates containing the biofilm were placed inside the device and treated for different time intervals as follows: 0, 2, 5, 6, 7, 8, 9, 10, and 15 min. As controls, non-plasma-treated samples were also placed in the SMD device for the same periods.
Furthermore, the disinfectant efficacy of 70% ethanol was tested on the Candida biofilm, as recently reported by Théraud et al. (30).
Quantification of inactivation efficacy.
After plasma treatment, the biofilm was scraped out of the 6-well plate using a sterile cell scraper (Sarstedt, Newtonk, NC). Each sample was sonicated for 3 min in an ultrasonic bath sonicator (USR 30H; Merck Eurolab, Germany) at a frequency of 35 kHz to disrupt the biofilm, and the CFU assay was used to determine the survivors by the technique of Miles et al. for viable counts (24). Serially diluted aliquots (20 μl) of treated and untreated samples were plated on Sabouraud dextrose agar, and the number of CFU per milliliter was determined after 24 h of incubation at 37°C.
Statistical methods.
All results are shown as medians, including the 25% and 75% quartiles, which were calculated from the values of at least three independent experiments. Each experiment was conducted in triplicate (three wells of a 6-well plate, corresponding to a biofilm area of 28.9 cm2) with Prism 4 for Windows (GraphPad Software Inc., San Diego, CA). The calculation (reduction in CFU/ml) was then compared with that for the untreated controls (non-plasma treated). In Fig. 3 and 4, medians on or below the dotted red or green line represent ≥99.9% efficacy or ≥99.999% Candida cell killing, corresponding to at least >3 or 5 orders of magnitude of log10 reduction compared with the matching untreated controls (non-plasma treated). A reduction of at least 3 orders of magnitude of log10 viable median numbers of Candida cells was considered biologically relevant with regard to the guidelines for hand hygiene (3).
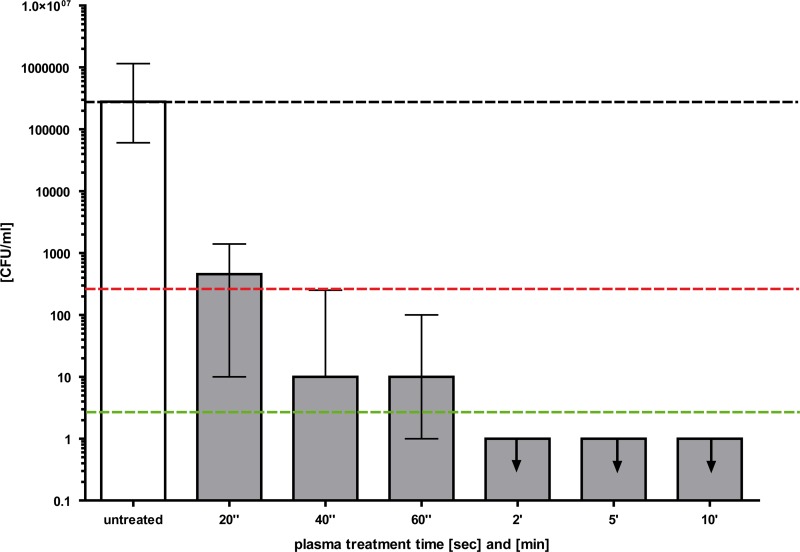
Fig 3.
Proof of concept of inactivation of C. albicans by cold atmospheric plasma. Twenty microliters of serially diluted free-floating C. albicans suspensions was applied to agar plates according to the Miles and Misra technique and dried for 45 min. Cold atmospheric plasma treatment was performed using different time intervals. Surviving colonies were counted 24 h later. Black dashed line, baseline of viable C. albicans per milliliter; red dashed line, reduction of 3 log10 steps in viable C. albicans (99.9%); green dashed line, reduction of 5 log10 steps in viable C. albicans (99.999%) (n = 3; median ± interquartile range). The arrows mark the appropriate upper limits.
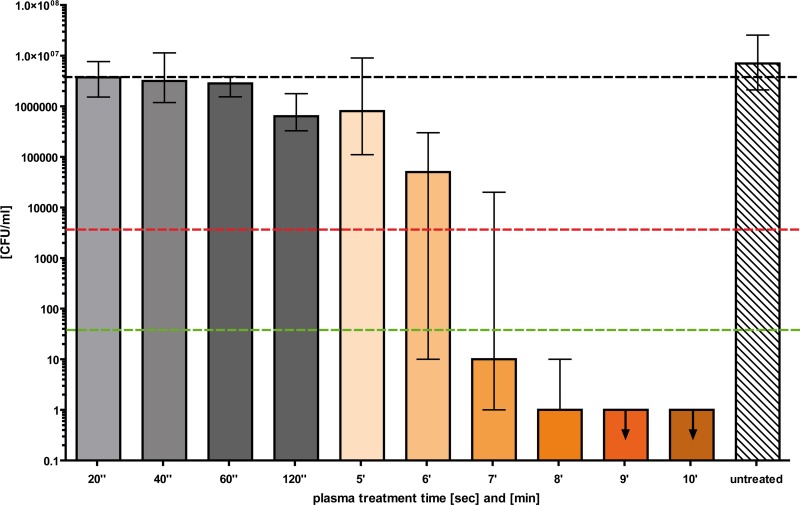
Fig 4.
Cold atmospheric plasma treatment of C. albicans biofilm. C. albicans biofilms were grown on the surfaces of 6-well plates for 24 h. After washing the biofilm to remove free-floating cells, the biofilm was dried for 30 min. Cold atmospheric plasma treatment was done using different time intervals (20 s to 10 min). A CFU assay was performed immediately after the plasma treatment, and colonies were counted 24 h later. Black dashed line, baseline of viable C. albicans per milliliter; red dashed line, reduction of 3 log10 steps in viable C. albicans (99.9%); green dashed line, reduction of 5 log10 steps in viable C. albicans (99.999%) (n = 3; median ± interquartile range). The arrows mark the appropriate upper limits.
RESULTS
SMD plasma device.
An SMD plasma device was used for all experiments. During the experiments, the door (as shown in Fig. 1) of the plasma device was closed, i.e., almost no gas exchange was able to take place. Thermal effects on the Candida biofilm can be ruled out in this study because the increase in the gas temperature during 10 min of application did not exceed 4°C (data not shown). As mentioned in Materials and Methods, the UV light emission was mainly observed from the N2 positive system between 280 and 420 nm (Fig. 2). Furthermore, emission in the UVC region resulting from the NO γ system was detected. Nevertheless the main content of the measured UV radiation refers to the UVA wavelength range between 320 and 400 nm. The UV power density of the plasma was 25 nW/cm2. The main components produced by the SMD plasma device that are relevant for biomedical applications are shown in Table 1. The ozone concentration in the device (inside the box with the door closed) after 60 s of plasma production was approximately 500 ppm. The NO2 concentration was approximately 3 ppm.
Plasma rapidly kills planktonic Candida cells: proof of concept.
First, the susceptibility of Candida to the SMD plasma device was determined by applying plasma to planktonic Candida cells plated on Sabouraud dextrose agar plates. The fungicidal effect of plasma is shown in Fig. 3. A killing efficacy of 99.9% of viable Candida cells was achieved with a plasma treatment time of 40 s. The reduction in viable Candida cells was increased further up to 5 log10 steps when the plasma treatment time was 5 min. No fungicidal effect was observed for the untreated controls.
Killing efficacy of plasma against Candida biofilms.
The Candida biofilms formed on inanimate surfaces were exposed to plasma treatment for different times (Fig. 4). A biofilm surface area of 28.9 cm2 was treated with plasma, corresponding to three single wells of a 6-well plate. The plasma application to Candida biofilms resulted in successful inactivation of Candida cells within the biofilm, which increased with increasing treatment time (Fig. 4). After 7 min, more than 99.9% of Candida cells were inactivated; 8 min resulted in a reduction of 6 log10 steps, which corresponds to a killing efficacy of 99.9999%. Again, no fungicidal effect was observed for the untreated controls. Note that the inactivation efficacy also depends on the distance between the electrode and the biofilm (data not shown). The tested distances ranged from 6 to 10 mm. All the results in the study refer to the most effective distance of 6 mm. In a second set of experiments, 70% ethanol was tested for biofilm inactivation. Reduction efficacies of only 1.5 log10, 2.8 log10, and >3 log10 were achieved with treatment times of 5 min, 7 min, and 10 min, respectively (data not shown).
DISCUSSION
Candida growing as a biofilm produces a broad range of infections, ranging from non-life-threatening mucocutaneous illnesses to severe invasive diseases (4, 27). This study demonstrates the efficacy of SMD plasma for killing C. albicans growing in biofilms. The results of the study clearly show that cold atmospheric plasma treatment is an attractive potential approach for inactivation of Candida biofilms growing on inanimate surfaces. A reduction of up to 99.9999% was achieved in a time-dependent manner (8 min) without any direct contact with the biofilm. A biofilm surface area of 28.9 cm2 was efficiently inactivated. This area is 72 times greater than the biofilm surface area of a 96-well plate, which was used in other studies (19). The possibility of removing large areas of biofilm in one step, without screening the surface, clearly exhibits advantages for industrial applications. Koban et al. measured a reduction factor of 5.2 log10 steps in a Candida biofilm grown in a 96-well microtiter plate with a 10-min plasma treatment using a DBD plasma device (19). As stated in the introduction, the great advantage of SMD plasma over DBD is that no direct contact with the surface is necessary and no current passes through the plate and the biofilm.
The main species that contribute to the inactivation of the biofilm using SMD plasma in ambient air are reactive species and charged particles. As indicated above, the density of the charged particles that interact with the biofilm is low due to excitation, dissociation, attachment, and recombination processes on the way from the electrode to the sample (25). Therefore, the main impact on the biofilm results from the reactive species produced. Measurements of the ozone produced in the device after a treatment time of 60 s showed a value of approximately 500 ppm. The NO2 concentration was approximately 3 ppm. As for the ozone concentration, Kowalski et al. reported that 15 s with 1,500 ppm of ozone was necessary for a 3 log10 reduction in Escherichia coli cells plated on agar (21). In our study, the reduction in C. albicans (and E. coli [data not shown]) cells is even faster with a smaller amount of ozone. This leads to the conclusion that the plasma agents produced have synergetic effects and therefore result in faster inactivation of pathogens. Various inactivation experiments with bacteria showed that the UV photons (gained by treating a planktonic bacterial sample with plasma filtered by a quartz glass) do not have any bactericidal property up to 120 s (data not shown). Therefore, we conclude that the UV radiation alone does not contribute to the fungicidal property. The electric field produced by the plasma can cause stress on the cell walls of fungi. However, we could not observe any inactivation of fungi due to the electric field produced by the plasma (data not shown). As stated above, the electrical current through the sample is negligible.
In recent years, opportunistic pathogens in the genus Candida have been the main cause of fungal infections, especially biofilm-mediated infections (4, 23). Therefore, contamination of inanimate surfaces by Candida may lead to the formation of a Candida biofilm, and if left untreated or not disinfected, it may become a risk in health care services. Candida biofilms are more resistant to standard antifungal agents than free-floating Candida cells (7). This study supports this statement, as we clearly showed that planktonic Candida cells can be killed more easily and using shorter plasma treatment times than cells growing in biofilms. Therefore, we conclude that not only the number of cells per milliliter, but also the growing conditions, planktonic or biofilm, limit inactivation. Single Candida cells can survive for a longer time embedded in a biofilm and grow at lower rates, and their metabolic and antifungal responses are often different from those of their planktonic counterparts. Furthermore, we were able to show that the disinfectant ethanol (70%) was less effective in killing the Candida biofilm, which is in agreement with published data (30). Théraud et al. demonstrated that the overall efficacy of antiseptics and disinfectants against yeast isolates is different when the cells are grown under planktonic or biofilm conditions (30). Eight out of nine agents investigated (10% iodine polyvinylpyrrolidone, 2% sodium lauryl sufate, 0.5% and 0.05% chlorhexidine digluconate, 3% hydrogen peroxide, 70% ethanol, 0.5% alkylamine, 0.5% alkylamine plus sodium hypochlorite, and UV radiation at 365 nm) were ineffective against Candida growing in biofilms (30). Chlorhexidine digluconate at a concentration of 0.5% was the only antifungal agent that was active on both planktonic Candida cell suspensions and C. albicans growing in biofilms. Today, chlorhexidine digluconate is used as a gold standard antiseptic in the oral cavity, but some risks must be considered (12). Chlorhexidine has a mutagenic potential, possesses a neurotoxic side effect, and can induce hypersensitivity (22). The Japanese Ministry of Health recommended in 1984 the use of chlorhexidine be avoided. Therefore, the long-term use of chlorhexidine has to be questioned (1).
As stated in the introduction, the high antifungal resistance of biofilms is a multifactor process in which an antifungal drug with only a single mechanism of action is unlikely to be effective (2). However, the mechanism of action of cold atmospheric plasma is different from that of an antifungal agent. Typically, an antifungal drug acts by using the lock-and-key principle. This means that a specific antifungal agent has to fit in a certain way inside or outside a Candida cell to induce an antifungal effect. In contrast, cold atmospheric plasma produced by the device results in the generation of reactive oxygen and nitrogen species, as well as atomic O and N and hydrogen peroxide interacting with water vapor. All these species are capable of inducing oxidative and radical damage to the biofilm during the plasma treatment. Therefore, rapid fungicidal disinfection is possible. In that case, no specific interaction is necessary to induce the antifungal effect.
Overall, the use of cold atmospheric plasma for disinfection of inanimate surfaces could lead to a major development preventing biofilm-associated Candida infections in the future. Therefore, the advantage of the concept of a contact-free application of plasma to sterilize Candida biofilm-contaminated surfaces (and items) in health care settings might have a positive impact on preventing community-acquired and nosocomial infections in the medical field, and also in the food industry, where time saving is critical to achieve efficient disinfection in the future.
ACKNOWLEDGMENTS
The excellent technical assistance of Judith Heider is gratefully acknowledged.
Tim Maisch was supported by a grant (M.TT.A.EXT00002) from the Max Planck Institute for Extraterrestrial Physics, Garching, Germany.
Footnotes
Published ahead of print 30 March 2012
REFERENCES
- 1. Autio-Gold J. 2008. The role of chlorhexidine in caries prevention. Oper. Dent. 33:710–716 [DOI] [PubMed] [Google Scholar]
- 2. Biel MA. 2010. Photodynamic therapy of bacterial and fungal biofilm infections. Methods Mol. Biol. 635:175–194 [DOI] [PubMed] [Google Scholar]
- 3. Boyce JM, Pittet D. 2002. Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am. J. Infect. Control 30:S1–S46 [DOI] [PubMed] [Google Scholar]
- 4. Chandra J, et al. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chandra J, et al. 2001. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J. Dent. Res. 80:903–908 [DOI] [PubMed] [Google Scholar]
- 6. Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Douglas LJ. 2003. Candida biofilms and their role in infection. Trends Microbiol. 11:30–36 [DOI] [PubMed] [Google Scholar]
- 8. Ermolaeva SA, et al. 2011. Bactericidal effects of non-thermal argon plasma in vitro, in biofilms and in the animal model of infected wounds. J. Med. Microbiol. 60:75–83 [DOI] [PubMed] [Google Scholar]
- 9. Fux CA, Costerton JW, Stewart PS, Stoodley P. 2005. Survival strategies of infectious biofilms. Trends Microbiol. 13:34–40 [DOI] [PubMed] [Google Scholar]
- 10. Ganz T. 2005. Defensins and other antimicrobial peptides: a historical perspective and an update. Comb. Chem. High Throughput Screen. 8:209–217 [DOI] [PubMed] [Google Scholar]
- 11. Ganz T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710–720 [DOI] [PubMed] [Google Scholar]
- 12. Greenstein G, Berman C, Jaffin R. 1986. Chlorhexidine. An adjunct to periodontal therapy. J. Periodontol. 57:370–377 [DOI] [PubMed] [Google Scholar]
- 13. Hoyle BD, Costerton JW. 1991. Bacterial resistance to antibiotics: the role of biofilms. Prog. Drug Res. 37:91–105 [DOI] [PubMed] [Google Scholar]
- 14. Hury S, Vidal DR, Desor F, Pelletier J, Lagarde T. 1998. A parametric study of the destruction efficiency of Bacillus spores in low pressure oxygen-based plasmas. Lett. Appl. Microbiol. 26:417–421 [DOI] [PubMed] [Google Scholar]
- 15. Jabra-Rizk MA, Falkler WA, Meiller TF. 2004. Fungal biofilms and drug resistance. Emerg. Infect. Dis. 10:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jori G, et al. 2006. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg. Med. 38:468–481 [DOI] [PubMed] [Google Scholar]
- 17. Joshi SG, et al. 2010. Control of methicillin-resistant Staphylococcus aureus in planktonic form and biofilms: a biocidal efficacy study of nonthermal dielectric-barrier discharge plasma. Am. J. Infect. Control 38:293–301 [DOI] [PubMed] [Google Scholar]
- 18. Kelly-Wintenberg K, et al. 1998. Room temperature sterilization of surfaces and fabrics with a one atmosphere uniform glow discharge plasma. J. Ind. Microbiol. Biotechnol. 20:69–74 [DOI] [PubMed] [Google Scholar]
- 19. Koban I, et al. 2010. Treatment of Candida albicans biofilms with low-temperature plasma induced by dielectric barrier discharge and atmospheric pressure plasma jet. New J. Physics 12:1–15 [Google Scholar]
- 19a. Kogelschatz U. 2003. Dielectric-barrier discharges: their history, discharge physics, and industrial applications. Plasma Chem. Plasma Process 23:1–46 [Google Scholar]
- 20. Kong MG, et al. 2009. Plasma medicine: an introductory review. New J. Physics 11:1–35 [Google Scholar]
- 21. Kowalski WJ, Bahnfleth WP, Whittam TS. 1998. Bactericidal effects of high airborne ozone concentrations on Escherichia coli and Staphylococcus aureus. Ozone Sci. Eng. 20:205–221 [Google Scholar]
- 22. Krautheim AB, Jermann TH, Bircher AJ. 2004. Chlorhexidine anaphylaxis: case report and review of the literature. Contact Dermatitis 50:113–116 [DOI] [PubMed] [Google Scholar]
- 23. Lamagni TL, Evans BG, Shigematsu M, Johnson EM. 2001. Emerging trends in the epidemiology of invasive mycoses in England and Wales (1990–9). Epidemiol. Infect. 126:397–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miles AA, Misra SS, Irwin JO. 1938. The estimation of the bactericidal power of the blood. J. Hyg. (London) 38:732–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morfill GE, Shimizu T, Steffes B, Schmidt H-U. 2009. Nosocomial infections—a new approach towards preventive medicine using plasmas. New J. Physics 11:10 [Google Scholar]
- 26. Radford DR, Sweet SP, Challacombe SJ, Walter JD. 1998. Adherence of Candida albicans to denture-base materials with different surface finishes. J. Dent. 26:577–583 [DOI] [PubMed] [Google Scholar]
- 27. Ramage G, Saville SP, Thomas DP, Lopez-Ribot JL. 2005. Candida biofilms: an update. Eukaryot. Cell 4:633–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rotrosen D, Calderone RA, Edwards JE., Jr 1986. Adherence of Candida species to host tissues and plastic surfaces. Rev. Infect. Dis. 8:73–85 [DOI] [PubMed] [Google Scholar]
- 29. Shimizu T, Zimmermann JL, Morfill GE. 2011. The bactericidal effect of surface micro-discharge plasma under different ambient conditions. New J. Physics 13:1–7 [Google Scholar]
- 30. Theraud M, Bedouin Y, Guiguen C, Gangneux JP. 2004. Efficacy of antiseptics and disinfectants on clinical and environmental yeast isolates in planktonic and biofilm conditions. J. Med. Microbiol. 53:1013–1018 [DOI] [PubMed] [Google Scholar]
- 31. Uppuluri P, Chaturvedi AK, Lopez-Ribot JL. 2009. Design of a simple model of Candida albicans biofilms formed under conditions of flow: development, architecture, and drug resistance. Mycopathologia 168:101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Uppuluri P, Pierce CG, Lopez-Ribot JL. 2009. Candida albicans biofilm formation and its clinical consequences. Future Microbiol. 4:1235–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vazquez JA, et al. 1998. Nosocomial Candida glabrata colonization: an epidemiologic study. J. Clin. Microbiol. 36:421–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zimmermann J, et al. 2011. Effects of cold atmospheric plasmas on adenoviruses in solution. J. Phys. D 44:1–9 [Google Scholar]