Abstract
Five essential oils (EOs), namely, clove oil (CLO), eucalyptus oil (EUO), garlic oil (GAO), origanum oil (ORO), and peppermint oil (PEO), were tested in vitro at 3 different doses (0.25, 0.50, and 1.0 g/liter) for their effect on methane production, fermentation, and select groups of ruminal microbes, including total bacteria, cellulolytic bacteria, archaea, and protozoa. All the EOs significantly reduced methane production with increasing doses, with reductions by 34.4%, 17.6%, 42.3%, 87%, and 25.7% for CLO, EUO, GAO, ORO, and PEO, respectively, at 1.0 g/liter compared with the control. However, apparent degradability of dry matter and neutral detergent fiber also decreased linearly with increasing doses by all EOs except GAO. The concentrations of total volatile fatty acids were not affected by GAO, EUO, or PEO but altered linearly and quadratically by CLO and ORO, respectively. All the EOs also differed in altering the molar proportions of acetate, propionate, and butyrate. As determined by quantitative real-time PCR, all the EOs decreased the abundance of archaea, protozoa, and major cellulolytic bacteria (i.e., Fibrobacter succinogenes, Ruminococcus flavefaciens, and R. albus) linearly with increasing EO doses. On the basis of denaturing gradient gel electrophoresis analysis, different EOs changed the composition of both archaeal and bacterial communities to different extents. The Shannon-Wiener diversity index (H′) was reduced for archaea by all EOs in a dose-dependent manner but increased for bacteria at low and medium doses (0.25 and 0.50 g/liter) for all EOs except ORO. Due to the adverse effects on feed digestion and fermentation at high doses, a single EO may not effectively and practically mitigate methane emission from ruminants unless used at low doses in combinations with other antimethanogenic compounds.
INTRODUCTION
Livestock contributes about 18% to the global anthropogenic greenhouse gas (GHG) emissions, accounting for about 37% of the total anthropogenic methane and 65% of global anthropogenic nitrous oxide (22). Of the total anthropogenic methane (5.9 × 109 metric tons CO2 equivalent), approximately 30% is contributed by enteric methane emission, mostly from fermentation of feeds in the rumen (22). Besides, methane production in the rumen represents a significant feed energy loss (2 to 12%), depending upon types of diets (26). Concerns over the substantial contribution to global warming, climate change, and feed energy loss have stimulated a plethora of scientific studies aimed at lowering enteric methane production by ruminants using different mitigation options (21, 39).
In recent studies, a variety of compounds and substances have been evaluated for their ability to reduce methane production in the rumen (4, 9, 39), including nitrate and organic nitro compounds (3, 58). However, most of them have inconsistent efficacy or are toxic to host animals at concentrations that are effective in mitigating methane production (4, 9, 30, 39). Additionally, concerns also arose over potential toxicity to the final products. Therefore, feed additives of plant origin are desired. In recent years, essential oils (EOs) have been widely evaluated as feed additives in improving microbial metabolism in the rumen, such as moderation of starch and protein degradation, increasing efficiency of fermentation, and inhibition of methanogenesis (12, 32, 40). Supplementation of EOs to dairy cows has also resulted in increased milk yield and feed efficiency (23, 27, 49). Although EOs have shown some promise in inhibiting the methanogenic archaea and methane production in the rumen (12, 37), adverse effects on fiber digestion and fermentation have also been reported, with the magnitude of these adverse effects varying depending upon the types and doses of EOs and diet composition (12, 28). However, no systematic comparative studies on EOs have been reported. In addition, the effects of EOs on the rumen microbiome and important microbial populations have not been comparatively evaluated. This study investigated the effects of five types of EOs on methane production; fermentation characteristics; abundance of total bacteria, archaea, protozoa, and cellulolytic bacteria; and diversity of bacteria and archaea using an in vitro model.
MATERIALS AND METHODS
Essential oils.
Five different types of EOs, i.e., clove oil (CLO; from Eugenia spp.), eucalyptus oil (EUO; from Eucalyptus globulus), garlic oil (GAO; from Allium sativum L.), origanum oil (ORO; from Thymus capitatus L. Hoffmanns & Link), and peppermint oil (PEO; from Mentha piperita L.), were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO) and used in this study. Each of the EOs was used at 3 doses: low at 0.10 g/liter, medium at 0.25 g/liter, and high at 1.0 g/liter of in vitro fermentation medium. A control without EOs was included in parallel.
Ruminal inoculum and in vitro incubations.
The ruminal inoculum for the in vitro incubations was collected from two fistulated lactating Jersey cows at 9 h after the morning feeding. The total mixed ration (TMR) of the cows was composed (percent dry matter [DM]) of corn silage (33%), a mixture of alfalfa and grass hay (8.5%), and a concentrate mixture (58.5%). The cows were fed the TMR (17.5 kg) twice a day at 6 a.m. and 6 p.m. From each of the two cows, about 500 ml of rumen sample was collected into a 500-ml bottle, leaving no headspace in the sample bottles. The samples were brought to the laboratory within 10 min (about 3 km away) and then placed into an anaerobic chamber containing N2 (95%), H2 (3%), and CO2 (2%). The rumen fluid was obtained after squeezing the rumen content through 4 layers of cheesecloth. Equal volumes of the rumen fluid collected from each of the cows were combined as the inoculum.
The in vitro incubation was carried out in 120-ml serum bottles in triplicate for each dose of each EO and the control. Ground alfalfa hay and a dairy concentrate mixture (consisting mainly of ground corn [33.2%], soybean meal [14.2%]), AminoPlus (Ag Processing Inc., USA) [15.5%], distillers' grains [19.8%], wheat middlings [11.3%]), in a ratio of 50:50 were used as the substrate. The in vitro buffered medium was prepared anaerobically (33). Inside the anaerobic chamber, 30 ml of this anaerobic medium and 10 ml of the rumen fluid inoculum were dispensed to each of the serum bottles containing 400 mg of the ground substrate. After they were sealed with butyl rubbers plus crimped aluminum seals, the serum bottles were incubated at 39°C for 24 h in a water bath with occasional manual shaking.
Sampling and analyses of biogas and VFAs.
At the end of the 24 h of incubation, gas pressure in the culture bottles was measured using a manometer (Traceable; Fisher Scientific) to determine total gas production. Then, 10 ml of headspace gas was collected into a tube filled with distilled water by displacement. The liquid samples (1 ml) were individually collected into microcentrifuge tubes and preserved at −20°C for microbial analysis. The pH values of the in vitro cultures were recorded using a pH meter. The remaining culture volume was filtered through filter bags (Ankom Technology) to determine the degradability of the added substrate. The filtrates were sampled in microcentrifuge tubes for volatile fatty acid (VFA) and ammonia analyses. If not analyzed immediately, all the samples were stored at −20°C until further processing.
The concentrations of methane in the gas samples were determined using a gas chromatograph (HP 5890 series; Agilent Technologies) equipped with a thermal conductivity detector and an HP-PLOT Q capillary column as described previously (58). The VFA concentrations in the cultures were also analyzed by a gas chromatograph (GC; HP 5890 series; Agilent Technologies) fitted with a flame ionization detector and a Chromosorb W AW packed-glass column (Supelco) (58). The concentrations of ammonia in the fermentation cultures were measured by a calorimetric method (16). The apparent DM degradability of the substrate was determined after drying the residues collected in the fiber bags and the initial substrate at 105°C in a hot air oven for 24 h (24). The residues in the fiber bags and the initial substrate were also analyzed for neutral detergent fiber (NDF) content (51), and true and NDF degradability were then calculated (7).
DNA extraction.
Metagenomic DNA was extracted from 0.5 ml of each homogenized sample using the repeated bead beating (on a beadbeater; BioSpec Products, Bartlesville, OK) and column purification (using a QIAamp DNA stool minikit; Qiagen, Valencia, CA) method (56). The DNA quality was evaluated using agarose gel (1%) electrophoresis, and DNA yield was quantified using a Quant-iT double-stranded DNA (dsDNA) broad-range assay kit (Invitrogen, Carlsbad, CA) and a Stratagene Mx3000p machine (La Jolla, CA). The DNA samples were stored at −20°C until analysis.
qPCR analyses.
The population sizes of total bacteria were quantified using a TaqMan assay, while those of archaea and major cultured cellulolytic bacterial species (i.e., Fibrobacter succinogenes, Ruminococcus albus, and R. flavefaciens) were quantified using SYBR-based quantitative real-time PCR (qPCR), respective specific primers, and a Stratagene Mx3000p machine (La Jolla, CA) as reported previously (47, 58). The protozoan populations were quantified using protozoan-specific primers as described previously (48). To minimize potential bias, instead of a single strain, sample-derived qPCR standards were prepared using the respective specific PCR primer set and a composite metagenomic DNA sample that were prepared by pooling equal amounts of all the metagenomic DNA samples (55). The standards were purified using a PCR purification kit (Qiagen) and quantified using a Quant-iT dsDNA broad-range assay kit (Invitrogen). For each of the standards, 16S rRNA (rrs) gene copy number concentrations were calculated on the basis of the length of the PCR products and the mass concentrations. Tenfold serial dilutions were prepared in Tris-EDTA (TE) buffer prior to qPCR assays. To eliminate the effect from potential primer dimers in the SYBR-based qPCR assays, the fluorescence signal was acquired at 86°C, at which primer dimers were completely denatured and thus not detected, and used in quantifying populations of the microbial groups or species (55). The qPCR assay for each species or group was performed in triplicate for both the standards and the metagenomic DNA samples using the same master mix and the same qPCR plate. The absolute abundances were expressed as number of rrs gene copies/ml of culture samples.
DGGE.
The microbiome in each of the cultures was examined using denaturing gradient gel electrophoresis (DGGE) as described previously (54, 56). Briefly, the V3 region of the 16S rRNA gene of bacteria and archaea was amplified using bacterium- and archaeon-specific primers with a 40-bp GC clamp attached to the 5′ end of the forward primers. To eliminate artifactual double DGGE bands, a final elongation step at 72°C for 30 min was included at the end of the PCR (25). The PCR products were confirmed using agarose (1.2%) gels and resolved using polyacrylamide gels (8%) with a denaturant gradient of between 40% and 60% (54, 57). Following staining with SYBR green I (Invitrogen), the images were captured using a FlourChem imaging system (Alpha Innotech, San Leandro, CA) and analyzed with BioNumerics software (Applied Maths, Inc., TX). A principal component analysis (PCA) based on the intensity and migration of the bands was performed using the PC-ORD program (31) as described previously (19). Biodiversity indices were calculated for each of the cultures as follows (57): (i) the Shannon-Wiener index, H′ = −∑(ni/N) ln(ni/N) (43), (ii) the Simpson dominance index, λ = ∑(ni/N)2 (44), and (iii) the evenness index, e = H′/lnS (41), where S is the total number of bands, ni is the intensity of ith band, and N is the sum of the intensity of all bands of each sample.
Statistical analysis.
The data on rumen fermentation characteristics, sizes of the populations quantified, and microbial diversity indices were analyzed using the mixed model procedure of SAS (44) in a 5 (EOs)-by-4 (doses) factorial design. Because dose × EO interactions were significant for most of the parameters, data were then analyzed among doses of each EO to test the dose effects. Orthogonal polynomial contrasts were used to examine the linear, quadratic, and cubic effects of the increasing doses of EOs. The IML procedure of the SAS program (42) was used to correct the contrast coefficients of orthogonal polynomial because of unequal intervals of EO doses.
RESULTS
Total gas and methane production, degradability, pH, and ammonia concentrations.
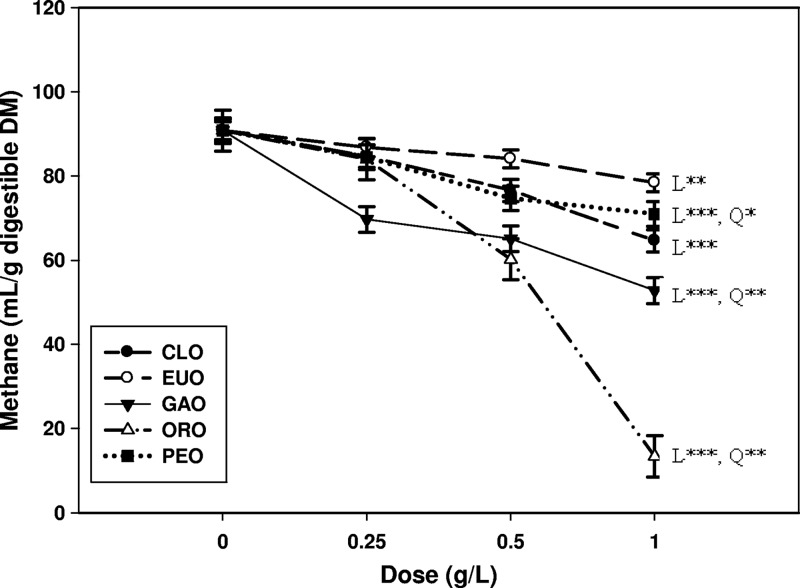
Total gas and methane production by the ruminal cultures decreased linearly with increasing doses of all EOs (Table 1). The most pronounced inhibition on methane production was noted for ORO, with the inhibition magnitude decreasing in the following order at the high dose (1.0 g/liter): ORO > GAO > CLO > PEO > EUO. Methane production in terms of ml/g digestible DM was also decreased by all EOs, but the dose-responses differed, with CLO, EUO, and PEO exhibiting a linear effect and GAO and ORO exhibiting linear and quadratic effects (Fig. 1). Similarly, apparent substrate DM degradability was decreased linearly with increasing EO doses (Table 1). However, true DM and NDF degradabilities were not (P > 0.10) affected by EOs, except for linear decreases in NDF degradability by ORO. Inclusion of CLO or ORO in the cultures decreased ammonia concentrations, whereas other EOs did not affect ammonia concentrations in the cultures (Table 1). The pH of the cultures was, in general, increased linearly when each of the EOs was added (Table 1).
Table 1.
Effects of different doses of essential oils on gas and methane production, degradability of feeds, pH, and ammonia concentration in vitroa
| Essential oil and dose (g/liter) | Vol (ml) |
Degradability (%) |
Ammonia concn (mM) | pH | |||
|---|---|---|---|---|---|---|---|
| Total gas | Methane | Apparent DM | NDF | True DM | |||
| Control, 0 | 59.8 | 26.1 | 68.7 | 33.9 | 72.2 | 28.4 | 5.47 |
| CLO | |||||||
| 0.25 | 54.4 | 23.2 | 66.2 | 30.8 | 71.0 | 31.3 | 5.49 |
| 0.50 | 57.0 | 21.5 | 66.3 | 31.9 | 71.4 | 23.3 | 5.52 |
| 1.00 | 49.3 | 17.1 | 63.7 | 27.0 | 69.6 | 20.0 | 5.56 |
| SEM | 1.25 | 0.88 | 0.44 | 1.46 | 0.91 | 1.84 | 0.006 |
| Contrast | L,*** C** | L*** | L,*** C* | NS | NS | L,*** C** | L*** |
| EUO | |||||||
| 0.25 | 59.8 | 25.4 | 69.8 | 36.7 | 73.3 | 27.9 | 5.46 |
| 0.50 | 57.5 | 24.0 | 68.2 | 32.8 | 71.8 | 27.1 | 5.48 |
| 1.00 | 53.6 | 21.5 | 66.4 | 27.2 | 69.6 | 27.8 | 5.52 |
| SEM | 0.75 | 0.74 | 0.46 | 2.13 | 0.81 | 2.27 | 0.005 |
| Contrast | L*** | L*** | L,*** Q,* C* | NS | NS | NS | L,*** Q,** C** |
| GAO | |||||||
| 0.25 | 56.8 | 20.1 | 69.8 | 37.0 | 73.4 | 26.2 | 5.49 |
| 0.50 | 57.1 | 18.7 | 69.1 | 36.9 | 73.4 | 27.7 | 5.51 |
| 1.00 | 53.5 | 14.9 | 68.0 | 33.0 | 71.9 | 27.7 | 5.54 |
| SEM | 0.72 | 0.91 | 0.41 | 1.32 | 0.51 | 2.19 | 0.005 |
| Contrast | L,*** C* | L*** | NS | NS | NS | NS | L*** |
| ORO | |||||||
| 0.25 | 53.0 | 22.8 | 65.5 | 27.4 | 69.7 | 24.7 | 5.51 |
| 0.50 | 45.8 | 16.0 | 63.9 | 25.1 | 68.8 | 18.6 | 5.58 |
| 1.00 | 12.3 | 3.4 | 62.1 | 25.2 | 68.8 | 10.8 | 6.04 |
| SEM | 1.54 | 1.04 | 0.27 | 1.68 | 0.65 | 3.14 | 0.012 |
| Contrast | L,*** Q*** | L*** | L,*** Q*** | L,*** Q** | L,*** Q** | L** | L,*** Q*** |
| PEO | |||||||
| 0.25 | 57.0 | 23.9 | 68.4 | 32.3 | 71.6 | 27.5 | 5.49 |
| 0.50 | 54.5 | 20.8 | 67.0 | 29.6 | 70.5 | 23.8 | 5.53 |
| 1.00 | 53.0 | 19.4 | 65.9 | 32.1 | 71.5 | 23.7 | 5.58 |
| SEM | 1.04 | 0.64 | 0.22 | 1.55 | 0.61 | 2.30 | 0.005 |
| Contrast | L*** | L,*** Q** | L,*** C** | NS | NS | NS | L*** |
Data were analyzed using dose levels of 0, 0.25, 0.50, and 1.0 g/liter for each essential oil. CLO, clove oil; EUO, eucalyptus oil; GAO, garlic oil; ORO, origanum oil; PEO, peppermint oil; NS, not significant. Only significant (***, P ≤ 0.01; **, P ≤ 0.05; *, P ≤ 0.10) linear (L), quadratic (Q), and cubic (C) effects are shown.
Fig 1.
Effects of different doses of essential oils on in vitro methane production (ml/g digested DM). CLO, clove oil; EUO, eucalyptus oil; GAO, garlic oil; ORO, origanum oil; PEO, peppermint oil. Significant (***, P < 0.01; **, P < 0.05; *, P < 0.10) linear (L) and quadratic (Q) effects of essential oils are shown.
Volatile fatty acid concentrations.
The EO supplementations showed mixed effects on the concentrations of total VFAs in the ruminal cultures (Table 2). The VFA concentrations were decreased by CLO and ORO but were not affected by GAO or PEO. Total VFA concentrations tended to increase (P < 0.10) linearly with increasing doses of EUO. The molar proportion of acetate was not affected by CLO but was decreased by EUO and GAO. In contrast, the molar proportion of acetate was increased by ORO and PEO. Supplementation with GAO increased the molar proportion of propionate in the cultures, while the other EOs decreased that measurement. The acetate-to-propionate (A/P) ratio was increased by CLO, ORO, and PEO, but to different extents. However, GAO and EUO decreased the A/P ratio. The molar proportion of butyrate increased with increased doses for all EOs except ORO, which showed mixed effects (Table 2). The dose-response of the molar proportion of isovalerate differed among the EOs (Table 2): a linear increase for EUO and PEO, a linear decrease for ORO, quadratic and cubic effects for CLO, and no effect for GAO. The molar percentage of valerate was lowered by EUO and GAO but not affected by CLO, while ORO and PEO had nonlinear effects.
Table 2.
Effects of different doses of essential oils on in vitro VFA production from fermentation of feeds incubated with rumen fluid from cattlea
| Essential oil and dose (g/liter) | Total VFA (mM) | Molar proportion of VFA (mol/100 mol) |
A/P | |||||
|---|---|---|---|---|---|---|---|---|
| Acetate | Propionate | Isobutyrate | Butyrate | Isovalerate | Valerate | |||
| Control, 0 | 99.5 | 55.5 | 25.0 | 1.60 | 13.2 | 2.43 | 2.29 | 2.22 |
| CLO | ||||||||
| 0.25 | 97.3 | 55.6 | 24.9 | 1.40 | 13.6 | 2.31 | 2.28 | 2.24 |
| 0.50 | 101.0 | 54.9 | 24.1 | 1.66 | 14.3 | 2.83 | 2.16 | 2.28 |
| 1.00 | 91.9 | 55.7 | 22.5 | 1.45 | 15.8 | 2.13 | 2.36 | 2.47 |
| SEM | 1.60 | 0.35 | 0.28 | 0.12 | 0.34 | 0.13 | 0.11 | 0.026 |
| Contrast | L,*** Q,* C* | NS | L*** | NS | L*** | Q,** C** | NS | L,*** Q** |
| EUO | ||||||||
| 0.25 | 105.3 | 54.0 | 24.7 | 1.68 | 14.8 | 2.53 | 2.29 | 2.19 |
| 0.50 | 107.6 | 52.4 | 24.1 | 1.87 | 16.6 | 2.89 | 2.11 | 2.18 |
| 1.00 | 106.8 | 50.3 | 23.5 | 1.74 | 19.4 | 2.92 | 2.07 | 2.14 |
| SEM | 2.68 | 0.47 | 0.18 | 0.13 | 0.31 | 0.14 | 0.049 | 0.024 |
| Contrast | L* | L*** | L*** | NS | L*** | L** | L*** | L** |
| GAO | ||||||||
| 0.25 | 106.3 | 52.8 | 25.3 | 1.88 | 14.6 | 3.06 | 2.34 | 2.09 |
| 0.50 | 100.8 | 53.0 | 25.3 | 1.76 | 14.6 | 2.89 | 2.38 | 2.10 |
| 1.00 | 101.3 | 50.7 | 26.0 | 1.68 | 16.7 | 2.89 | 2.07 | 1.96 |
| SEM | 2.40 | 0.61 | 0.20 | 0.13 | 0.33 | 0.20 | 0.088 | 0.035 |
| Contrast | NS | L*** | L*** | NS | L*** | NS | L* | L*** |
| ORO | ||||||||
| 0.25 | 98.3 | 54.3 | 24.5 | 1.62 | 14.8 | 2.74 | 2.07 | 2.22 |
| 0.50 | 90.0 | 58.1 | 18.3 | 1.49 | 17.3 | 2.09 | 2.79 | 3.19 |
| 1.00 | 61.0 | 59.3 | 20.8 | 2.17 | 14.2 | 1.71 | 1.85 | 2.85 |
| SEM | 2.75 | 0.57 | 0.30 | 0.17 | 0.32 | 0.23 | 0.13 | 0.068 |
| Contrast | L,*** Q*** | L,*** C*** | L,*** Q,*** C*** | L** | L,** Q,*** C*** | L** | L,* Q,*** C*** | L,*** Q,*** C*** |
| PEO | ||||||||
| 0.25 | 103.4 | 53.1 | 25.5 | 1.66 | 15.0 | 2.82 | 1.95 | 2.09 |
| 0.50 | 105.7 | 53.9 | 24.5 | 1.46 | 14.5 | 3.07 | 2.50 | 2.22 |
| 1.00 | 103.3 | 56.0 | 21.5 | 1.91 | 15.0 | 3.11 | 2.42 | 2.60 |
| SEM | 3.04 | 0.71 | 0.41 | 0.14 | 0.40 | 0.16 | 0.17 | 0.062 |
| Contrast | NS | Q** | L,*** Q*** | NS | L** | L*** | C* | L** Q** |
Data were analyzed using dose levels of 0, 0.25, 0.50, and 1.0 g/liter for each essential oil. CLO, clove oil; EUO, eucalyptus oil; GAO, garlic oil; ORO, origanum oil; PEO, peppermint oil; A/P, acetate to propionate ratio; NS, not significant. Only significant (***, P ≤ 0.01; **, P ≤ 0.05; *, P ≤ 0.10) linear (L), quadratic (Q), and cubic (C) effects are shown.
Population dynamics of bacteria, methanogens, and protozoa.
In accordance with methane production, archaeal populations were significantly decreased by all the EOs, but to different extents; i.e., ORO showed the greatest reduction, followed by PEO, GAO, CLO, and EUO in decreasing order of magnitude (Table 3). Total bacterial populations were linearly decreased with increasing doses of CLO, ORO, and PEO but were not influenced by GAO or EUO. However, all the EOs exhibited adverse effect on all the three rumen cellulolytic bacterial populations analyzed. The populations of F. succinogenes, R. flavefaciens, and R. albus were significantly reduced by all EOs, but to different magnitudes, depending on the doses and species. The F. succinogenes population suffered from more inhibition than the populations of R. flavefaciens and R. albus for all EOs at any of EO doses. The greatest inhibition to F. succinogenes was noted for ORO and PEO (by about 4 log units), followed by CLO and EUO (by about 3 log units), and GAO (by 2 log units). The inhibition to R. flavefaciens was the greatest for CLO (by nearly 3 log units), followed by PEO and ORO (by >2 log units), GAO (more than 1 log unit), and EUO (by <1 log unit). The abundance of the R. albus population was reduced in the following order: ORO > CLO > GAO > PEO > EUO. All EOs also exhibited antiprotozoal activity, though to different magnitudes, with ORO and PEO decreasing protozoa the greatest (by nearly 3 log units), followed by CLO (by 2 log units) and EUO and GAO (by <1 log unit).
Table 3.
Effects of different doses of essential oils on abundance of rumen archaea, cellulolytic bacteria, and protozoa quantified by real-time PCRa
| Essential oil and dose (g/liter) | Abundance (log10 no. of copies of rrs gene/ml) |
|||||
|---|---|---|---|---|---|---|
| Archaea | Total bacteria | F. succinogenes | R. flavefaciens | R. albus | Protozoa | |
| Control, 0 | 7.31 | 11.46 | 7.41 | 7.62 | 6.09 | 8.27 |
| CLO | ||||||
| 0.25 | 6.82 | 11.41 | 5.95 | 7.41 | 6.14 | 8.45 |
| 0.50 | 7.08 | 11.39 | 4.37 | 5.81 | 4.79 | 7.77 |
| 1.00 | 6.41 | 10.94 | 4.14 | 4.80 | 4.29 | 6.03 |
| SEM | 0.094 | 0.080 | 0.283 | 0.158 | 0.079 | 0.053 |
| Contrast | L,*** C*** | L*** | L,*** Q*** | L,*** C** | L,*** C*** | L,*** Q,*** C*** |
| EUO | ||||||
| 0.25 | 7.22 | 11.41 | 7.05 | 7.65 | 6.19 | 8.42 |
| 0.50 | 7.28 | 11.63 | 6.20 | 7.61 | 6.01 | 8.08 |
| 1.00 | 6.84 | 11.66 | 4.47 | 7.08 | 5.72 | 7.63 |
| SEM | 0.059 | 0.067 | 0.110 | 0.070 | 0.085 | 0.124 |
| Contrast | L,*** Q** | NS | L,*** Q** | L,*** Q** | L*** | L*** |
| GAO | ||||||
| 0.25 | 6.67 | 11.52 | 6.50 | 6.88 | 5.91 | 8.25 |
| 0.50 | 6.25 | 11.24 | 6.10 | 6.85 | 6.04 | 8.56 |
| 1.00 | 6.10 | 11.24 | 5.37 | 5.91 | 5.21 | 7.62 |
| SEM | 0.079 | 0.116 | 0.055 | 0.080 | 0.124 | 0.114 |
| Contrast | L,*** Q** | NS | L,*** Q,*** C** | L,*** C*** | L,*** Q* | L,*** Q*** |
| ORO | ||||||
| 0.25 | 6.18 | 11.18 | 4.95 | 5.91 | 5.29 | 7.34 |
| 0.50 | 5.81 | 10.67 | 3.92 | 5.10 | 4.23 | 5.60 |
| 1.00 | 4.50 | 9.78 | 3.59 | 5.03 | 4.02 | 5.36 |
| SEM | 0.046 | 0.126 | 0.221 | 0.134 | 0.207 | 0.173 |
| Contrast | L,*** Q,*** C*** | L*** | L,*** Q*** | L,*** Q*** | L,*** Q** | L,*** Q,*** C** |
| PEO | ||||||
| 0.25 | 6.21 | 11.51 | 6.02 | 6.83 | 5.86 | 8.13 |
| 0.50 | 5.83 | 11.03 | 4.13 | 5.46 | 4.69 | 7.30 |
| 1.00 | 5.78 | 10.96 | 3.63 | 5.32 | 4.51 | 5.53 |
| SEM | 0.061 | 0.085 | 0.078 | 0.068 | 0.110 | 0.097 |
| Contrast | L,*** Q,*** C*** | L,*** C** | L,*** Q,*** C*** | L,*** Q,*** C*** | L,*** Q,*** C*** | L,*** Q,*** C* |
Data were analyzed using dose levels of 0, 0.25, 0.50, and 1.0 g/liter for each essential oil. CLO, clove oil; EUO, eucalyptus oil; GAO, garlic oil; ORO, origanum oil; PEO, peppermint oil; NS, not significant. Only significant (***, P ≤ 0.01; **, P ≤ 0.05; *, P ≤ 0.10) linear (L), quadratic (Q), and cubic (C) effects are shown.
Bacteria and archaeal biodiversity revealed by DGGE profiles.
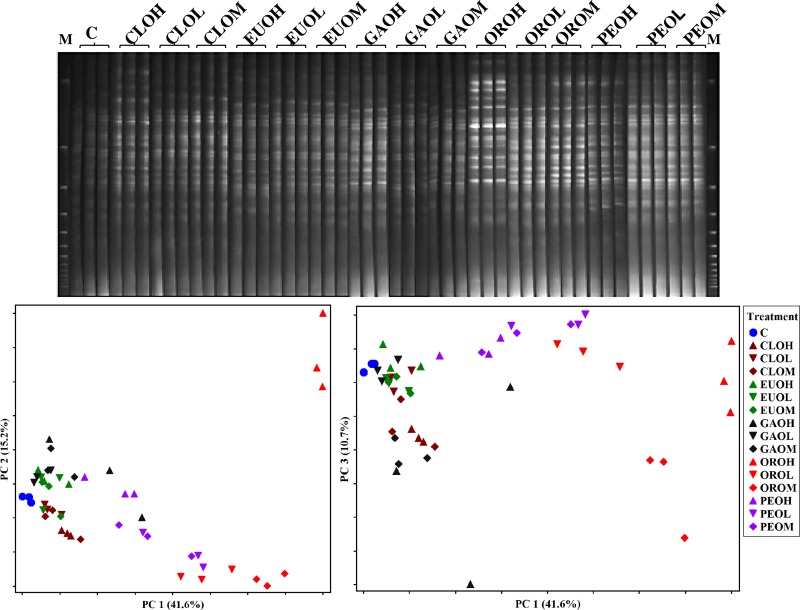
The DGGE banding patterns obtained for bacteria showed many bands (Fig. 2). A few intense bacterial bands were observed in the cultures receiving ORO and PEO at medium and high doses. The PCA of the DGGE profiles indicated that inclusion of EOs in the ruminal cultures resulted in apparently different microbial communities (Fig. 2). The first principal component (PC1), which explained 41.6% of the variability, showed that ORO and PEO changed the bacterial communities differently than the rest of the EOs and the control. The doses of ORO and PEO also separated the bacterial communities along PC1. The second principal component (PC2), which explained 15.2% of the variations, indicated that the bacterial community of the ORO culture at the high dose differed from that of the other EO cultures. The third principal component (PC3) (7.1% variations) separated CLO (medium and high doses) from GAO and ORO (medium dose).
Fig 2.
DGGE profile of total bacteria (top) and PCA plots of the DGGE profiles (bottom). The first three letters stand for the EO: CLO, clove oil; EUO, eucalyptus oil; GAO, garlic oil; ORO, origanum oil; PEO, peppermint oil. The fourth letter represents the dose of EO: H, high (1.0 g/liter); M, medium (0.5 g/liter); and L, low (0.25 g/liter). All the essential oils and the control (C) were used in triplicate. Lanes M, molecular size marker.
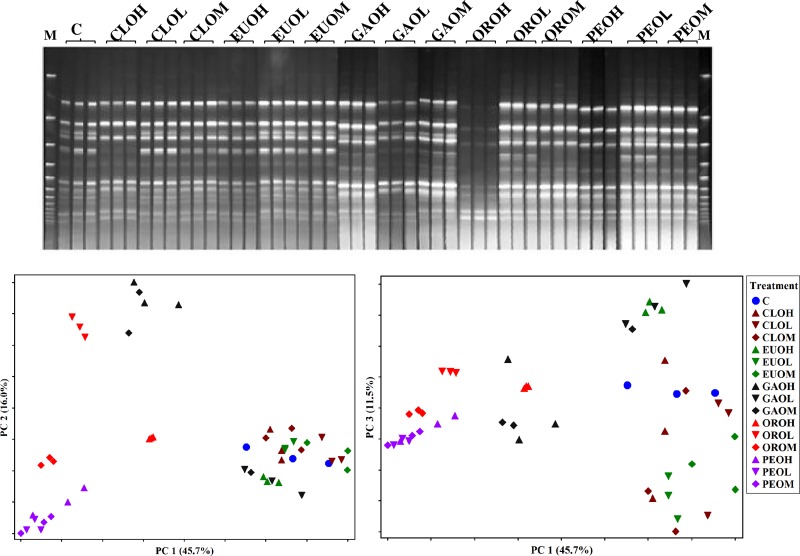
The archaeal community appeared to have only a small number of dominant groups (Fig. 3). Several DGGE bands weakened or disappeared in the cultures receiving ORO (medium and high doses), CLO (high dose), PEO (medium and high doses), and GAO (all doses). Accounting for 45.7% of the total variations, the PC1 showed that the archaeal communities in the ORO, PEO, and GAO (high-dose) cultures were distinctly different from those in the other EO cultures or the control. The PC2 explained 16.0% of the total variations and demonstrated that GAO (high dose) and ORO (low dose) changed the archaeal communities compared to the control. The PC3 (11.5% of total variations) separated archaeal communities of EUO (all doses) and GAO (low doses) from the control.
Fig 3.
DGGE profile of archaea (top) and PCA plots of the DGGE profile (bottom). The first three letters stand for the EO: CLO, clove oil; EUO, eucalyptus oil; GAO, garlic oil; ORO, origanum oil; PEO, peppermint oil. The fourth letter represents the dose of EO: H, high (1.0 g/liter); M, medium (0.5 g/liter); and L, low (0.25 g/liter). All the essential oils and the control (C) were used in triplicate. Lanes M, molecular size marker.
The Shannon-Wiener diversity index (H′) of bacteria was changed quadratically by CLO, EUO, and GAO or linearly decreased by ORO, whereas PEO did not alter this diversity index (Table 4). It was observed that the H′ of bacteria was greater at low and medium doses than at the control and the high doses for CLO, EUO, and GAO. The effects of EOs on the evenness of bacteria were mixed: quadratic responses for CLO, ORO, and PEO, a linear decrease for GAO, and a cubic one for EUO. The dominance index of bacteria was not affected by CLO or PEO but was changed quadratically by GAO and EUO and increased linearly by ORO with increasing doses. The H′ of the archaeal communities was significantly affected by CLO and ORO (linearly), EUO (quadratically), and GAO and PEO (linearly and quadratically). The highest H′ was noted for EUO at the medium dose, while the lowest H′ was noted for ORO at the high dose. The evenness scores were decreased linearly by GAO and ORO, changed quadratically by EUO, and tended (P < 0.10) to change by EUO and PEO. In contrast, all EOs increased the dominance index of archaeal communities, with the exception of a decreased dominance index for EUO at low and medium doses. This index was generally increased linearly (CLO and GAO) or quadratically (PEO and ORO) with increased EO doses.
Table 4.
Effects of different doses of essential oils on biodiversity indices from DGGE fingerprints of the rumen bacterial and archaeal communities in vitroa
| Essential oil and dose (g/liter) | Bacteria |
Archaea |
||||
|---|---|---|---|---|---|---|
| Shannon index | Evenness index | Dominance index | Shannon index | Evenness index | Dominance index | |
| Control, 0 | 2.76 | 0.964 | 0.069 | 2.20 | 0.895 | 0.130 |
| CLO | ||||||
| 0.25 | 2.87 | 0.942 | 0.065 | 2.16 | 0.869 | 0.137 |
| 0.50 | 2.85 | 0.933 | 0.066 | 2.05 | 0.855 | 0.160 |
| 1.00 | 2.79 | 0.952 | 0.068 | 1.96 | 0.879 | 0.162 |
| SEM | 0.041 | 0.0051 | 0.0029 | 0.037 | 0.010 | 0.0076 |
| Contrast | Q* | Q*** | NS | L*** | Q* | L*** |
| EUO | ||||||
| 0.25 | 2.86 | 0.948 | 0.063 | 2.30 | 0.917 | 0.113 |
| 0.50 | 2.91 | 0.967 | 0.058 | 2.33 | 0.901 | 0.113 |
| 1.00 | 2.84 | 0.960 | 0.063 | 2.12 | 0.844 | 0.150 |
| SEM | 0.039 | 0.0048 | 0.0026 | 0.034 | 0.007 | 0.0053 |
| Contrast | Q** | C* | Q** | L,* Q*** | L,*** Q*** | L,*** Q*** |
| GAO | ||||||
| 0.25 | 3.01 | 0.957 | 0.054 | 1.92 | 0.921 | 0.162 |
| 0.50 | 2.87 | 0.943 | 0.063 | 1.92 | 0.872 | 0.167 |
| 1.00 | 2.72 | 0.920 | 0.074 | 1.92 | 0.836 | 0.172 |
| SEM | 0.039 | 0.0041 | 0.0026 | 0.029 | 0.013 | 0.0057 |
| Contrast | L,* Q,*** C*** | L*** | L,** Q,*** C** | L,*** Q,*** C** | L,*** C* | L,*** Q** |
| ORO | ||||||
| 0.25 | 2.73 | 0.928 | 0.073 | 2.04 | 0.850 | 0.158 |
| 0.50 | 2.75 | 0.918 | 0.073 | 1.79 | 0.863 | 0.188 |
| 1.00 | 2.58 | 0.932 | 0.085 | 1.14 | 0.708 | 0.398 |
| SEM | 0.035 | 0.0053 | 0.0025 | 0.036 | 0.021 | 0.013 |
| Contrast | L*** | L,*** Q*** | L*** | L,*** Q** | L,*** Q* | L,*** Q*** |
| PEO | ||||||
| 0.25 | 2.82 | 0.946 | 0.066 | 2.18 | 0.877 | 0.138 |
| 0.50 | 2.79 | 0.943 | 0.068 | 1.91 | 0.871 | 0.170 |
| 1.00 | 2.84 | 0.949 | 0.064 | 2.00 | 0.912 | 0.151 |
| SEM | 0.031 | 0.0038 | 0.0020 | 0.032 | 0.014 | 0.0067 |
| Contrast | NS | L,* Q*** | NS | L,*** Q,*** C*** | Q* | L,** Q,** C* |
Data were analyzed using dose levels of 0, 0.25, 0.50, and 1.0 g/liter for each essential oil. CLO, clove oil; EUO, eucalyptus oil; GAO, garlic oil; ORO, origanum oil; PEO, peppermint oil; NS, not significant. Only significant (***, P ≤ 0.01; **, P ≤ 0.05; *, P ≤ 0.10) linear (L), quadratic (Q), and cubic (C) effects are shown.
DISCUSSION
Supplementation of ruminant diets with EOs can alter microbial populations, digestion and fermentation of diets, proteolysis, and methanogenesis in the rumen (12). Essential oils produced by different plant species can vary in chemical structures and stereochemistry as well as bioactive activities (10). In this study, five EOs with different chemical structures and stereochemistries were evaluated for their efficacy to mitigate methane production by in vitro ruminal cultures. The CLO contains eugenol (phenylpropanoid), EUO contains cineole (bicyclic monoterpinoid), GAO contains alliin and allicin (organosulfur compounds), ORO contains thymol (monoterpinoid monocyclic phenol), and PEO contains menthol (monoterpinoid monocyclic nonphenol). This study demonstrated that different EOs vary in their potencies in modulating rumen microbial populations and fermentation.
Several studies have documented reduction in methane production by EOs (1, 17, 28, 52). However, the in vivo study of Beauchemin and McGinn (5) did not reveal any effect on methanogenesis. In the present study, ORO was the most potent in lowering methane production, but it also had the greatest inhibition of feed digestion. The phenolic nature of ORO might explain its high potency in inhibiting both bacteria involved in feed digestion and methanogens. As the second most antimethanogenic EO, GAO did not adversely affect feed digestibility even at the highest dose tested, corroborating findings in other studies (36, 45). All the other EOs appeared to reduce feed digestibility differently. These results corroborate several previous studies (5, 38, 53). Thus, optimal doses of EOs for practical application should be determined.
Previous in vivo studies reported mixed effects of EOs on fermentation by the rumen microbiome, as demonstrated by an increase (14, 18, 52), no change (17, 29, 36), or a significant decrease in total VFA production (28). As reflected by total VFA concentrations and VFA profiles, the present study also showed that the effects of EOs on fermentation depend on the types and doses of EOs. It is also clear that EOs can affect individual VFAs differently. In the present study, all the EOs increased the molar proportion of butyrate, supporting the findings of several previous studies (11, 15). The Gram-positive bacterium Butyrivibrio fibrisolvens is a major cultured butyrate-producing bacterium ubiquitous in the rumen (46). However, this bacterial species is very sensitive to EOs (32). Some cryptic butyrate-producing bacteria might be less sensitive to EOs and contribute to the increased molar proportion of butyrate in the rumen cultures. Alternatively, predominant butyrate-utilizing bacteria might have been inhibited by EOs or other fermentation products, such as hydrogen gas. Indeed, the concentrations of hydrogen gas increased (calculated by deducting nitrogen, methane, and carbon dioxide from total gas) in the headspace of the ruminal cultures when methane production was inhibited by EO (data not shown), and butyrate utilization and growth of butyrate-utilizing bacteria can be inhibited when hydrogen gas accumulates (2).
The results of this study showed that different EOs affected aminogenesis to different extents. Decreased ammonia concentrations in the cultures of CLO and ORO probably resulted from inhibition of deamination of amino acids, a premise substantiated by the reduced proportion of branched-chain VFAs (isobutyrate and isovalerate). Borchers (8) reported that in vitro incubation of casein in rumen fluid supplemented with thymol (1.0 g/liter) resulted in accumulation of amino acids and reduction in ammonia concentration, corroborating that deamination was inhibited. Clostridium sticklandii, Peptostreptococcus anaerobius, and C. aminophilum are known hyper-ammonia-producing bacteria (HAB), with the former two species being very sensitive to EO inhibition (32). The inability of EUO and GAO to reduce ammonia concentrations in the cultures suggests that different EOs have different potencies to inhibit proteolysis and aminogenesis. In future studies, populations of HAB need to be quantified to help understand how EOs affect aminogenesis by ruminal microbes.
Several mechanisms have been proposed to explain the antimicrobial properties of EOs, with chemical structures and physical properties being thought to be important to determine their antimicrobial potency (10, 20). The presence and relative position of a hydroxyl group in the phenolic structures of EOs (e.g., thymol and eugenol) were proposed to influence the antimicrobial potency of EOs (20, 50). The greater potency of ORO (containing a phenol) than PEO (containing a cyclohexane) shown in this study corroborates the importance of the phenolic ring to the antimicrobial activities of EOs (50).
Gram-positive bacteria are thought to be more susceptible to EOs than Gram-negative bacteria due to the lack of a protecting outer membrane surrounding the cell wall (10, 20). However, the present study did not show a reduction in the population of F. succinogenes, a Gram-negative species, than significantly less than that of R. flavefaciens or R. albus, both Gram-positive bacteria. It remains to be elucidated if EOs act on other cellular structures. The primary mode of action of EOs on archaea is also likely on the cell membranes. The action of GAO on archaea, however, might include additional mechanisms because GAO was more inhibitory to archaea than to bacteria in the ruminal cultures. It has been hypothesized that some of the organosulfur compounds present in GAO may particularly inhibit SH-containing enzymes essential to metabolic activities, especially the synthesis of specific isoprenoid side chains in archaeal lipid (11, 12).
All the EOs reduced the abundance of protozoa, though to different extents. Differences in chemical structures and properties of EOs might be among the reasons explaining these variations. In the literature, the effects of EOs on ruminal protozoa are mixed. Newbold et al. (34) and Benchaar et al. (6) reported that ruminal protozoan counts were not affected in sheep and dairy cows when fed 110 and 750 mg/day of a mixture of EOs, respectively. Clove EOs, however, decreased total protozoa, small entodiniomorphs, and holotrichs, but not large entodiniomorphs (36). Cardozo et al. (13) even observed increases in holotrichs in beef heifers when fed a mixture of cinnamaldehyde (180 mg/day) and eugnol (90 mg/day). Future studies are needed to elucidate the relationship between the structure and properties of EOs and their antiprotozoal potencies.
Although EOs have been evaluated and shown to reduce methane production by the rumen microbiome, their effects on rumen bacterial and archaeal diversity were poorly documented. In this study, all the EOs decreased the H′ of bacteria at the high dose but not at the low dose (except for ORO). The evenness index for bacteria was generally reduced with increasing dose of EO. These results implied that EOs can increase bacterial species richness in the rumen at low doses. In contrast, the H′ of archaea was decreased by all the EOs at all the doses. The decreased species evenness and increased species dominance suggest that some archaeal species might have dominated while others were diminished when exposed to EOs. This finding is consistent with a phylogenetic study where sheep fed supplemental GAO, juniper berry oil, or cinnamaldehyde had an increased abundance of methanogens related to Methanosphaera stadtmanae, Methanobrevibacter smithii, and some uncultured groups but a decreased abundance of a Methanobrevibacter ruminantium-related cluster (35). The PCA analysis showed that the species composition of both bacteria and archaea was significantly changed by all the EOs but was changed differently by different EOs. Comprehensive metagenomic studies can help detail such changes in species composition.
In summary, this study showed that EOs can significantly decrease methane production, ammonia production, and the abundance and diversity of archaea with increasing doses but that they also, especially, ORO, exert adverse effects on ruminal feed digestion and fermentation. A single EO at a low dose does not likely depress methane production in the rumen significantly. A combination of several EOs at low doses or a combination of EOs with other antimethanogenic agents may be effective in mitigating methane emission from ruminants. The findings of the present study may be used in determining the types and doses of such EO combinations in future studies.
ACKNOWLEDGMENTS
This work was supported in part by an OARDC grant (2010-007). A. K. Patra's tenure at The Ohio State University was supported by a BOYSCAST fellowship from the Department of Science and Technology, India.
Footnotes
Published ahead of print 6 April 2012
REFERENCES
- 1. Agarwal N, Shekhar C, Kumar R, Chaudhary LC, Kamra DN. 2009. Effect of peppermint (Mentha piperita) oil on in vitro methanogenesis and fermentation of feed with buffalo rumen liquor. Anim. Feed Sci. Technol. 148:321–327 [Google Scholar]
- 2. Ahring BK, Westermann P. 1987. Thermophilic anaerobic degradation of butyrate by a butyrate-utilizing bacterium in coculture and triculture with methanogenic bacteria. Appl. Environ. Microbiol. 53:429–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson RC, et al. 2010. Effect of nitroethane, dimethyl-2-nitroglutarate and 2-nitro-methyl-propionate on ruminal methane production and hydrogen balance in vitro. Bioresour. Technol. 101:5345–5349 [DOI] [PubMed] [Google Scholar]
- 4. Beauchemin KA, Kreuzer M, O'Mara F, McAllister TA. 2008. Nutritional management for enteric methane abatement: a review. Aust. J. Exp. Agric. 48:21–27 [Google Scholar]
- 5. Beauchemin KA, McGinn SM. 2006. Methane emissions from beef cattle: effects of fumaric acid, essential oil, and canola oil. J. Anim. Sci. 84:1489–1496 [DOI] [PubMed] [Google Scholar]
- 6. Benchaar C, et al. 2007. Effects of essential oils on digestion, ruminal fermentation, rumen microbial populations, milk production, and milk composition in dairy cows fed alfalfa silage or corn silage. J. Dairy Sci. 90:886–897 [DOI] [PubMed] [Google Scholar]
- 7. Blümmel M, Steingass H, Becker K. 1997. The relationship between in vitro gas production, in vitro microbial biomass yield and 15N incorporation and its implications for the prediction of voluntary feed intake of roughages. Br. J. Nutr. 77:911–921 [DOI] [PubMed] [Google Scholar]
- 8. Borchers R. 1965. Proteolytic activity of rumen fluid in vitro. J. Anim. Sci. 24:1033–1038 [Google Scholar]
- 9. Buddle BM, et al. 2011. Strategies to reduce methane emissions from farmed ruminants grazing on pasture. Vet. J. 188:11–17 [DOI] [PubMed] [Google Scholar]
- 10. Burt S. 2004. Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 94:223–253 [DOI] [PubMed] [Google Scholar]
- 11. Busquet M, Calsamiglia S, Ferret A, Kamel C. 2006. Plant extracts affect in vitro rumen microbial fermentation. J. Dairy Sci. 89:761–771 [DOI] [PubMed] [Google Scholar]
- 12. Calsamiglia S, Busquet M, Cardozo PW, Castillejos L, Ferret A. 2007. Invited review: essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 90:2580–2595 [DOI] [PubMed] [Google Scholar]
- 13. Cardozo PW, Calsamiglia S, Ferret A, Kamel C. 2006. Effects of alfalfa extract, anise, capsicum and a mixture of cinnamaldehyde and eugenol on ruminal fermentation and protein degradation in beef heifers fed a high concentrate diet. J. Anim. Sci. 84:2801–2808 [DOI] [PubMed] [Google Scholar]
- 14. Castillejos L, Calsamiglia S, Ferret A, Losa R. 2005. Effects of a specific blend of essential oil compounds and the type of diet on rumen microbial fermentation and nutrient flow from a continuous culture system. Anim. Feed Sci. Technol. 119:29–41 [Google Scholar]
- 15. Castillejos L, Calsamiglia S, Ferret A. 2006. Effect of essential oil active compounds on rumen microbial fermentation and nutrient flow in in vitro systems. J. Dairy Sci. 89:2649–2658 [DOI] [PubMed] [Google Scholar]
- 16. Chaney AL, Marbach EP. 1962. Modified reagents for determination of urea and ammonia. Clin. Chem. 8:130–132 [PubMed] [Google Scholar]
- 17. Chaves AV, et al. 2008. Effects of essential oils on proteolytic, deaminative and methanogenic activities of mixed ruminal bacteria. Can. J. Anim. Sci. 89:97–104 [Google Scholar]
- 18. Chaves AV, Stanford K, Gibson LL, McAllister TA, Benchaar C. 2008. Effects of carvacrol and cinnamaldehyde on intake, rumen fermentation, growth performance, and carcass characteristics of growing lambs. Anim. Feed Sci. Technol. 145:396–408 [Google Scholar]
- 19. Cressman MD, et al. 2010. Interactions between the microbiotas in the litter and in the intestines of commercial broiler chickens. Appl. Environ. Microbiol. 76:6572–6582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dorman HJD, Deans SG. 2000. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 88:308–316 [DOI] [PubMed] [Google Scholar]
- 21. Eckard RJ, Grainger C, de Klein CAM. 2010. Options for the abatement of methane and nitrous oxide from ruminant production: a review. Livest. Sci. 130:47–56 [Google Scholar]
- 22. FAO 2006. Livestock's long shadow. Environmental issues and options. Food and Agriculture Organization of the United Nations, Rome, Italy [Google Scholar]
- 23. Giannenas I, et al. 2011. Effects of essential oils on milk production, milk composition, and rumen microbiota in Chios dairy ewes. J. Dairy Sci. 94:5569–5577 [DOI] [PubMed] [Google Scholar]
- 24. Howitz W, Latimer GW., Jr (ed). 2007. Official methods of analysis, 18th ed, revision 2. AOAC International, Gaithersburg, MD [Google Scholar]
- 25. Janse I, Bok J, Zwart G. 2004. A simple remedy against artifactual double bands in denaturing gradient gel electrophoresis. J. Microbiol. Methods 57:279–281 [DOI] [PubMed] [Google Scholar]
- 26. Johnson KA, Johnson DA. 1995. Methane emissions from cattle. J. Anim. Sci. 73:2483–2492 [DOI] [PubMed] [Google Scholar]
- 27. Kung L, Jr, Williams P, Schmidt RJ, Hu W. 2008. A blend of essential plant oils used as an additive to alter silage fermentation or used as a feed additive for lactating dairy cows. J. Dairy Sci. 91:4793–4800 [DOI] [PubMed] [Google Scholar]
- 28. Macheboeuf D, Morgavi DP, Papon Y, Mousset JL, Arturo-Schaan M. 2008. Dose-response effects of essential oils on in vitro fermentation activity of the rumen microbial population. Anim. Feed Sci. Technol. 145:335–350 [Google Scholar]
- 29. Malecky M, Broudiscou LP, Schmidely P. 2009. Effects of two levels of monoterpene blend on rumen fermentation, terpene and nutrient flows in the duodenum and milk production in dairy goats. Anim. Feed Sci. Technol. 154:24–35 [Google Scholar]
- 30. Martin C, Morgavi DP, Doreau M. 2010. Methane mitigation in ruminants: from microbe to the farm scale. Animal 4:351–365 [DOI] [PubMed] [Google Scholar]
- 31. McCune B, Mefford MJ. 2006. PC-ORD. Multivariate analysis of ecological data, version 5.10. MjM Software, Gleneden Beach, OR [Google Scholar]
- 32. McIntosh FM, et al. 2003. Effects of essential oils on ruminal microorganisms and their protein metabolism. Appl. Environ. Microbiol. 69:5011–5014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Menke KH, Steingass H. 1988. Estimation of the energetic feed value from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 28:7–55 [Google Scholar]
- 34. Newbold CJ, McIntosh FM, Williams P, Losa R, Wallace RJ. 2004. Effects of a specific blend of essential oil compounds on rumen fermentation. Anim. Feed Sci. Technol. 114:105–112 [Google Scholar]
- 35. Ohene-Adjei S, et al. 2008. Evidence of increased diversity of methanogenic archaea with plant extract supplementation. Microb. Ecol. 56:234–242 [DOI] [PubMed] [Google Scholar]
- 36. Patra AK, Kamra DN, Agarwal N. 2010. Effects of extracts of spices on rumen methanogenesis, enzyme activities and fermentation of feeds in vitro. J. Sci. Food Agric. 90:511–520 [DOI] [PubMed] [Google Scholar]
- 37. Patra AK, Saxena J. 2010. A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in ruminants. Phytochemistry 71:1198–1222 [DOI] [PubMed] [Google Scholar]
- 38. Patra AK. 2010. Meta-analyses of effects of phytochemicals on rumen fermentation characteristics and digestibility associated with methanogenesis. J. Sci. Food Agric. 90:2700–2708 [DOI] [PubMed] [Google Scholar]
- 39. Patra AK. 2012. Enteric methane mitigation technologies for ruminant livestock: a synthesis of current research and future directions. Environ. Monitor. Assess. 184:1929–1952 [DOI] [PubMed] [Google Scholar]
- 40. Patra AK. 2011. Effects of essential oils on rumen fermentation, microbial ecology and ruminant production. Asian J. Anim. Vet. Adv. 6:416–428 [Google Scholar]
- 41. Pielou EC. 1966. The measurement of diversity in different types of biological collections. J. Theor. Biol. 13:131–144 [Google Scholar]
- 42. SAS 2001. Statistical Analysis Systems software, version 8. SAS Institute Inc., Cary, NC [Google Scholar]
- 43. Shannon CE. 1948. The mathematical theory of communication. Bell Syst. Tech. J. 27:379–423, 623–656 [Google Scholar]
- 44. Simpson EH. 1949. Measurement of diversity. Nature 163:688 [Google Scholar]
- 45. Soliva CR, Amelchanka SLSL, Duval SM, Kruzer M. 2011. Ruminal methane inhibition potential of various pure compounds in comparison with garlic oil as determined with a rumen simulation technique (Rusitec). Br. J. Nutr. 106:114–122 [DOI] [PubMed] [Google Scholar]
- 46. Stewart CS, Flint HJ, Bryant MP. 1997. The rumen bacteria, p 10–72 In Hobson PN, Stewart CS. (ed), The rumen microbial ecosystem, 2nd ed Blackie Academic and Professional, New York, NY [Google Scholar]
- 47. Stiverson J, Morrison M, Yu Z. 2011. Populations of select cultured and uncultured bacteria in the rumen of sheep and the effect of diets and ruminal fractions. Int. J. Microbiol. 2011:750613 doi:10.1155/2011/750613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sylvester JT, Karnati SK, Yu Z, Morrison M, Firkins JL. 2004. Development of an assay to quantify rumen ciliate protozoal biomass in cows using real-time PCR. J. Nutr. 134:3378–3384 [DOI] [PubMed] [Google Scholar]
- 49. Tassoul MD, Shaver RD. 2009. Effect of a mixture of supplemental dietary plant essential oils on performance of periparturient and early lactation dairy cows. J. Dairy Sci. 92:1734–1740 [DOI] [PubMed] [Google Scholar]
- 50. Ultee A, Bennink MHJ, Moezelaar R. 2002. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 68:1561–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597 [DOI] [PubMed] [Google Scholar]
- 52. Wang CJ, Wang SP, Zhou H. 2009. Influences of flavomycin, ropadiar, and saponin on nutrient digestibility, rumen fermentation, and methane emission from sheep. Anim. Feed Sci. Technol. 148:157–166 [Google Scholar]
- 53. Yang WZ, Ametaj BN, Benchaar C, He ML, Beauchemin KA. 2010. Cinnamaldehyde in feedlot cattle diets: intake, growth performance, carcass characteristics, and blood metabolites. J. Anim. Sci. 88:1082–1092 [DOI] [PubMed] [Google Scholar]
- 54. Yu Z, Garcia-Gonzalez R, Schanbacher FL, Morrison M. 2008. Evaluations of different hypervariable regions of archaeal 16S rRNA genes in profiling of methanogens by archaea-specific PCR and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 74:889–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yu Z, Michel FC, Jr, Hansen G, Wittum T, Morrison M. 2005. Development and application of real-time PCR assays for quantification of genes encoding tetracycline resistance. Appl. Environ. Microbiol. 71:6926–6933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yu Z, Morrison M. 2004. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 36:808–812 [DOI] [PubMed] [Google Scholar]
- 57. Yu Z, Morrison M. 2004. Comparisons of different hypervariable regions of rrs genes for use in fingerprinting of microbial communities by PCR-denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 70:4800–4806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhou Z, Meng Q, Yu Z. 2011. Effects of methanogenic inhibitors on methane production and abundance of methanogen and cellulolytic bacteria in in-vitro ruminal cultures. Appl. Environ. Microbiol. 77:2634–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]