Abstract
Termites are well-known cellulose decomposers and can give researchers insights into how to utilize lignocellulosic biomass in the actual scenario of energy consumption. In this work, an endogenous β-glucosidase from the midgut of the higher termite Nasutitermes takasagoensis was purified to homogeneity by Ni2+ affinity chromatography and its properties were characterized. This β-glucosidase (G1mgNtBG1), which belongs to glycoside hydrolase family 1, is a homotrimer in its native form, with a molecular mass of 169.5 kDa, as demonstrated by gel filtration chromatography. The enzyme displayed maximum activity at pH 5.5 and had broad substrate specificities toward several saccharides, including cellobiose. G1mgNtBG1 showed a relatively high temperature optimum of 65°C and one of the highest levels of glucose tolerance among several β-glucosidases already characterized, with a Ki of 600 mM glucose. To examine the applicability of G1mgNtBG1 in biomass conversion, we compared the thermostability and glucose tolerance of G1mgNtBG1 with those of Novozym 188. We found that G1mgNtBG1 was more thermostable after 5 h of incubation at 60°C and more resistant to glucose inhibition than Novozym 188. Furthermore, our result suggests that G1mgNtBG1 acts synergistically with Celluclast 1.5 L in releasing reducing sugars from Avicel. Thus, G1mgNtBG1 seems to be a potential candidate for use as a supplement in the hydrolysis of biomass.
INTRODUCTION
Worries regarding the current crisis of climate change and the depletion of fossil fuels make the use of bioethanol an attractive option for both combating global warming and reducing dependence on fossil fuels. Bioethanol from lignocellulosic biomass does not compete with food production, since available materials come from plentiful and inexpensive agricultural wastes and wood (1). Cellulose, which is a homopolysaccharide composed of β-d-glucopyranose units, accounts for around 40 to 50% by weight of plant biomass and is a potential feedstock for biomass conversion. The approach for bioethanol production involves the conversion of lignocellulosic biomass to sugars using complex enzyme cocktails and finally the fermentation of those sugars to ethanol by yeast or bacteria. Enzymatic hydrolysis of cellulose requires the synergistic action of cellulases, such as endoglucanase (EC 3.2.1.4), cellobiohydrolase (EC 3.2.1.91), and β-glucosidase (EC 3.2.1.21) (2). Among cellulases, β-glucosidase is essential for the final step of cellulose saccharification because it reduces the inhibition of endoglucanase and cellobiohydrolase through hydrolysis of cellobiose and cello-oligosaccharides (5, 18). However, β-glucosidases themselves are also subject to product inhibition by glucose. Thus, the identification and production of β-glucosidases insensitive to or stimulated by glucose are of special interest in biomass conversion (22, 32, 36).
Termites are well known as efficient cellulose decomposers; 74 to 99% of the cellulose ingested is hydrolyzed (23). The common wisdom is that termites feed on wood only with the aid of symbiotic protists in their hindguts. However, termites have evolved their own system to degrade cellulose, independently of whether or not they maintain a relationship with symbiotic protists (34). To date, only a few endogenous cellulases of termite origin have been biochemically characterized (13, 25, 27, 32, 37). The properties of endogenous β-glucosidases from three termite species, Macrotermes muelleri (25), Neotermes koshunensis (20, 29, 32), and Reticulitermes flavipes (27), have been characterized. Regarding the wood-feeding higher termite Nasutitermes takasagoensis, only the site of expression of endogenous β-glucosidases has been reported (28, 30). However, detailed analysis of β-glucosidases from N. takasagoensis has not been performed.
Here we report the heterologous expression in Pichia pastoris of an endogenous β-glucosidase from the midgut of N. takasagoensis, here called G1mgNtBG1, which belongs to glycoside hydrolase family 1 (refer to the CAZy database at http://www.cazy.org/). We also characterized the recombinant protein and compared it with the most common commercial β-glucosidase preparation from Aspergillus niger, Novozym 188 (3, 9, 11). The effect of the addition of G1mgNtBG1 or Novozym 188 to Celluclast 1.5 L, a commercial cellulase preparation from Trichoderma reesei, on the degradation of Avicel was studied. This work might introduce an alternative and effective enzyme that can be used as a supplement in biomass processing.
MATERIALS AND METHODS
Microorganisms.
N. takasagoensis cDNA was used as the donor of the target gene for β-glucosidase (GenBank accession no. AB508958). P. pastoris KM71 (his4 aox1::ARG4; Invitrogen) was used as the host for the expression of G1mgNtBG1, and Escherichia coli DH5α was used in all other DNA manipulations.
Cloning, heterologous expression in P. pastoris, and purification of G1mgNtBG1.
G1mgNtBG1 was expressed using the plasmid pBGP3 (31). The mature region of mgntbg1 was amplified by PCR with the MunI site (underlined in the primer sequence presented) added at the 5′ ends of the forward and reverse primers used (forward, 5′-CAATTGCAAAATAACACTACGTTTCCAG-3′; reverse, 5′-CAATTGTTAATCTAAGAAGCGGTCTG-3′). The amplified DNA fragment was phosphorylated and inserted into the EcoRV site of pBluescript II SK(+), resulting in the construction of pBS-mgNtBG1. After subcloning, this fragment was isolated by digestion with MunI and ligated to EcoRI-digested, dephosphorylated pBGP3, resulting in the generation of pBGP3-mgNtBG1. Transformation of P. pastoris was done by electroporation according to the standard method (8). For collection of the culture supernatant of the P. pastoris transformant strain, we used the method previously described (31). Purification was performed by using the Ni2+-nitrilotriacetic acid (NTA) purification system according to the instructions given by the manufacturer (Qiagen).
Enzyme assays and protein determination.
β-Glucosidase activity was routinely assayed with 10 μM p-nitrophenyl-β-d-glucopyranoside (pNPG; Sigma, St. Louis, MO) as the substrate as previously described (32). For substrate specificity analyses, 10 mM p-nitrophenyl-β-d-fucopyranoside (pNPFuc), p-nitrophenyl-β-d-lactopyranoside (pNPLac), and p-nitrophenyl-β-d-galactopyranoside (pNPGal) were assayed under the same conditions as pNPG. The release of p-nitrophenol (pNP) was measured at A410. G1mgNtBG1 activity was also determined by the release of glucose from natural substrates using glucose oxidase-mutarotase reagent (Glucose CII Test Wako; Wako Pure Chemical Co., Tokyo, Japan) according to a previously described method (29). The substrates used were 2% (wt/vol) laminaribiose, 2% laminarin, 2% gentiobiose, 2% sophorose, 2% salicin, 2% lactose, 1% carboxymethyl cellulose (CMC), 1% Avicel, 30 mM cellobiose, 30 mM cellotriose, 30 mM cellotetraose, 30 mM cellopentaose, and 30 mM cellohexaose. Each substrate was dissolved in 50 mM sodium acetate buffer (pH 5.5) and incubated with the enzyme. The release of glucose was measured at A505. In all analyses, 1 U was defined as the amount of enzyme that releases 1 μmol of product per min under the conditions used. Cellulase activity was determined according to the method previously described (12), and reducing sugars were measured at A540 by 3,5-dinitrosalicylic acid (DNS) assay (19). The protein concentration was measured at A595 by using the Bio-Rad protein assay (Bio-Rad) based on the Bradford method (6) and with bovine serum albumin (TaKaRa, Tokyo, Japan) as the standard. Specific activity was expressed in units per milligram of protein. Celluclast 1.5 L (cellulases) and Novozym 188 (β-glucosidase) produced by T. reesei and A. niger, respectively, were purchased from Sigma.
G1mgNtBG1 properties. (i) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
For SDS-PAGE analyses, proteins were separated on a 10% polyacrylamide gel and detected by Western blot assay using specific antibodies or stained with Coomassie brilliant blue (CBB). In the Western blot assay, anti-c-Myc mouse monoclonal antibody (1:1,000 dilution; Clontech) and a peroxidase-labeled anti-mouse IgG antibody (1:500 dilution; Vector) were used as the primary and second antibodies, respectively. Proteins were detected through a luminescent image analyzer (LAS-4000miniEPUV; Fujifilm, Tokyo, Japan) using enhanced chemiluminescence detection reagents (Pierce).
(ii) Protein deglycosylation.
Protein deglycosylation analysis was done by incubating the purified concentrated enzyme with endoglycosidase H (Endo H; New England BioLabs) according to the manufacturer's specifications.
(iii) Effects of temperature and pH on G1mgNtBG1 activity.
The temperature optimum and stability of G1mgNtBG1 were determined by incubating 0.03 U of the purified enzyme ml−1 for 30 min at temperatures ranging from 25°C to 80°C with or without substrate, respectively. The stability of G1mgNtBG1 and Novozym 188 was also analyzed in a time course experiment for 5 h at 55°C and 60°C. Residual β-glucosidase activity was measured according to the standard assay procedure. The pH optimum of G1mgNtBG1 was measured by incubating 0.03 U of the purified enzyme ml−1 with 10 mM pNPG in universal buffer (7) over a pH range of 2.5 to 9.0 at 45°C for 30 min. To test its stability, purified G1mgNtBG1 was incubated in universal buffer without a substrate over a pH range of 2.5 to 10 at 45°C for 30 min. The remaining activities were measured according to the standard assay procedure. The maximum activities obtained were considered 100%.
(iv) Kinetic analysis.
Kinetic constants (Km and Vmax) were analyzed by a Hanes-Woolf plot using linear regression techniques. Kinetic inhibition (Ki) was calculated from a Dixon plot.
(v) Effect of glucose on β-glucosidase activities.
Different concentrations of glucose ranging from 0.1 to 1.0 M were incubated with 0.03 U of purified GlmgNtBG1 and Novozym 188 to test the effect of end product inhibition. After 30 min of incubation, β-glucosidase activities were measured according to the standard assay procedure. The maximum activity (100%) was considered to be when no glucose was added to the reaction mixture.
Effect of addition of β-glucosidases to Celluclast 1.5 L on the hydrolysis of Avicel.
In a sterile 2-ml tube, 1% (wt/vol) Avicel and 0.02% (wt/vol) sodium azide were mixed with 1.5 ml of 50 mM sodium acetate buffer (pH 5.0). To the substrate, around 0.4 filter paper unit of Celluclast 1.5 L was added either alone (control) or together with Novozym 188 or G1mgNtBG1 (0.004 U). After incubation at 50°C for 8, 24, or 48 h, samples were centrifuged at 500 × g for 10 min at room temperature. Reducing sugars were analyzed using DNS reagent.
RESULTS
Heterologous expression of G1mgNtBG1 in P. pastoris.
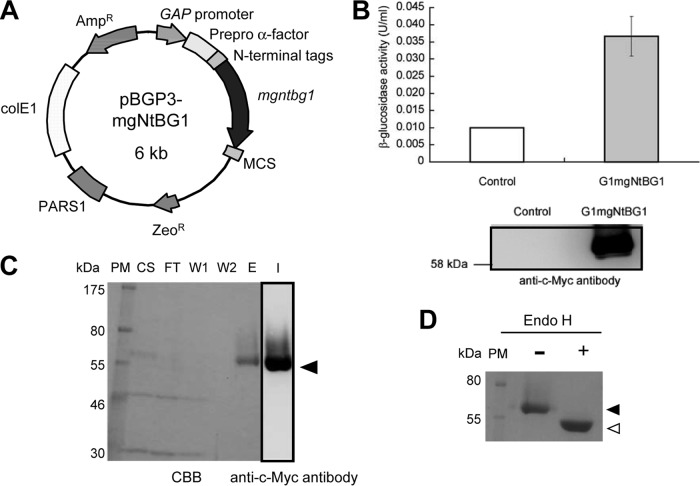
G1mgNtBG1 was expressed under the control of the strong constitutive GAP promoter and with the prepro-α-factor signal sequence that directs the secretion of the protein into the medium (Fig. 1A). The production of G1mgNtBG1 in the culture supernatant of the transformant strain was checked by enzyme activity (Fig. 1B, top) and a Western blot assay using anti-c-Myc antibody (Fig. 1B, bottom). A small amount of β-glucosidase activity was detected in the culture supernatant of the control P. pastoris strain, although no immunoreactive band was observed by Western blot analysis (Fig. 1B).
Fig 1.
Cloning, expression, purification, and deglycosylation assay. (A) Expression plasmid. (B) Production of G1mgNtBG1 was analyzed by an enzyme activity assay (upper graphic) and a Western blot assay with anti-c-Myc antibody (bottom panel) and compared with that of the control. (C) Purification was performed by Ni2+-NTA chromatography. Fractions were resolved by SDS-PAGE, and the gels were either stained with CBB or transferred and immunoblotted with anti-c-Myc antibody. PM, protein marker; CS, culture supernatant; FT, flowthrough; W1 and W2, wash fractions 1 and 2; E, elution fraction; I, immunoblotting. The arrowhead indicates the G1mgNtBG1 band. (D) G1mgNtBG1 was treated (+) or not treated (−) with Endo H, separated by SDS-PAGE, and stained with CBB. The reduction in size from 60 kDa (closed arrowhead) to 56 kDa (open arrowhead) indicates the presence of the posttranslational modification by N-glycosylation.
Enzyme purification.
Purification of G1mgNtBG1 was done in a single-step using Ni2+-NTA chromatography with the aid of an N-terminal 6×His sequence (Fig. 1C). The purity of G1mgNtBG1 was confirmed by CBB staining (Fig. 1C, left side). A Western blot assay using anti-c-Myc antibody shows that the band that appears in the elution fraction reacts with the antibody (Fig. 1C, lane I). The purity increased by 6.7-fold and the recovery was 83% compared to the culture supernatant of the transformant strain. The specific activity was 5.83 U/mg.
Posttranslational modification and molecular mass determination.
A deglycosylation experiment was performed to elucidate the cause of the difference between the apparent and predicted molecular masses (60 and 54 kDa, respectively) of G1mgNtBG1, mainly because G1mgNtBG1 possesses four N-linked glycosylation sites (Asn21, Asn22, Asn315, and Asn404) in its mature region. The purified enzyme was treated with Endo H and compared to the untreated enzyme (Fig. 1D). After Endo H treatment, the band shifted from 60 to 56 kDa and the smear disappeared, which indicates that N-glycosylation increased the molecular mass by around 4 kDa. The native molecular mass of purified G1mgNtBG1 was analyzed by gel filtration, which demonstrated that G1mgNtBG1 was eluted at a molecular mass of around 169.5 kDa, indicating that G1mgNtBG1 is a trimeric protein (data not shown).
Effects of temperature and pH on the activity and stability of G1mgNtBG1.
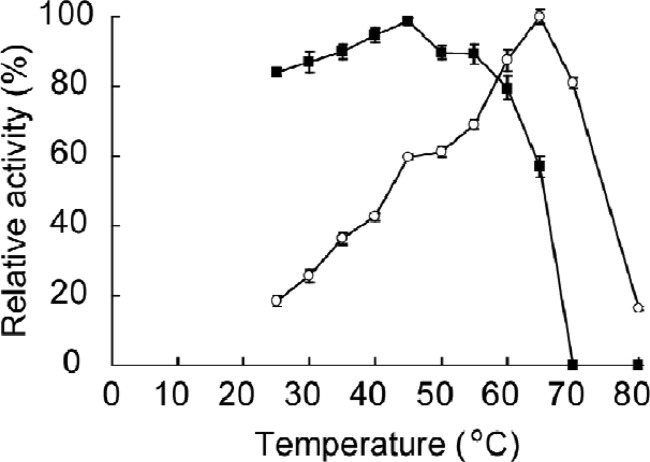
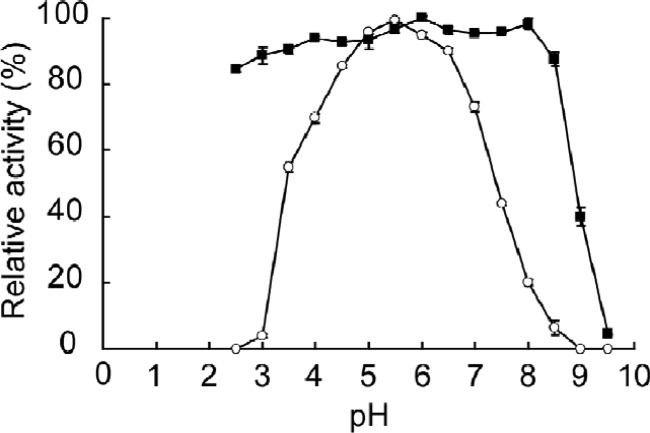
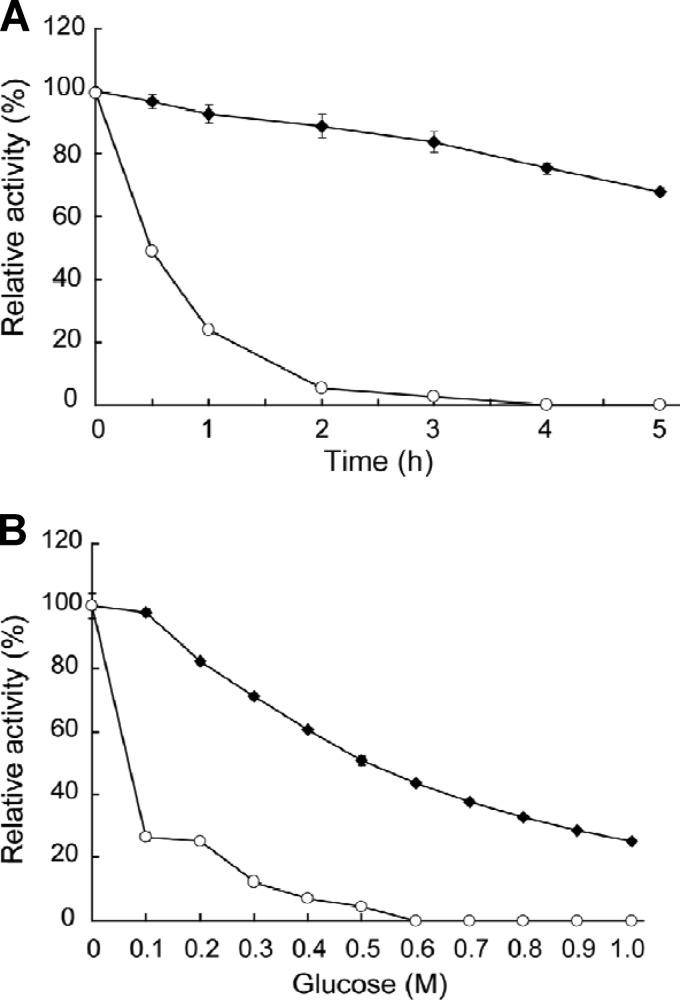
The maximum activity of G1mgNtBG1 was observed at 65°C (Fig. 2) and pH 5.5 (Fig. 3) under the assay conditions used. More than 80% of its maximum activity was retained at temperatures of up to 60°C. Total inactivation of the enzyme was observed at temperatures above 70°C (Fig. 2). G1mgNtBG1 was stable over a broad pH range of 2.5 to 8.5 (Fig. 3). When we compared G1mgNtBG1 and Novozym 188 for thermostability in a time course experiment, we found that both enzymes were stable at 55°C after 5 h of incubation (data not shown). However, after 1 h of incubation at 60°C, G1mgNtBG1 retained 93% of its total activity while Novozym 188 was quickly inactivated and retained only 24% of its total activity (Fig. 4A).
Fig 2.
Effect of temperature on the activity and stability of purified G1mgNtBG1. For activity testing (open circles), the enzyme was incubated at various temperatures and its activity was measured by the standard assay procedure. For stability testing (closed squares), the enzyme solution was incubated in 50 mM sodium acetate buffer (without substrate) at various temperatures and the residual activity was measured. Data are expressed as the mean ± the standard deviation of three independent experiments.
Fig 3.
Effect of pH on the activity and stability of purified G1mgNtBG1. For activity testing (open circles), the enzyme was incubated in universal buffer at various pH values and its activity was measured by the standard assay procedure. For stability testing (closed squares), the enzyme solution was incubated in universal buffer at various pHs (without substrate) for 30 min at 45°C and the residual activity was measured. Data are expressed as the mean ± the standard deviation of three independent experiments.
Fig 4.
Comparison of G1mgNtBG1 and Novozym 188. (A) For stability testing, G1mgNtBG1 (closed diamonds) and Novozym 188 (open circles) were incubated in 50 mM sodium acetate buffer (without substrate) at 60°C for the times indicated and their residual activities were measured. (B) Several concentrations of glucose were incubated with G1mgNtBG1 (closed diamonds) and Novozym 188 (open circles), and their activities were measured according to the standard assay method using pNPG as the substrate. Data are expressed as the mean ± the standard deviation of three independent experiments.
Substrate specificity.
To examine the substrate specificity of G1mgNtBG1, the enzyme was incubated with selected aryl glycosides, saccharides, and oligosaccharides (Table 1). pNPFuc (βFuc) was the most preferred substrate among the aryl glycosides tested, and its relative activity was taken as 100%. pNPG, the substrate used in the standard assay procedure, was hydrolyzed at 37.5% pNPFuc. The other two aryl glycosides, pNPLac and pNPGal, were also hydrolyzed (13.3% and 9.1% of pNPFuc, respectively).
Table 1.
Relative activity of G1mgNtBG1 on various substrates
| Substrate | Linkage of glycosyl group | Mean relative activity (%) ± SDa |
|---|---|---|
| Aryl glycosides | ||
| pNPFuc | βFuc | 100.0 ± 0.44 |
| pNPG | βGlc | 37.5 ± 0.45 |
| pNPLac | βLac | 13.3 ± 0.31 |
| pNPGal | βGal | 9.1 ± 0.42 |
| Saccharides | ||
| Laminaribiose | (β-1,3) Glc | 100.0 ± 2.18 |
| Sophorose | (β-1,2) Glc | 92.2 ± 1.83 |
| Lactose | (β-1,4) Gal | 69.9 ± 2.95 |
| Salicin | βGlc | 11.7 ± 0.84 |
| Gentiobiose | (β-1,6) Glc | 10.3 ± 0.16 |
| Laminarin | (β-1,3; β-1,6) Glc | 6.5 ± 0.58 |
| CMC | (β-1,4) Glc | 0.0 ± 0.00 |
| Avicel | (β-1,4) Glc | 0.0 ± 0.00 |
| Cellobiose | (β-1,4) Glc | 98.2 ± 2.29 |
| Cellotriose | (β-1,4) Glc | 32.2 ± 4.00 |
| Cellotetraose | (β-1,4) Glc | 24.5 ± 2.34 |
| Cellopentaose | (β-1,4) Glc | 19.7 ± 3.55 |
| Cellohexaose | (β-1,4) Glc | 15.5 ± 1.35 |
The release of sugars was measured at A410 for aryl glycosides and at A505 for saccharides. The relative activity of the most preferentially hydrolyzed substrates was taken as 100%. Each value is the mean of triplicate experiments.
Among the saccharides, laminaribiose (β-1,3-linked glucose) was the most preferred substrate, while gentiobiose (β-1,6-linked glucose; 10.3% laminaribiose) was the least preferred, followed by salicin (βGlc; 11.7%), lactose (β-1,4-glucose-linked galactose; 69.9%), and sophorose (β-1,2-linked glucose; 92.2%). Usually, β-1,6 glycosidic linkage is less susceptible to hydrolysis by β-glucosidases (25). G1mgNtBG1 had very little activity toward laminarin (6.5%), a β-1,3- and β-1,6-linked polyglycan. No activity against CMC and Avicel, which were the β-1,4-linked polyglycans tested, was found. G1mgNtBG1 was able to hydrolyze all of the cello-oligosaccharides tested: cellobiose (98.2%), cellotriose (32.2%), cellotetraose (24.5%), cellopentaose (19.7%), and cellohexaose (15.5%). It was observed by thin-layer chromatography that G1mgNtBG1 also possesses transglycosylation activity, because products at least one glucose unit longer than the original substrates were observed when cello-oligosaccharides (from cellobiose to cellohexaose) were reacted with G1mgNtBG1 (see Fig. S1 in the supplemental material).
Effects of several cations and reagents on the activity of G1mgNtBG1.
We tested the effects of various metal ions and reagents on G1mgNtBG1 activity (data not shown). Significant inactivation by Fe2+ and Cu2+ (4.8 and 38.3%, respectively) was observed. On the other hand, G1mgNtBG1 was significantly stimulated by Mn2+ (139%) and slightly affected by EDTA (85.6%), dimethyl sulfoxide (86.0%), and dithiothreitol (77.6%).
Effects of glucose on G1mgNtBG1 and Novozym 188 activities.
The effect of glucose, added at concentrations ranging from 0.1 to 1 M, on the activities of G1mgNtBG1 and Novozym 188 was examined using pNPG as the substrate (Fig. 4B). Glucose dose dependently inhibited the activity of G1mgNtBG1. G1mgNtBG1 retained 50% of its activity at 0.5 M glucose but retained only about 20% of its activity when glucose was added at 1 M. Novozym 188 was more severely inhibited by glucose, with 26% of its activity retained at 0.1 M glucose and only 5% retained at 0.5 M glucose.
Kinetic analysis.
The Km and Vmax values of G1mgNtBG1 toward pNPG were 0.67 mM and 8 U/mg of protein, respectively (data not shown). The Ki value of glucose was 600 mM (data not shown).
Effects of addition of β-glucosidases to Celluclast 1.5 L on the hydrolysis of Avicel.
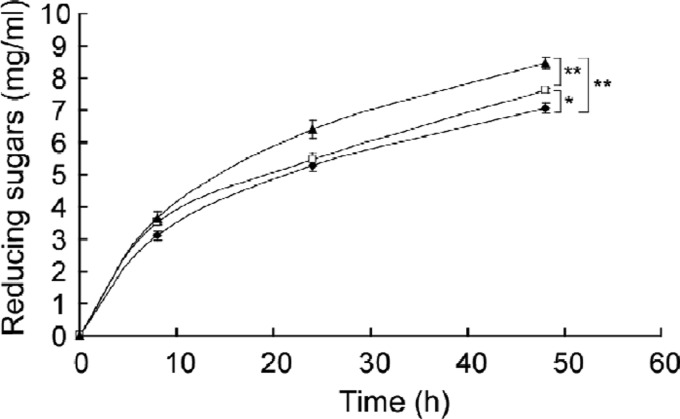
The effects of addition of β-glucosidases to Celluclast 1.5 L on the hydrolysis of Avicel were studied (Fig. 5). After 8 h of incubation with Celluclast 1.5 L alone (closed diamonds) or Celluclast 1.5 L plus either G1mgNtBG1 (closed triangles) or Novozym 188 (closed squares), similar amounts of reducing sugars of ca. 3.1 to 3.6 mg/ml were released. After 48 h of incubation, however, the addition of β-glucosidases to Celluclast 1.5 L resulted in the release of more reducing sugars than with Celluclast 1.5 L alone. Addition of G1mgNtBG1 was more effective at releasing the reducing sugars than that of Novozym 188 (P < 0.01). Incubation with G1mgNtBG1 alone did not result in the release of reducing sugars (data not shown).
Fig 5.
Enhanced production of reducing sugars from Avicel by addition of β-glucosidases. Enzyme solution was incubated at 50°C for the times indicated. As a control, Celluclast 1.5 L (closed diamonds) was mixed with the substrate. Addition of G1mgNtBG1 (closed triangles) and Novozym 188 (open squares) to Celluclast 1.5 L enhanced the production of reducing sugars (*, P < 0.05; **, P < 0.01 [Student's t test]). Reducing sugar was measured with DNS reagent. Data are expressed as the mean ± the standard deviation of three independent experiments.
DISCUSSION
It was demonstrated that the termite N. takasagoensis possesses four different sequences for β-glucosidases in the salivary glands (sgNtBG1 to sgNtBG4) and three in the midgut (mgNtBG1 to mgNtBG3) (28). We report here the successful heterologous expression in P. pastoris, purification, and characterization of an endogenous β-glucosidase from the midgut (G1mgNtBG1) of the higher termite N. takasagoensis. This is only the fourth endogenous β-glucosidase to be characterized from more than 3,000 species of termites (14). G1mgNtBG1 shares 80.4% and 70.8% amino acid sequence homology with β-glucosidases from the salivary glands of the termites R. flavipes (RfBGluc-1) and N. koshunensis (G1NkBG), respectively (25, 30). Salivary β-glucosidase A from M. muelleri (23) is another β-glucosidase characterized.
β-Glucosidase is a key enzyme in the hydrolysis of cellulose; it alleviates the inhibition of cellobiohydrolases and endoglucanases by cellobiose and, at the same time, liberates the fermentable sugar glucose. Characterization of G1mgNtBG1 has revealed that this enzyme has some desired properties for the conversion of cellulosic biomass to glucose, as discussed below, which may facilitate the production of bioethanol.
First, in contrast to most β-glucosidases which are inhibited by the end product, glucose, with Ki values ranging from 0.3 to 100 mM, G1mgNtBG1 is highly resistant to glucose inhibition, with a Ki of 600 mM. For example, β-glucosidases from Fomitopsis palustris, Sporotrichum thermophile, Daldinia eschscholzii, Fusarium oxysporum, and Streptomyces sp. strain QM-B814 were competitively inhibited by glucose, with Ki values of 0.35, 0.5, 0.79, 2.05, and 65 mM, respectively (4, 10, 15, 21, 35). The highest Ki values found in the literature were 1.4 M (Candida peltata; 26) and 1.36 M (Aspergillus oryzae; 24). Recently, a β-glucosidase from the lower termite N. koshunensis was reported to be not only tolerant to but also stimulated by glucose at concentrations ranging from 0.2 to 0.6 M (32). Thus, β-glucosidases from termites appear to be good candidates as supplements in the cellulolytic processes, especially in the case of separate hydrolysis and fermentation processes, where glucose remains in the reaction mixture.
Second, additional advantageous features of G1mgNtBG1 are its temperature and pH stabilities. G1mgNtBG1 was thermostable at up to 60°C (Fig. 2) under the assay conditions used, which is higher than the thermostability reported for other termite β-glucosidases (23, 25, 30). The thermostability of β-glucosidases from various sources is in the range between 40°C and 110°C (16). Usually, the hydrolysis of cellulose is performed under mild conditions at around 45°C. Finding β-glucosidases with higher temperature stability is of special interest in bioethanol production, because thermostable enzymes are easily handled, stored, and transported (33) and thus potentially decrease the cost of hydrolysis. With respect to pH, G1mgNtBG1 is stable over a broad pH range of 2.5 to 8.5 (Fig. 3). Pretreatment of lignocellulosic biomass, a prior step of hydrolysis during cellulose deconstruction, is carried out under acidic and high-temperature conditions. Thus, the use of enzymes stable at acidic pH might accelerate the process, since hydrolysis can be conducted as soon as pretreatment is finished.
It is worth noting that β-glucosidases have a wide range of substrate specificities, due to the abundance of β-linked d-glycosyl residues in nature (17). Like G1NkBG (30), G1mgNtBG1 has broad substrate specificity and hydrolyzes both natural and synthetic substrates (Table 1). Since wood is the main diet source for termites, it is intuitive that cellobiose was hydrolyzed by all β-glucosidases (25, 27, 32). Activities of both laminaribiase and cellobiase emphasize the importance of G1mgNtBG1 in the hydrolysis of biomass.
The biochemical characterization of G1mgNtBG1 toward its applicability in biomass conversion showed it to display some advantages over the β-glucosidase preparation most commonly used in industry, Novozym 188. First, G1mgNtBG1 was more thermostable than Novozym 188 after 5 h of incubation at 60°C (Fig. 4A). Second, G1mgNtBG1 was far more glucose tolerant than Novozym 188 (Fig. 4B). Third, G1mgNtBG1 was more effective than Novozym 188 at releasing reducing sugars when mixed with Celluclast 1.5 L to degrade Avicel (Fig. 5). G1mgNtBG1 alone did not hydrolyze Avicel after 48 h of incubation (data not shown). Hence, the result further suggests that G1mgNtBG1 serves as an enzyme that shows better synergism with other cellulolytic components, probably by removing the inhibitory effect of cello-oligosaccharides. We hypothesize that with greater production of glucose, which reaches some milligrams per milliliter, Novozym 188 starts to be inhibited by glucose and the reaction rates gets slower.
In conclusion, with its high glucose tolerance, relatively high thermostability, broad pH stability, and efficient hydrolysis of cello-oligosaccharides, G1mgNtBG1 has the potential to be used as a supplement in the enzymatic hydrolysis of cellulose.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry, Japan. We thank the Ministry of Education, Culture, Sports, Science, and Technology of Japan for the scholarship received by C.A.U.
We are also thankful to the anonymous reviewers for their constructive comments.
Footnotes
Published ahead of print 20 April 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Balat M, Balat H, Oz C. 2008. Progress in bioethanol processing. Prog. Energy Combustion Sci. 34:551–573 [Google Scholar]
- 2. Béguin P, Aubert JP. 1994. The biological degradation of cellulose. FEMS Microbiol. Rev. 13:25–58 [DOI] [PubMed] [Google Scholar]
- 3. Berlin A, et al. 2005. Evaluation of novel fungal cellulase preparations for ability to hydrolyze softwood substrates—evidence for the role of accessory enzymes. Enzyme Microb. Technol. 37:175–184 [Google Scholar]
- 4. Bhat KM, Gaikwad JS, Maheshwari R. 1993. Purification and characterization of an extracellular beta-glucosidase from the thermophilic fungus Sporotrichum thermophile and its influence on cellulase activity. J. Gen. Microbiol. 139:2825–2832 [Google Scholar]
- 5. Bhatia Y, Mishra S, Bisaria VS. 2002. Microbial beta-glucosidases: cloning, properties, and applications. Crit. Rev. Biotechnol. 22:375–407 [DOI] [PubMed] [Google Scholar]
- 6. Bradford MM. 1976. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 7. Britton HTS, Robinson RA. 1931. Universal buffer solutions and the dissociation constant of Veronal. J. Chem. Soc. 1456–1462 [Google Scholar]
- 8. Cereghino JL, Cregg JM. 2000. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol. Rev. 24:45–66 [DOI] [PubMed] [Google Scholar]
- 9. Chauve M, et al. 2010. Comparative kinetic analysis of two fungal beta-glucosidases. Biotechnol. Biofuels 3(1):3 doi:10.1186/1754-6834-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christakopoulos P, et al. 1994. Purification and characterization of an extracellular beta-glucosidase with transglycosylation and exo-glucosidase activities from Fusarium oxysporum. Eur. J. Biochem. 224:379–385 [DOI] [PubMed] [Google Scholar]
- 11. Dekker RFH. 1986. Kinetic, inhibition, and stability properties of a commercial beta-d-glucosidase (cellobiase) preparation from Aspergillus niger and its suitability in the hydrolysis of lignocellulose. Biotechnol. Bioeng. 28:1438–1442 [DOI] [PubMed] [Google Scholar]
- 12. Ghose TK. 1987. Measurement of cellulase activities. Pure Appl. Chem. 59:257–268 [Google Scholar]
- 13. Hirayama K, Watanabe H, Tokuda G, Kitamoto K, Arioka M. 2010. Purification and characterization of termite endogenous beta-1,4-endoglucanases produced in Aspergillus oryzae. Biosci. Biotechnol. Biochem. 74:1680–1686 [DOI] [PubMed] [Google Scholar]
- 14. Hongoh Y. 2011. Toward the functional analysis of uncultivable, symbiotic microorganisms in the termite gut. Cell. Mol. Life Sci. 68:1311–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karnchanatat A, et al. 2007. Purification and biochemical characterization of an extracellular beta-glucosidase from the wood-decaying fungus Daldinia eschscholzii (Ehrenb.:Fr.) Rehm. FEMS Microbiol. Lett. 270:162–170 [DOI] [PubMed] [Google Scholar]
- 16. Kengen SW, Luesink EJ, Stams AJ, Zehnder AJ. 1993. Purification and characterization of an extremely thermostable beta-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus. Eur. J. Biochem. 213:305–312 [DOI] [PubMed] [Google Scholar]
- 17. Ketudat Cairns JR, Esen A. 2010. β-Glucosidases. Cell. Mol. Life Sci. 67:3389–3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. 2002. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 66:506–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller GL. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31:426–428 [Google Scholar]
- 20. Ni J, Tokuda G, Takehara M, Watanabe H. 2007. Heterologous expression and enzymatic characterization of beta-glucosidase from the drywood-eating termite, Neotermes koshunensis. Appl. Entomol. Zool. 42:457–463 [Google Scholar]
- 21. Parry NJ, et al. 2001. Biochemical characterization and mechanism of action of a thermostable beta-glucosidase purified from Thermoascus aurantiacus. Biochem. J. 353:117–127 [PMC free article] [PubMed] [Google Scholar]
- 22. Pérez-Pons JA, Rebordosa X, Querol E. 1995. Properties of a novel glucose-enhanced beta-glucosidase purified from Streptomyces sp. (ATCC 11238). Biochim. Biophys. Acta 1251(2):145–153 [DOI] [PubMed] [Google Scholar]
- 23. Prins RA, Kreulen DA. 1991. Comparative aspects of plant cell wall digestion in insects. Anim. Feed Sci. Technol. 32:101–118 [Google Scholar]
- 24. Riou C, Salmon JM, Vallier MJ, Gunata Z, Barre P. 1998. Purification, characterization, and substrate specificity of a novel highly glucose-tolerant beta-glucosidase from Aspergillus oryzae. Appl. Environ. Microbiol. 64:3607–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rouland C, Matoub M, Mora P, Petek F. 1992. Properties of two β-glucosidases purified from the termite Macrotermes muelleri and from its symbiotic fungus Termitomyces sp. Carbohydr. Res. 233:273–278 [Google Scholar]
- 26. Saha BC, Bothast RJ. 1996. Production, purification, and characterization of a highly glucose-tolerant novel beta-glucosidase from Candida peltata. Appl. Environ. Microbiol. 62:3165–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scharf ME, et al. 2010. Functional and translational analyses of a beta-glucosidase gene (glycosyl hydrolase family 1) isolated from the gut of the lower termite Reticulitermes flavipes. Insect Biochem. Mol. Biol. 40:611–620 [DOI] [PubMed] [Google Scholar]
- 28. Tokuda G, Miyagi M, Makiya H, Watanabe H, Arakawa G. 2009. Digestive beta-glucosidases from the wood-feeding higher termite, Nasutitermes takasagoensis: intestinal distribution, molecular characterization, and alteration in sites of expression. Insect Biochem. Mol. Biol. 39:931–937 [DOI] [PubMed] [Google Scholar]
- 29. Tokuda G, Saito H, Watanabe H. 2002. A digestive beta-glucosidase from the salivary glands of the termite, Neotermes koshunensis (Shiraki): distribution, characterization and isolation of its precursor cDNA by 5′- and 3′-RACE amplifications with degenerate primers. Insect Biochem. Mol. Biol. 32:1681–1689 [DOI] [PubMed] [Google Scholar]
- 30. Tokuda G, Watanabe H, Matsumoto T, Noda H. 1997. Cellulose digestion in the wood-eating higher termite, Nasutitermes takasagoensis (Shiraki): distribution of cellulases and properties of endo-beta-1,4-glucanase. Zool. Sci. 14:83–93 [DOI] [PubMed] [Google Scholar]
- 31. Uchima CA, Arioka M. 2012. Expression and one-step purification of recombinant proteins by using an alternative episomal vector for expression of N-tagged heterologous proteins in Pichia pastoris. Biosci. Biotechnol. Biochem. 76:368–371 [DOI] [PubMed] [Google Scholar]
- 32. Uchima CA, Tokuda G, Watanabe H, Kitamoto K, Arioka M. 2011. Heterologous expression and characterization of a glucose-stimulated beta-glucosidase from the termite Neotermes koshunensis in Aspergillus oryzae. Appl. Microbiol. Biotechnol. 89:1761–1771 [DOI] [PubMed] [Google Scholar]
- 33. Venturi LL, Polizeli MD, Terenzi HF, Furriel RD, Jorge JA. 2002. Extracellular beta-d-glucosidase from Chaetomium thermophilum var. coprophilum: production, purification and some biochemical properties. J. Basic Microbiol. 42:55–66 [DOI] [PubMed] [Google Scholar]
- 34. Watanabe H, Tokuda G. 2010. Cellulolytic systems in insects. Annu. Rev. Entomol. 55:609–632 [DOI] [PubMed] [Google Scholar]
- 35. Yoon J-J, Kim K-Y, Cha C-J. 2008. Purification and characterization of thermostable beta-glucosidase from the brown-rot basidiomycete Fomitopsis palustris grown on microcrystalline cellulose. J. Microbiol. 46:51–55 [DOI] [PubMed] [Google Scholar]
- 36. Zanoelo FF, Polizeli M, Terenzi HF, Jorge JA. 2004. Beta-glucosidase activity from the thermophilic fungus Scytalidium thermophilum is stimulated by glucose and xylose. FEMS Microbiol. Lett. 240:137–143 [DOI] [PubMed] [Google Scholar]
- 37. Zhang D, Lax AR, Bland JM, Allen AB. 2011. Characterization of a new endogenous endo-beta-1,4-glucanase of Formosan subterranean termite (Coptotermes formosanus). Insect Biochem. Mol. Biol. 41:211–218 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.