Abstract
The currently used microbial decontamination method for spacecraft and components uses dry-heat microbial reduction at temperatures of >110°C for extended periods to prevent the contamination of extraplanetary destinations. This process is effective and reproducible, but it is also long and costly and precludes the use of heat-labile materials. The need for an alternative to dry-heat microbial reduction has been identified by space agencies. Investigations assessing the biological efficacy of two gaseous decontamination technologies, vapor hydrogen peroxide (Steris) and chlorine dioxide (ClorDiSys), were undertaken in a 20-m3 exposure chamber. Five spore-forming Bacillus spp. were exposed on stainless steel coupons to vaporized hydrogen peroxide and chlorine dioxide gas. Exposure for 20 min to vapor hydrogen peroxide resulted in 6- and 5-log reductions in the recovery of Bacillus atrophaeus and Geobacillus stearothermophilus, respectively. However, in comparison, chlorine dioxide required an exposure period of 60 min to reduce both B. atrophaeus and G. stearothermophilus by 5 logs. Of the three other Bacillus spp. tested, Bacillus thuringiensis proved the most resistant to hydrogen peroxide and chlorine dioxide with D values of 175.4 s and 6.6 h, respectively. Both low-temperature decontamination technologies proved effective at reducing the Bacillus spp. tested within the exposure ranges by over 5 logs, with the exception of B. thuringiensis, which was more resistant to both technologies. These results indicate that a review of the indicator organism choice and loading could provide a more appropriate and realistic challenge for the sterilization procedures used in the space industry.
INTRODUCTION
When a spacecraft visits regions of the solar system which may potentially hold biological interest, Planetary Protection (1967 Outer Space Treaty) precautions must be observed to protect any present and future life detection activities (2). Planetary protection precautions require that flight systems must be assembled, tested, and launched under conditions to control the forward contamination of pristine extraterrestrial environments by terrestrial microorganisms and indeed backward contamination during Earth return missions.
Spacecraft and their components are constructed and assembled in high classification clean room facilities (30, 38) similar to that used in medical (12, 16, 42) and industrial pharmaceutical (31) applications. Although it might be expected that the contaminating organisms of these facilities will be the result of human activity (30), the highly desiccated and nutrient-limited environment of spacecraft assembly clean rooms demonstrate selective pressure toward oligotrophic organisms that can persist in the environment (30). Of these organisms, spore-forming bacteria are perhaps the best suited to persistence and survival (21, 26). For example, spore-forming Bacillus species are the most commonly isolated (26), but other aerobic and anaerobic bacterial species have also been detected, namely, Staphylococcus, Acinetobacter, Micrococcus, Clostridium, and Streptococcus spp. (36–38), as well as eukaryotic organisms such as yeasts and fungi (37). The continual microbial monitoring of the spacecraft assembly clean rooms has led to the characterization of two new Bacillus species: Bacillus odysseyi (27) and Bacillus nealsonii (41).
Since microorganisms that survive within spacecraft assembly facilities can potentially contaminate spacecraft components and thus ultimately their destinations, the bioburden needs to be reduced to safe levels to satisfy the tightly regulated Planetary Protection requirements before deployment (2). This cannot be done by sterile manufacture alone. Regular assays of spacecraft allows a baseline contamination level to be calculated which must be controlled and reduced by a decontamination process.
The current validated decontamination process used in the space industry is dry-heat microbial reduction (DHMR) (14), which has been used on spacecraft and their components since the Viking lander missions in 1975. The spacecraft and components are heated to >110°C within a sealed dry oven for extended periods of time (e.g., up to 50 h for one cycle) (8, 40). This method has provided effective and repeatable decontamination which has been validated using thermocouples and biological indicator(s) (BI). The kinetics of heat inactivation of microorganisms is well established and therefore demonstrates a high degree of sterility assurance.
The use of heat-sensitive components such as used on the Beagle 2 Lander ruled out the use of DHMR, and so alternative technologies such as sporicides, gamma irradiation, and gas plasma (8, 11, 32) were used. These technologies had to be accompanied by evidence of their efficacy to fulfill National Aeronautics and Space Administration (NASA) requirements for this mission (32); the methods were not validated for continued use in the space industry. There has been much interest in developing alternative and validated low-temperature methods that would enable both larger modules and small components to be decontaminated. Such methods must operate at a low temperature (<60°C), be compatible with a number of different materials used within the space industry, leave no significant levels of residues, and be able to be carried out safely without uncontrolled exposure to the decontamination technology operators. The technology should also be scalable to allow the decontamination of various sizes of objects, from small components to entire spacecraft.
Low-temperature decontamination is widely used within the medical-device, pharmaceutical, and laboratory sectors (1, 3, 5, 13, 18, 25). Gaseous hydrogen peroxide (10, 17, 20, 29) and chlorine dioxide technologies (6, 10, 19, 34, 39) have been shown to be effective against a wide range of bacterial and viral organisms.
This investigation set out to assess alternatives to dry-heat microbial reduction by investigating low-temperature decontamination technologies for the surface decontamination of heat-sensitive spacecraft components. The technologies were selected after an extensive literature review and scoring matrix to determine the most appropriate for this application. Two of the technologies tested for their biological efficacy, material compatibility, and residue formation are described here.
MATERIALS AND METHODS
Technology selection.
A technical review of current gaseous decontamination technologies used in laboratories, pharmaceutical environments, and health care settings was carried out to determine the most suitable technologies to be used here (data not included). The technologies selected were methods utilizing vapor hydrogen peroxide (VHP; Steris, Inc., United Kingdom) and gaseous chlorine dioxide (ClO2 [ClorDiSys], supplied by Primatec, United Kingdom).
Microorganisms.
Commercially available biological indicators preloaded with >106 spores of Bacillus atrophaeus ATCC 9372 (SGM Biotech, USA) and Geobacillus stearothermophilus ATCC 7953 (Steris) sealed within Tyvek and Mylar pouches were selected for use after a prestudy trial (data not shown).
In addition, three naturally occurring organisms (NOO), previously isolated from spacecraft clean room assembly facilities—Bacillus megaterium (2c1 European Space Agency [ESA] organism reference [clean room isolate]), Bacillus safensis (DSM 19292), and Bacillus thuringiensis (E24 ESA organism reference [clean room isolate])—were chosen and supplied in spore suspensions (DLR, Germany). Biological indicators using these spore suspensions were produced using each NOO spore suspension; 10 μl of >108 spores/ml of each spore suspension was pipetted and dried (22 ± 3°C for 2 h) onto clean stainless steel coupons (Grade 316; M-Tech Diagnostics, United Kingdom). After drying the coupons were then heat sealed into Tyvek and Mylar pouches (SGM Biotech) and kept at 4 ± 2°C for <48 h prior to exposure. These NOO biological indicators were exposed to three cycles of the most efficacious cycle from each of the decontamination technologies, determined from the results of the commercial BI exposure.
Exposure chamber.
All processes were undertaken in the environmental chamber facility of the Health Protection Agency (HPA), Porton Down, United Kingdom. The facility consisted of an ante room connected to a sealable room with an internal volume of 20.7 m3. The anteroom was used for preparation and collection of samples.
The gaseous decontamination generators were connected to the chamber using locking gas ports on the exterior wall. A fan (100 liters/min; CED, United Kingdom) was placed into the chamber (at floor level) to assist with the mixing of the decontaminant. A viewing window from chamber to ante room had two glove ports beneath, which enabled BI to be placed into phosphate-buffered saline (PBS) at specific time points to stop the exposure to the decontaminant.
The chamber was set up with sensor probes to detect the concentration of the gaseous decontaminant, the relative humidity, and the temperature in the immediate vicinity of the BI coupons. These sensor probes were used to ascertain when the maximum concentration of gaseous decontaminant had been achieved for the VHP and ClO2 generators. The VHP probes were self-contained units, VHP ARD system sensing units, which incorporated a Fluke digital thermometer, a Digitron relative humidity probe, and an ATI VHP electrochemical sensor. The ClO2 sensor was integral to the generator; gas from the chamber was returned to the sensor (Optex model AF26-S15; Primatec) from the sample area by tubing. A separate probe containing the relative humidity and temperature sensors (Vaisala model HMD4DY; Primatec) was connected to the generator and placed in the chamber. All probes were calibrated prior to use.
Generators.
The generator supplied by Steris used during the study was a VHP-1000ARD generator. This generator dehumidified the air within the chamber to 20% during the dehumidification phase. The conditioning phase was then begun when 35% liquid hydrogen peroxide (Steris) was injected into the chamber to achieve the required concentration of VHP in the air. The VHP concentration was maintained above the set point during the decontamination phase for the required period of time. The aeration phase allowed the removal of VHP from the chamber to a safe limit for entry to remove the exposed coupons. An external heater (Dragon Two; Delonghi, United Kingdom) was placed in the chamber to increase the temperature to 35°C during the study.
A ClorDiSys Minidox M generator was supplied for the study (PrimaTec, United Kingdom). The generator operated by entering a preconditioning phase where a humidifier was used to increase the relative humidity within the chamber to >65%. The conditioning phase held the relative humidity for a preset time. During the charge phase the generator produced and injected the chlorine dioxide gas into the chamber. The gas concentration was held at a predetermined level within the chamber in the exposure phase. The aeration phase was used to remove the chlorine dioxide gas from the chamber.
The generators were operated by trained HPA staff. The VHP generator was installed and programmed with multiple cycles (Table 1) by the company engineers. The two different technologies were studied at different temperatures with VHP at 35°C and ClO2 at 25°C due to problems with condensation at the higher temperature on the photometer lens in the ClO2 generator (external to the chamber and therefore at a lower temperature) causing the decontamination cycle to continually abort. This problem was ameliorated but not totally resolved by reducing the chamber's temperature to 25°C, but it caused time delays that only allowed one ClO2 concentration to be studied in the investigation. The VHP generator was also operated outside of the chamber but did not suffer from the same problems because the sensors were within the chamber. The sampling points were chosen after a short study using each generator was undertaken (data not shown).
Table 1.
Decontamination cycle parameters
| Technology | Generator | Decontaminant concn (ppm) | Exposure period (min) | Temp (°C) |
|---|---|---|---|---|
| VHP | ARD-1000 | 750 | 20 | 35 |
| ARD-1000 | 625 | 50 | 35 | |
| ARD-1000 | 500 | 45 | 35 | |
| ClO2 | Minidox M | 396 | 60 | 25 |
Experimental procedure.
For each sterilization cycle, 18 of each type of commercially produced biological indicator coupons (Steris and SGM Biotech, Ltd.) were prepared. Fifteen of the coupons were attached by their pouches to a supporting frame that rested on a table inside the test chamber within reach of the glove ports. Three coupon pouches were retained as the unexposed controls within the laboratory. The frame was then placed within a sealed box, held at positive pressure to the exposure chamber. When the peak decontaminant concentration was reached, the box was opened, exposing the BI and starting the exposure period.
The three unexposed BI controls of each organism, representing the zero time point, were opened and placed into universal tubes (Sterilin, United Kingdom) containing 5 ml of PBS and 0.1% Tween 80 (VWR, United Kingdom) and four glass beads (3 mm in diameter; VWR) using sterile disposable forceps (SLS, United Kingdom).
At each of the additional five time points, the BI within the Tyvek pouches (n = 3) were aseptically deposited into universal tubes containing 5 ml of PBS and 0.1% Tween 80 and four glass beads (3 mm in diameter) using sterile disposable forceps. Previous experiments determined that there was no loss of viability to the BI caused by absorption of hydrogen peroxide into the containers even over extended periods of opening (5 min maximum opening) (data not included). The order in which the BI were taken during each sampling period was alternated (i.e., in one cycle organism A would be taken before organism B, and then in the next cycle organism B would be taken before organism A) to achieve an even exposure for each organism over the set of triplicate cycles. The chamber was aerated after the final coupon had been taken. The samples were removed to the laboratory where they were processed within 1 h after the end of the exposure period.
The universals were ultrasonicated (5 min) within a water bath (Branson series 5510; 42 KHz, input power of 185 W) to aid removal of the spores from the coupons. The universal tubes containing the coupons were then removed and vortexed (5 min) (Heidolph Multireax; SLS), after which a further 5 ml of sterile water (Aguettant, United Kingdom) was added, and the samples were vortexed for an additional 5 min.
Serial dilutions (1 in 10) to 10−4 were prepared using sterile water. Aliquots (100 μl) of the appropriate dilution were pipetted and spread onto Trypticase soy agar (TSA [bioMérieux, Marcy l'Etoile, France]) in duplicate. The plates were incubated at the recommended temperature (60°C for G. stearothermophilus and 37°C for all four other organisms used) for 48 h, after which the colonies were enumerated; the samples were then refrigerated from 2 to 6°C, while the plates were incubated. If no organisms were detected after plating 100 μl of the neat sample dilution, then the entire sample was filtered through a 0.2-μm-pore-size Cyclopore track etched membrane (Whatman, USA). Once the sample had been filtered, the filter was placed directly onto a TSA plate and incubated at the appropriate temperature for 48 h. A total of nine BI were processed for each time point (i.e., three replicates per run × three runs).
Data analysis.
The data were expressed in terms of the survival fraction, which was calculated as the proportion of organisms recovered after a set exposure period divided by the unexposed population: survival fraction = N/N0. These data were processed in SigmaPlot 10.0, where the data was log transformed and then graphically analyzed. Average values (n = 9, three replicates per run × three runs) were plotted with error bars representing geometric standard deviations.
To avoid biasing the results by the low levels of spore recovery from the later sample points, an arbitrary cutoff point for the data was used for regression calculation. Any data set(s) with no detectable spores in six of the nine filtered samples and only one spore in the remaining three samples was treated as having no spores detectable and was removed from the regression calculation. A linear regression was plotted to calculate the D values (time taken for a 1-log reduction in spore numbers) (Table 2).
Table 2.
Comparison of values for each microorganism exposed to VHP and ClO2 technologies
| Organism | Decontaminant (concn [ppm]) | D value (mg/liter) |
|---|---|---|
| G. stearothermophilus | VHP (500) | 585.4 |
| VHP (625) | 493.3 | |
| VHP (750) | 159.8 | |
| ClO2 (396) | 726.7 | |
| B. atrophaeus | VHP (500) | 92.7 |
| VHP (625) | 76.9 | |
| VHP (750) | 48.4 | |
| ClO2 (396) | 924.4 | |
| B. megaterium | VHP (750) | 45.8 |
| ClO2 (396) | 757.8 | |
| B. safensis | VHP (750) | 68.6 |
| ClO2 (396) | 627.8 | |
| B. thuringiensis | VHP (750) | 175.4 |
| ClO2 (396) | 2.38 × 104 |
RESULTS
Exposure to VHP caused a rapid inactivation of B. atrophaeus spores with a 6-log reduction in survival fraction within 6 min and a 5-log reduction in G. stearothermophilus in 20 min (Fig. 1). A steady decrease in the survival of B. thuringiensis spores was witnessed over the 20-min exposure period, with a 6-log reduction in the survival fraction by the end of the exposure period.
Fig 1.
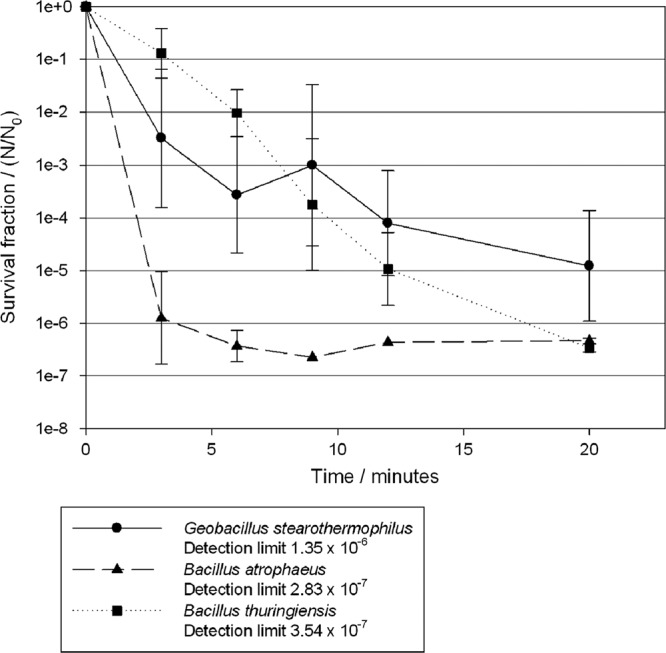
Survival fractions of G. stearothermophilus (●), B. atrophaeus (▲), and B. thuringiensis (■) after exposure to VHP at a concentration of 750 ppm for 20 min.
Exposure to ClO2 caused a steady reduction in the survival fraction of G. stearothermophilus of ∼5 logs in 60 min (Fig. 2). The survival fraction of B. atrophaeus also reduced by 5 logs at 60 min. B. thuringiensis numbers were not significantly reduced during the 60-min exposure period.
Fig 2.
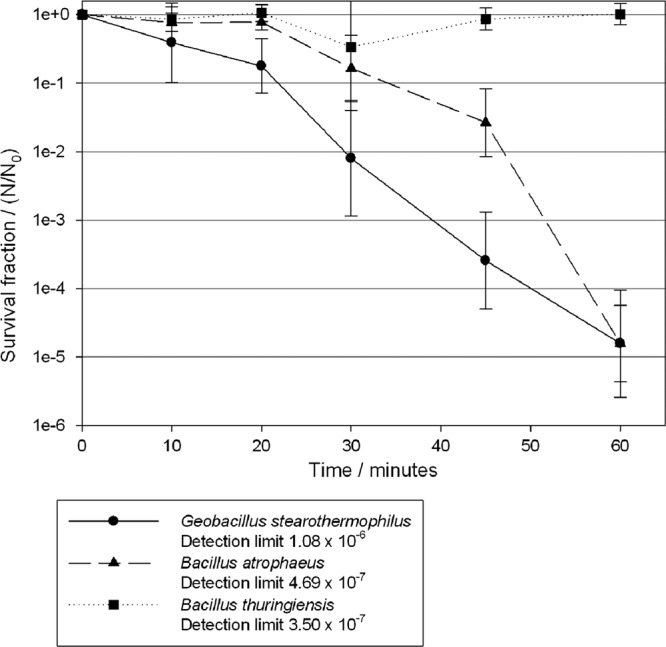
Survival fractions of G. stearothermophilus (●), B. atrophaeus (▲), and B. thuringiensis (■) after exposure to ClO2 at a concentration of 396 ppm for 60 min.
The D values (Table 2) for G. stearothermophilus decreased from 585.4 (500 ppm) to 159.8 s (750 ppm). A greater D value of 726.7 s was observed when ClO2 at a concentration of 396 ppm was used. With B. atrophaeus the D value was 48.4 s at 750 ppm and 92.7 s at 500 ppm. The D value for B. atrophaeus exposed to ClO2 was 924.4 s. B. megaterium and B. safensis had D values that closely matched that of B. atrophaeus when exposed to VHP (750 ppm) and that of G. stearothermophilus when exposed to ClO2. The D values for B. thuringiensis were greater than those for both B. atrophaeus and G. stearothermophilus when exposed to both VHP and ClO2, which were 175.4 s and 6.6 h, respectively.
DISCUSSION
Prior to the launch of spacecraft bound for planetary bodies, the microbial load must be monitored, controlled, and potentially reduced in order to satisfy Planetary Protection guidelines to ensure any risks of forward contamination to other celestial bodies are minimized (2). While the current validated method uses DHMR, the increase in the number of thermolabile materials being used in spacecraft today has led to a requirement for the development of alternative low-temperature surface decontamination technologies. We investigated here the biological efficacy of two low-temperature gaseous decontamination technologies using a range of biological spore indicators.
The VHP and ClO2 systems achieved a 5-log reduction in the recovery of the biological indicators used within 20 and 60 min, respectively (Fig. 1 and 2). The results are expressed in graphs as survival fractions, allowing linear regressions to be drawn and D values to be calculated. As indicated in Materials and Methods, these results were adjusted to take account of the low numbers of spores that were recovered from a small number of samples that may otherwise have led to a bias and skewing of the D values. D values were first used for heat sterilization and describe this process by first-order kinetics (4). However, gaseous disinfection is a more complex process that requires a decontaminant to penetrate into a biofilm of microorganisms dried onto a surface and to cause irreversible damage to these spores. The use of D values is a simplified description of the inactivation kinetics, and the use of D values produced from the linear regression may lead to an incomplete decontamination procedure. Therefore, it is recommended that the D values should be used as guidelines for the overestimation of exposure periods rather than exact times. For example, the D value for B. thuringiensis was 175.4 s compared to that for G. stearothermophilus (159.8 s). However, after a 20-min exposure there was a difference of >1 log in the survival fractions of the two organisms, with G. stearothermophilus exhibiting greater survival (Fig. 2) as the rate of killing for G. stearothermophilus slowed over the last few time points.
This retention of viability in a small subsection of spores that remain resistant to gaseous disinfectants is a phenomenon known as “trailing” (33). Various explanations for this include: (i) the presence of a subpopulation of hyper-resistant spores, (ii) the occlusion of spores by layering or other factors, and/or (iii) the possibility of cross-contamination. However, each of these explanations can be regarded as a product of the experimental situation and has limited relevance to a natural situation wherein contamination on space hardware will be of a much lower magnitude in terms of density (26, 36, 37).
We have demonstrated here that G. stearothermophilus spores were the more resistant of the two commercially available indicators for VHP, whereas B. atrophaeus was more resistant to ClO2 (Table 2). These results are in line with the recommendations from the respective companies, G. stearothermophilus for VHP (Steris) (22, 24) and B. atrophaeus for ClO2 (ClorDiSys) (28), for organisms to be used as biological indicators to validate their processes, respectively. Two of the naturally occurring organisms, B. megaterium and B. safensis, demonstrated lower resistance to the decontamination technologies compared to the recommended biological indicators. However, B. thuringiensis exhibited a level of resistance comparable to that of G. stearothermophilus when exposed to VHP, with respective D values of 175.4 and 159.8 s. In the case of ClO2, B. thuringiensis exhibited a greatly increased resistance compared to B. atrophaeus, with D values of 6.6 h and 924.4 s, respectively (Fig. 2). The resistance of B. thuringiensis to ClO2 gas has previously been demonstrated (15), a study wherein 106 spores of B. thuringiensis were dried onto paper, wood, and epoxy surfaces and then exposed to ClO2 (5,400 ppm) in a sealed container for 720 min. In this case, there was a single injection of ClO2 in the exposure chamber, and the concentration decreased with time. A minimum of 10,800 ppm of ClO2 was required to completely inactivate the spores on paper and wood (15). Microbial reduction using VHP has previously been demonstrated to be dependent on the initial microbial loading on coupons, e.g., MS2 coliphage at concentrations of 1010 PFU (33). However, the initial loading with B. thuringiensis in the present study was considerably lower (106 PFU). These results indicate that further work is required to determine the mode of resistance of B. thuringiensis and to determine whether it is species or, indeed, strain specific.
There was an increase in the rate of killing after the first 20-min period for B. atrophaeus and G. stearothermophilus exposed to ClO2, which may be explained by the mode of operation of the generator (Fig. 2). The ClO2 technology uses an external humidifier to raise the humidity within the chamber to >65% during the preconditioning phase prior to ClO2 injection. The increase in the humidity above that normally found in the chamber may allow the water vapor to microcondense onto surfaces and penetrate into a dried population of microorganisms. Chlorine dioxide readily dissolves in water (28); if this water has condensed onto the surfaces and surrounds the spores, then there will be greater penetration into the coupons and a quicker kill. In the present study the biological indicators were kept within a positively pressurized box during the conditioning phase and only exposed at the peak ClO2 concentration. This suggests that the initial slow reduction in survival fraction may be a lack of penetration of water vapor during the preconditioning and conditioning phases, followed by absorption of the ClO2 into the dried spore population on the coupons.
While the VHP system produced more rapid kills, for example, with G. stearothermophilus (D value of 159.8 s) compared to the ClO2 system (D value of 726.7 s), respectively, the kill time for the ClO2 system was still within the expected range (PrimaTec, unpublished data). Indeed, the concentrations of ClO2 used in a decontamination cycle are normally far higher (5 to 30 mg/liter) than the concentration used here (1.1 mg/liter, 396 ppm) (9, 15, 19, 23), and the higher levels decrease the kill time for the biological indicators (19). However, the increased ClO2 concentration could potentially lead to greater damage of sensitive spacecraft materials through the deposition of chemical residues (based on current material compatibility and residue analysis data).
Biological indicators are widely used to demonstrate the efficacy of decontamination cycles (35, 43). Microbiological indicators are produced with 106 microorganisms dried within a 1-cm2 area (7). This high point loading is not representative of environments where the level of contamination may be lower, e.g., in spacecraft assembly clean rooms where the density of microorganisms on surfaces is at very low levels, i.e., approximately 0 to 4 CFU/cm2 (26, 36, 37). A biological indicator could be produced using the standard organism G. stearothermophilus or using B. thuringiensis spores, which both have comparable levels of resistance to 750 ppm of VHP in terms of D values but with a reduced loading concentration. Therefore, a more appropriate biological indicator for this setting may contain a lower loading of spores, i.e., 103 or 104, dried onto a larger area (10 cm2). This indicator would then present a realistic but stringent challenge for gaseous decontamination technology. The combination of the decontamination cycle at the highest concentrations shown here and the actual low surface contamination in spacecraft assembly facilities shows that the D values produced within the present study can be used as effective guidelines to ensure a safe decontamination.
In conclusion, we have demonstrated that low-temperature gaseous decontamination technologies can be used as an appropriate alternative to the existing decontamination procedure of DHMR and, on the basis of the biological efficacy and material compatibility results, VHP has been chosen by the European Space Agency as an alternative to DHMR.
ACKNOWLEDGMENTS
We acknowledge the European Space Agency for the funding of this project (AO/1–5434/07/NL/EK).
We thank technical officer Gerhard Kminek for assistance. We also acknowledge the commercial companies Steris and ClorDiSys for the provision of the equipment and their engineers for its support and installation.
The views expressed here are those of the authors, not those of the HPA or any other funding source.
Footnotes
Published ahead of print 6 April 2012
REFERENCES
- 1. Andersen BM, et al. 2006. Decontamination of rooms, medical equipment and ambulances using an aerosol of hydrogen peroxide disinfectant. J. Hosp. Infect. 62:149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anonymous 2002. COSPAR planetary protection policy. Workshop on Planetary Protection. Committee on Space Research, International Council for Science, Paris, France [Google Scholar]
- 3. Bates CJ, Pearse R. 2005. Use of hydrogen peroxide vapour for environmental control during a Serratia outbreak in a neonatal intensive care unit. J. Hosp. Infect. 61:364–366 [DOI] [PubMed] [Google Scholar]
- 4. Block SS. 2001. Disinfection, sterilization, and preservation, 5th ed Lippincott/The Williams & Wilkins Co, Philadelphia, PA [Google Scholar]
- 5. Bolister NJ, Johnson HE, Wathes CM. 1992. The ability of airborne Klebsiella pneumoniae to colonize mouse lungs. Epidemiol. Infect. 109:121–131 [PMC free article] [PubMed] [Google Scholar]
- 6. Canter DA. 2005. Addressing residual risk issues at anthrax cleanups: how clean is safe? J. Toxicol. Environ. Health A 68:1017–1032 [DOI] [PubMed] [Google Scholar]
- 7. Cheney JE, Collins CH. 1995. Formaldehyde disinfection in laboratories: limitations and hazards. Br. J. Biochem. Sci. 52:195–201 [PubMed] [Google Scholar]
- 8. Committee on Preventing the Forward Contamination of Mars 2006. Preventing the forward contamination of Mars. National Academy Press, Washington, DC [Google Scholar]
- 9. Czarneski MA, Lorcheim P. 2005. Isolator decontamination using chlorine dioxide gas. Pharm. Tech. 29:124–133 [Google Scholar]
- 10. Davies A, Pottage T, Bennett A, Walker J. 2011. Gaseous and air decontamination technologies for Clostridium difficile in the healthcare environment. J. Hosp. Infect. 77:199–203 [DOI] [PubMed] [Google Scholar]
- 11. Debus A. 2006. The European standard on planetary protection requirements. Res. Microbiol. 157:13–18 [DOI] [PubMed] [Google Scholar]
- 12. Dye C, Scheele S, Dolin P, Pathania V, Raviglione M. 1999. Global burden of tuberculosis: estimated incidence, prevalence and mortality by country. JAMA 282:677–686 [DOI] [PubMed] [Google Scholar]
- 13. French GL, et al. 2004. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): a comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J. Hosp. Infect. 57:31–37 [DOI] [PubMed] [Google Scholar]
- 14. Hall LB, Bruch CW. 1965. Procedures necessary for the prevention of planetary contamination. Life Sci. Space Res. 3:48–62 [PubMed] [Google Scholar]
- 15. Han Y, Applegate B, Linton RH, Nelson PE. 2003. Decontamination of Bacillus thuringiensis spores on selected surfaces by chlorine dioxide gas. J. Environ. Health 66:16–21 [PubMed] [Google Scholar]
- 16. Hannan MM, Azadian BS, Gazzard BG, Hawkins DA, Hoffman PN. 2000. Hospital infection control in an era of HIV infection and multidrug resistant tuberculosis. J. Hosp. Infect. 44:5–11 [DOI] [PubMed] [Google Scholar]
- 17. Heckert RA, et al. 1997. Efficacy of vaporized hydrogen peroxide against exotic animal viruses. Appl. Environ. Microbiol. 63:3916–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jahnke M, Lauth G. 1997. Biodecontamination of a large volume filling room with hydrogen peroxide. Pharm. Eng. 17:96–108 [Google Scholar]
- 19. Jeng DK, Woodworth AG. 1990. Chlorine dioxide gas sterilization under square-wave conditions. Appl. Environ. Microbiol. 56:514–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kahnert A, et al. 2005. Decontamination with vaporized hydrogen peroxide is effective against Mycobacterium tuberculosis. Lett. Appl. Microbiol. 40:448–452 [DOI] [PubMed] [Google Scholar]
- 21. Kempf MJ, Chen F, Kern R, Venkateswaran K. 2005. Recurrent isolation of hydrogen peroxide-resistant spores of Bacillus pumilus from a spacecraft assembly facility. Astrobiology 5:391–405 [DOI] [PubMed] [Google Scholar]
- 22. Klapes NA, Vesley D. 1990. Vapor-phase hydrogen peroxide as a surface decontaminant and sterilant. Appl. Environ. Microbiol. 56:503–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knapp JE, et al. 1986. Chlorine dioxide as a gaseous sterilant. Med. Device Diagn. Ind. 8:48–50 [Google Scholar]
- 24. Krause J, McDonnell G, Riedesel H. 2001. Biodecontamination of animal rooms and heat-sensitive equipment with vaporized hydrogen peroxide. Contemp. Top. Lab. Anim. Sci. 40:18–21 [PubMed] [Google Scholar]
- 25. Krishnan U, et al. 1994. Variations in quantitative respirator fit factors due to fluctuations in leak size during fit testing. Am. Ind. Hyg. Assoc. J. 55:309–314 [DOI] [PubMed] [Google Scholar]
- 26. La Duc MT, Kern R, Venkateswaran K. 2004. Microbial monitoring of spacecraft and associated environments. Microb. Ecol. 47:150–158 [DOI] [PubMed] [Google Scholar]
- 27. La Duc MT, Satomi M, Venkateswaran K. 2004. Bacillus odysseyi sp. nov., a round-spore-forming bacillus isolated from the Mars Odyssey spacecraft. Int. J. Syst. Evol. Microbiol. 54:195–201 [DOI] [PubMed] [Google Scholar]
- 28. Luftman HS, Regits MA. 2008. Bacillus atrophaeus and Geobacillus stearothermophilus biological indicators for chlorine dioxide gas decontamination. J. Appl. Biosafety. 13:143–157 [Google Scholar]
- 29. McDonnell G, Grignol G, Antloga K. 2002. Vapour-phase hydrogen peroxide decontamination of food contact surfaces. Dairy Food Environ. Sanitat. 22:868–873 [Google Scholar]
- 30. Moissl C, et al. 2007. Molecular bacterial community analysis of clean rooms where spacecraft are assembled. FEMS Microbiol. Ecol. 61:509–521 [DOI] [PubMed] [Google Scholar]
- 31. Nagarkar PP, Ravetkar SD, Watve MG. 2001. Oligophilic bacteria as tools to monitor aseptic pharmaceutical production units. Appl. Environ. Microbiol. 67:1371–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pillinger JM, Pillinger CT, Sancisi-Frey S, Spry JA. 2006. The microbiology of spacecraft hardware: lessons learned from the planetary protection activities on the Beagle 2 spacecraft. Res. Microbiol. 157:19–24 [DOI] [PubMed] [Google Scholar]
- 33. Pottage T, Richardson C, Parks S, Walker JT, Bennett AM. 2010. Evaluation of hydrogen peroxide gaseous disinfection systems to decontaminate viruses. J. Hosp. Infect. 74:55–61 [DOI] [PubMed] [Google Scholar]
- 34. Rastogi VK, et al. 2011. Systematic evaluation of the efficacy of chlorine dioxide in decontamination of building interior surfaces contaminated with anthrax spores. Appl. Environ. Microbiol. 76:3343–3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sansoe-Bourget E. 2006. Risk assessment paradigm: an opportunity for rationalizing the choice of biological indicator during the validation of isolator biodecontamination cycles. PDA J. Pharm. Sci. Technol. 60:156–163 [PubMed] [Google Scholar]
- 36. Schuerger AC. 2003. Survival of endospores of Bacillus subtilis on spacecraft surfaces under simulated Martian environments: implications for the forward contamination of Mars. Icarus 165:253–276 [DOI] [PubMed] [Google Scholar]
- 37. Schuerger AC. 2008. Use of non-thermal atmospheric plasmas to reduce the viability of Bacillus subtilis on spacecraft surfaces. Int. J. Astro. 7:47–57 [Google Scholar]
- 38. Stieglmeier M, Wirth R, Kminek G, Moissl-Eichinger C. 2009. Cultivation of anaerobic and facultatively anaerobic bacteria from spacecraft-associated clean rooms. Appl. Environ. Microbiol. 75:3484–3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang J, et al. 2005. Door-opening motion can potentially lead to a transient breakdown in negative-pressure isolation conditions: the importance of vorticity and buoyancy airflows. J. Hosp. Infect. 61:283–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Task Group on the Forward Contamination of Europa 2000. Preventing the forward contamination of Europa. National Academy Press, Washington, DC [Google Scholar]
- 41. Venkateswaran K, et al. 2003. Bacillus nealsonii sp. nov., isolated from a spacecraft-assembly facility, whose spores are gamma-radiation resistant. Int. J. Syst. Evol. Microbiol. 53:165–172 [DOI] [PubMed] [Google Scholar]
- 42. Walker JT, Dickinson J, Sutton JM, Raven ND, Marsh PD. 2007. Cleanability of dental instruments: implications of residual protein and risks from Creutzfeldt-Jakob disease. Br. Dent. J. 203:395–401 [DOI] [PubMed] [Google Scholar]
- 43. Wright AM, Hoxey EV, Soper CJ, Davies DJG. 1995. Biological indicators for low temperature steam and formaldehyde sterilization: the effect of defined media on sporulation, growth index, and formaldehyde resistance of spores of Bacillus stearothermophilus strains. J. Appl. Bacteriol. 79:432–438 [DOI] [PubMed] [Google Scholar]


