Abstract
The ability of microorganisms to perform dissimilatory (per)chlorate reduction is, for most species, known to be oxygen sensitive. Consequently, bioremediation processes for the removal of oxochlorates will be disturbed if oxygen is present. We measured the expression of chlorite dismutase and chlorate reductase in the presence of different terminal electron acceptors in the chlorate reducer Ideonella dechloratans. Enzyme activity assays and mRNA analyses by real-time quantitative reverse transcription (qRT)-PCR were performed on cell extracts from cells grown aerobically with and without chlorate and on cells grown anaerobically in the presence of chlorate. Our results showed that both chlorite dismutase and chlorate reductase are expressed during aerobic growth. However, transfer to anaerobic conditions with chlorate resulted in significantly enhanced enzyme activities and mRNA levels for both enzymes. Absence of oxygen was necessary for the induction to occur, since chlorate addition under aerobic conditions produced neither increased enzyme activities nor higher relative levels of mRNA. For chlorite dismutase, the observed increase in activity was on the same order of magnitude as the increase in the relative mRNA level, indicating gene regulation at the transcriptional level. However, chlorate reductase showed about 200 times higher enzyme activity in anaerobically induced cells, whereas the increase in mRNA was only about 10-fold, suggesting additional mechanisms influence the enzyme activity.
INTRODUCTION
The use of chlorine oxyanions, such as chlorate (ClO3−) and perchlorate (ClO4−), in industry has resulted in contaminated soils and waters, threatening the environment. Chlorate, for example, is highly toxic to brown algae (25, 35), and perchlorate may have a direct influence on human health, as it affects the function of iodide transporters in the thyroid and mammary glands (9, 17, 38). The main source of chlorate contamination is chlorine dioxide (ClO2) bleaching in the pulp and paper industry (8), and the most efficient remediation techniques for both chlorate and perchlorate are based on degradation performed by microorganisms (13, 33, 43). More than 40 bacterial strains that are able to grow by dissimilatory perchlorate or chlorate reduction have been isolated (2, 6, 10, 13, 15, 18, 21, 22, 24, 36, 37, 39, 40). They are phylogenetically diverse, with members in the Alpha-, Beta-, Gamma-, and Epsilonproteobacteria (13, 15). Recently two members of the Firmicutes were also reported to be dissimilatory perchlorate reducers (3, 4). All the known strains are facultative anaerobes or microaerophiles. Perchlorate-respiring bacteria (PRB) can use both perchlorate and chlorate as terminal electron acceptors, whereas chlorate-respiring bacteria (CRB) use only chlorate. This reflects the presence of two different enzymes, a perchlorate reductase (Pcr) in PRB that reduces perchlorate to chlorite via chlorate and a chlorate reductase (Clr) in CRB that reduces chlorate to chlorite. The toxic chlorite formed by the action of (per)chlorate reductase is decomposed to molecular oxygen and chloride by the enzyme chlorite dismutase (Cld). The following pathway is widely accepted for dissimilatory (per)chlorate degradation: ClO4− → ClO3 − → ClO2− → O2 + Cl− (23, 24).
The oxygen produced by the action of chlorite dismutase during (per)chlorate respiration is believed to be used by a terminal oxidase in the (per)chlorate-respiring bacteria concomitant with (per)chlorate respiration (1, 24). However, oxygen at higher concentrations is known to inhibit (per)chlorate reduction (12, 24, 27, 34, 39). For some species, inhibition can be observed immediately upon introduction of oxygen to anaerobically grown cultures (12, 34). A concentration of dissolved oxygen as low as 1.6 μM has been reported to inhibit growth of Azospira sp. strain KJ (27). The ability to perform (per)chlorate reduction is restored when (per)chlorate is offered as the sole electron acceptor, but recovery phases extending from hours to several days have been observed (26, 39, 44). The CRB Pseudomonas sp. strain PDA is the only strain reported to constitutively express (per)chlorate-reducing capacity. Aerobically grown cultures of the strain do not show any lag period when they are transferred to chlorate-respiring growth conditions (44). The high oxygen sensitivity of microbial (per)chlorate reduction in combination with long lag phases after exposure to oxygen reduces the efficiency of chlorate or perchlorate removal. This can be overcome by adding excess electron donor to sustain the growth of non-(per)chlorate-reducing microorganisms, but such treatment has drawbacks, like increased cost and reduction of water quality (14).
Understanding the regulating mechanisms leading to the inhibitory effects of oxygen is essential in order to control and optimize microbial (per)chlorate degradation, but today, this knowledge is scanty. For some PRB, the activity of chlorite dismutase has been found to be absent or present only at a low level in aerobically grown cells, whereas it is induced by up to 50 times with (per)chlorate as the only terminal electron acceptor (24, 42). The PRB Dechloromonas agitata strain CKB has been investigated in further detail. Northern blot hybridization studies showed that the gene for chlorite dismutase (cld) was expressed under aerobic conditions and that it was highly induced in cells grown with perchlorate in the absence of oxygen (5), while the gene for subunit A of perchlorate reductase (pcrA) was expressed exclusively under anaerobic (per)chlorate-reducing conditions (7). Recently, it was also shown by real-time quantitative reverse transcription (qRT)-PCR that mRNA levels for both the cld and pcrA genes decreased upon transfer of anaerobic perchlorate-reducing cultures of the PRB Rhodocyclaceae strain JDS4 to aerobic conditions (16). Transcripts from both genes were detected even after loss of perchlorate-reducing ability, but it was not clear if it was due to de novo synthesis or long half-lives of the mRNAs.
However, transcription studies of the cld and chlorate reductase subunit A (clrA) genes have not been reported previously in a CRB. Here, we report on a study of enzyme activities and relative transcription levels in the CRB Ideonella dechloratans (22) grown in the presence of oxygen, chlorate, or a combination of these electron acceptors. The results show that both enzymes are regulated in an oxygen-dependent way at the transcriptional level and that the activity of chlorate reductase is also influenced by other oxygen-dependent mechanisms.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
I. dechloratans was obtained from the culture collection of Göteborg University, Göteborg, Sweden (CCUG 30977). Freeze cultures were inoculated in 5 ml PC medium (5 g tryptone and 2.5 g yeast extract per liter deionized water at pH 7.0) in 15-ml Falcon plastic tubes and grown aerobically, with the tubes in a horizontal position, at 180 rpm and 37°C for 16 to 18 h. These cultures were transferred to three different growth conditions: (i) PC medium with oxygen as the sole electron acceptor, (ii) PC medium with oxygen and sodium chlorate (10 mM) as electron acceptors, and (iii) a defined medium with acetic acid (25 mM) as the electron donor and carbon source and with sodium chlorate (10 mM) as the electron acceptor (22). Aerobic cultures (i and ii) were harvested at 2,000 × g for 10 min and transferred to fresh PC medium without (i) or with (ii) NaClO3 to a final concentration of 10 mM and incubated in partially filled flasks closed with foam stoppers at 180 rpm and 37°C. When indicated, defined medium with sodium chlorate (10 mM) was used for aerobic growth. Anaerobic cultures (iii) were obtained by harvesting the overnight cultures at 2,000 × g for 10 min, washing the pellet once with the defined medium, and thereafter transferring the cells to culture flasks completely filled with the same medium, closed with rubber stoppers, and incubated at 180 rpm and 37°C. To follow the induction of enzyme activity, cultures were harvested at different times from 3 to 24 h after the cells had been transferred to anaerobic growth conditions, and cell extracts were immediately prepared for activity measurements. For comparisons of enzyme activities and mRNA levels between the different growth conditions (i to iii), cells were harvested in mid-log phase at an optical density measured at 600 nm (OD600) of 0.4 to 0.6, resulting in a growth time, after subcultivation, of 3 to 4 h for the aerobic cultures and 20 to 24 h for the anaerobic cultures.
Preparation of cell extracts.
Cultures were harvested by centrifugation at 3,000 × g for 10 min, and the cells were resuspended in 1 to 3 ml of 25 mM bis-Tris propane buffer, pH 7.2, and broken with a Branson Sonifier 450 set at a duty cycle of 50% and an output of 3 for 3 min. During sonication, the samples were cooled in an ice bath with stirring. The cell homogenate was centrifuged for 90 s at 13,400 rpm (Eppendorf Mini Spin) at room temperature, and the supernatant was recovered and kept on ice until it was used for enzyme activity measurements. Activity measurements were performed within 2 h from preparation, and no decrease in enzyme activity was observed during that time.
Enzyme assays.
Chlorite dismutase activity was measured as chlorite-dependent oxygen evolution using a Clark-type electrode (Hansatech Instruments) (29). The reaction chamber contained 2 to 300 μl cell extract and 0.1 M sodium phosphate buffer at pH 7.2 with 5 mM EDTA in a total volume of 2 ml. The reaction was started by the addition of 20 μl sodium chlorite from a freshly prepared stock to a final concentration of 0.25 mM. Three measurements of the initial reaction rate were used for estimation of the enzyme activity for each sample. The activity was normalized to the total protein content and expressed as mmol O2 min−1 mg protein−1.
Chlorate reductase activity was measured as chlorate-dependent oxidation of reduced methyl viologen (MV) as described previously (31). The reaction mixture, containing 15 to 1,250 μl cell extract, 0.3 mM MV, and 25 mM bis-Tris propane buffer at pH 7.2 in a total volume of 3 ml, was placed in a quartz cuvette equipped with a rubber septum and flushed with nitrogen for 5 min prior to measurement. A small amount of nitrogen-flushed sodium dithionite solution was added with a syringe to the cuvette until an absorbance of ≥1 at 600 nm was reached. After recording a stable baseline, the reaction was initiated by the addition of 5 μl NaClO3 from a nitrogen-flushed stock solution to a final concentration of 1.7 mM. Anaerobic samples were measured in triplicate, while 1 to 3 measurements were performed on each aerobic sample. Initial rates of MV reoxidation were calculated by using an extinction coefficient of 1.3 × 104 M−1 cm−1 (32). Samples were normalized to the total protein content, and activity was expressed as μM oxidized MV min−1 mg protein−1.
Total protein content.
The total amount of protein in the cell extracts was determined using a Pierce BCA Protein Assay Kit (Thermo Scientific) following the microplate procedure with bovine serum albumin (BSA) as a standard. The absorbance was measured at 550 nm (EMax; Molecular Devices), and all sampling was performed in duplicate or triplicate.
RNA isolation.
Cultures of I. dechloratans, grown under the three different growth conditions described above, were harvested at an OD600 of about 0.5. Total RNA was extracted with an RNeasy Protect Bacteria Mini Kit (Qiagen), and residual DNA was removed by the addition of RNase-free DNase I (Qiagen), both at an on-column step and after purification. All steps followed the recommendations of the manufacturer. The quality of the RNA preparations was checked by absorbance measurements using a Shimadzu UV-1601PC spectrophotometer and by agarose gel electrophoresis. For all preparations, the A260/A280 ratios were between the expected values of 1.9 and 2.2, and the gels showed two distinct bands for 16S and 23S rRNA without degradation products (data not shown). The total RNA concentration was estimated from the A260 values.
Primer design.
PCR primers were designed for the target genes clrA (AJ566363) and cld (AJ296077) and the reference 16S rRNA gene (X72724) by using the PrimerSelect application of Lasergene (DNAStar). The specificity of the primer pairs was confirmed by performing colony PCR for I. dechloratans and analyzing the PCR products on agarose gels. Each PCR produced a single band on the gel that matched the expected product size. Primer pairs and expected product lengths are shown in Table 1.
Table 1.
Primer design for real-time qRT-PCR analysis
| Gene | Primer (5′→ 3′)a | Expected product length (bp) |
|---|---|---|
| 16SrRNA | F: CATCGGAACGTGCCCAGTAGTG | 116 |
| R: TGACATCGGCCGCTCCAATAG | ||
| cld | F: CACCGCGCTTTGCCTTCAT | 154 |
| R: GAGCCCCGTCGAGTGGTAGAG | ||
| clrA | F: GGACGAAGCGCTCACCGAAATC | 93 |
| R: TGCGAAAGGGCGCTTGGGAATA |
F, forward; R, reverse.
cDNA synthesis and real-time qRT-PCR.
RNA preparations were denatured at 65°C for 10 min and then cooled on ice to avoid the formation of secondary structures prior to cDNA synthesis. One microgram of DNase-treated total RNA was added to 10 μl of reaction mixture from a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems), including random hexamer primers, and prepared according to the instructions of the manufacturer. The total volume was adjusted to 20 μl by the addition of nuclease-free water. Samples without reverse transcriptase were prepared as negative controls to verify the absence of genomic DNA contamination. The reaction mixtures were incubated for 10 min at 25°C, followed by 120 min at 37°C and finally 5 min at 85°C.
Real-time qRT-PCR was performed on a Step One Plus instrument (Applied Biosystems). The reaction mixtures contained cDNA, 12.5 μl Fast SYBR Green Master Mix (Applied Biosystems), 0.2 μM gene-specific primers, and nuclease-free water to a final reaction volume of 25 μl. Reaction mixtures lacking cDNA and the controls from the cDNA synthesis were run as negative controls. The following thermocycler program was used: denaturing at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s, with each sample measured in triplicate. After every run, melting curve analysis was performed to check for primer dimers or nonspecific double-stranded DNA (dsDNA). The threshold cycles (CT values) of the samples were obtained from manual settings of the threshold signal as an intersection of the amplification curve in the linear region of the semilogarithmic plot. For estimation of the efficiency of the amplification step, cDNA from cultures grown anaerobically or aerobically without chlorate was diluted in 4 or 5 steps over a 104- to 105-fold range and amplified by the specific primers for 16S rRNA, clrA, and cld. The amplification efficiency for each cDNA and growth condition was determined from the slope of a plot of the log cDNA concentrations against the CT values and calculated as follows: amplification efficiency = 10(−1/slope) − 1 (11). To determine the relative amount of the target mRNA, the CT values from the clrA- and cld-specific reactions were normalized to the CT values of the reference gene, i.e., the 16S rRNA-specific reaction. The fold difference in target mRNAs between two growth regimes (A and B) was calculated by using the 2−ΔΔCT method (20): relative expression ratio = 2−[(CTtarget B − CTtarget A) − (CT16SrRNA B − CT16SrRNA A)].
RESULTS
Enzyme activities of Cld and Clr under aerobic and anaerobic growth conditions.
In order to investigate if I. dechloratans synthesizes active enzymes necessary for chlorate reduction in the presence of oxygen as the only electron acceptor, aerobically grown cultures were harvested in mid-log growth phase, and the enzyme activities of Cld and Clr were measured in cell extracts. The extracts showed a chlorite-dependent oxygen-evolving capacity corresponding to a Cld activity of 2.7 × 10−2 mmol O2 min−1 mg protein−1 and a chlorate-dependent MV-oxidizing capacity corresponding to a Clr activity of 9 × 10−3 μmol MV min−1 mg protein−1 (Table 2). The same procedure was performed with cells grown aerobically in the presence of chlorate to explore if the addition of the alternative electron acceptor influences the enzyme activity under aerobic conditions. In this case, a Cld activity of 1.9 × 10−2 mmol O2 min−1 mg protein−1 and a Clr activity of 5 × 10−3 μmol MV min−1 mg protein−1 was found (Table 2), i.e., activities similar to those in the cultures grown without chlorate. The measurements were repeated with cultures grown aerobically in the defined medium with the addition of chlorate. This resulted in a Cld activity of 2 × 10−2 mmol O2 min−1 mg protein−1 and a Clr activity of 1 × 10−2 μmol MV min−1 mg protein−1, showing that the choice of culture medium did not have any significant effect on the enzyme activities during aerobic growth.
Table 2.
Summary of enzyme activity measurements
| Terminal electron acceptor | Cld |
Clr |
||
|---|---|---|---|---|
| Mean sp act ± SD (mmol O2 min−1 mg−1) | No. of samples | Mean sp act ± SD (µmol MV min−1 mg−1) | No. of samples | |
| O2 | (2.7 ± 0.4) × 10−2 | 3 | (9 ± 6) × 10−3 | 3 |
| O2 and ClO3− | (1.9 ± 0.2) × 10−2 | 2 | 5 × 10−3 | 1 |
| ClO3− | (1.3 ± 0.2) × 10−1 | 3 | 2.4 ± 1.0 | 3 |
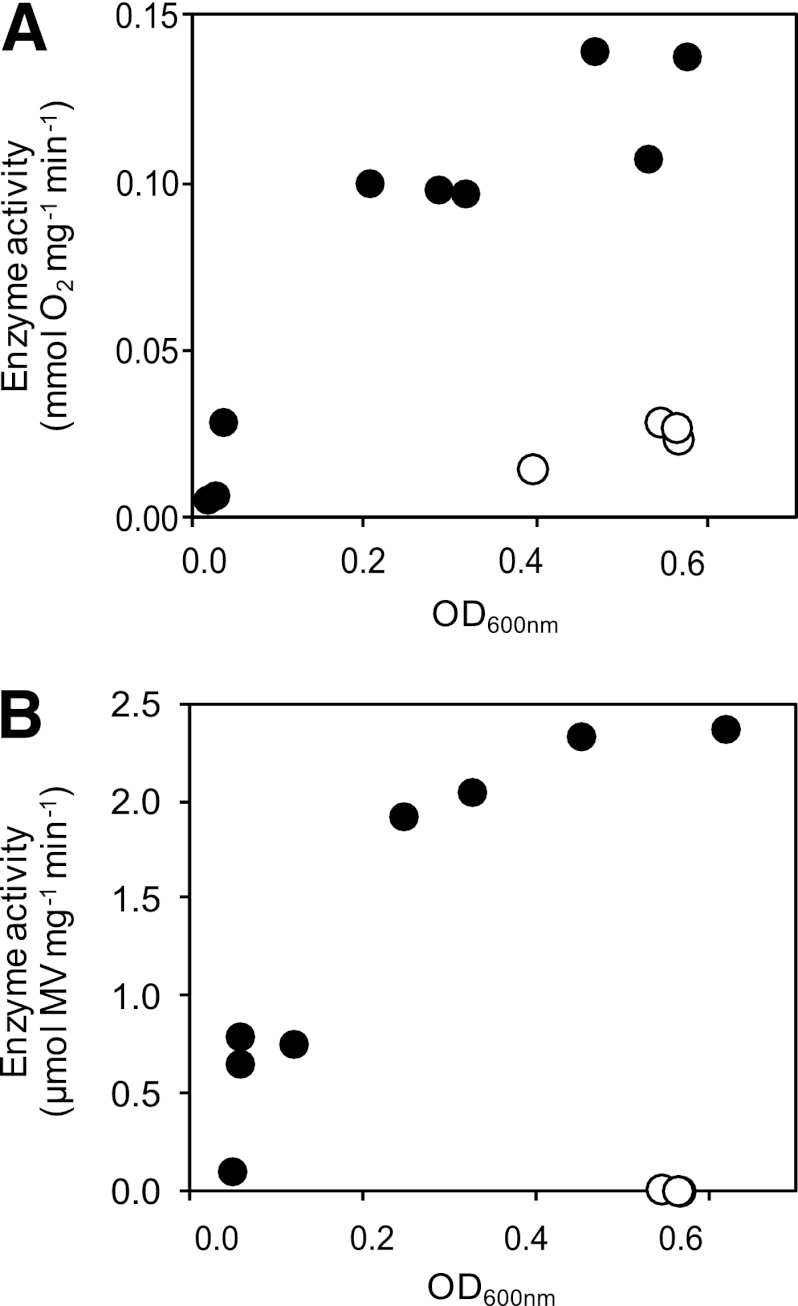
We also studied the possible induction of enzyme activity by transferring aerobically grown overnight cultures to anaerobic conditions with chlorate, as described in Materials and Methods, and measured the activities of Cld and Clr at times from 3 to 24 h after the transfer. Five hours after transfer to anaerobic conditions, the activity of Cld was still the same as for the aerobic cultures, but thereafter, it increased rapidly in the early logarithmic growth phase and reached a maximum at an OD600 of around 0.6 after about 20 h of anaerobic growth (Fig. 1A). The maximal Cld activity was estimated at 1.3 × 10−1 mmol O2 min−1 mg protein−1, which is about five times higher than for aerobically grown cells (Table 2). For Clr, activity 10 times higher than that for aerobic cells was found after only 3 h under anaerobic growth conditions (Fig. 1B). The activity continued to increase to a maximum of 2.4 μmol MV min−1 mg protein−1, i.e., more than 200 times higher than what was found in aerobic cultures (Table 2). Maximal activity was reached at an OD600 of about 0.6, the same as for the Cld activity.
Fig 1.
Enzyme activities of Cld (A) and Clr (B), normalized to total protein content, after transfer of aerobically grown cells to anaerobic (black circles) or aerobic (white circles) growth conditions.
Validation of the real-time qRT-PCR method.
A real-time qRT-PCR method was developed in order to compare the relative mRNA levels of the cld and clrA genes in samples from different growth regimes. Accurate comparison of target gene expression between different samples can be performed by normalizing the samples to a reference gene whose expression is invariable under the conditions studied. We evaluated the 16S rRNA gene of I. dechloratans as a reference gene for comparison of samples from cultures grown with oxygen and/or chlorate as a terminal electron acceptor. The fold change between 16S rRNA levels in two samples (A and B) with the same amount of total RNA, as estimated by absorbance measurements at 260 nm, was calculated from the ΔCT values (CT sample A − CT sample B) of the 16S rRNA-specific real-time qRT-PCRs by using the following formula: amount of RNAA/amount RNAB = 2−ΔCT. This resulted in the following ratios: anaerobic growth versus aerobic growth without chlorate, 0.95 ± 0.41; anaerobic growth versus aerobic growth with chlorate, 0.76 ± 0.08; and aerobic growth with chlorate versus aerobic growth without chlorate, 1.29 ± 0.33. The values are means of data from 6 independent experiments ± standard deviations. Since ratios close to 1 indicate that the relative amounts of 16S rRNA did not vary significantly between the culture conditions applied in this study, the 16S rRNA gene was chosen as a reference gene. However, the data do not exclude the possibility that the total amount of mRNA and the amount of 16S rRNA differ under different growth conditions.
Amplification efficiency was estimated as described in Materials and Methods. For all three primer pairs, calibration curves were linear over at least 4 orders of magnitude (R2 = 0.99), and efficiencies between 90 and 95% were obtained for all samples (Table 3). Thus, the two target genes, cld and clrA, and the reference 16S rRNA gene are amplified with similar efficiencies, and the efficiency is independent of the sample type. We also determined the amplification efficiencies of cDNAs generated from the gene-specific primers. Deviations from linearity and efficiency values above 110% were found in those samples (data not shown); therefore, the specific primers were not used for cDNA synthesis.
Table 3.
Amplification efficiency of real-time qRT-PCR
| Gene | Anaerobica |
Aerobicb |
||
|---|---|---|---|---|
| Slope | Efficiency (%) | Slope | Efficiency (%) | |
| 16S rRNA | −3.46 | 94.5 | −3.48 | 93.8 |
| cld | −3.47 | 94.1 | −3.50 | 92.9 |
| clrA | −3.57 | 90.4 | −3.57 | 90.6 |
Samples grown with chlorate as a terminal electron acceptor.
Samples grown with oxygen as a terminal electron acceptor.
Relative mRNA levels of the cld and clrA genes under aerobic and anaerobic growth conditions.
The observed induction of enzyme activities in an oxygen-depleted environment could be regulated at any level from gene transcription to protein activation. We used real-time qRT-PCR to investigate if the ability of chlorate reduction is controlled at the transcriptional level in I. dechloratans. The same growth conditions were studied as for the enzyme activity measurements, i.e., aerobic growth with and without chlorate and anaerobic growth with chlorate. All three growth conditions were analyzed in the same PCR run with primer pairs specific for 16S rRNA, cld, and clrA. It was found that samples from all growth regimes produced amplification curves with primers for both the cld and clrA genes (data not shown). Melting curve analyses verified a single amplification product in each reaction. No amplification or amplification with CT values >20 cycles higher than those of the gene-specific reactions appeared in the negative controls, indicating insignificant contamination with genomic or nonspecific DNA. Thus, the results show that both the cld and clrA genes are transcribed under aerobic, as well as anaerobic, growth, confirming the results from the enzyme activity measurements.
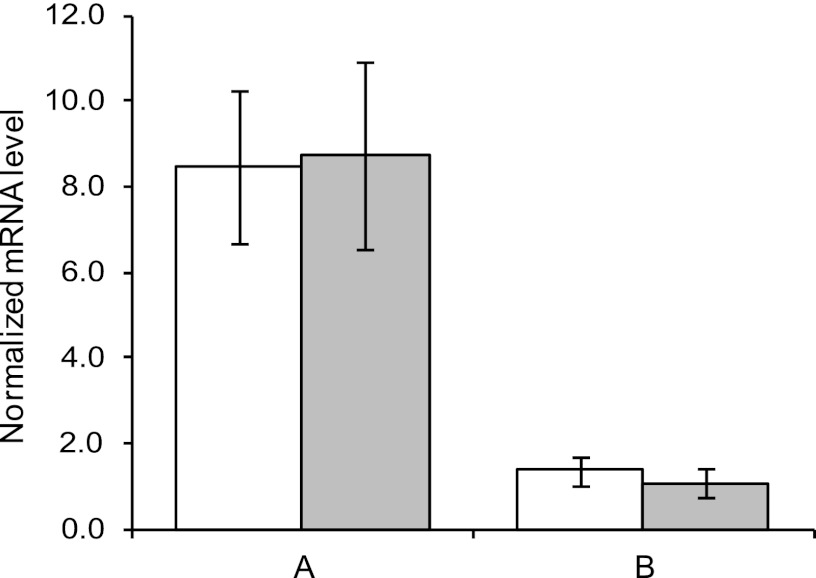
To compare mRNA levels of the cld and clrA genes between samples from two different growth conditions, the ΔCT value (CT target − CT 16SrRNA) was estimated for both samples, and the relative expression ratio was calculated as 2−ΔΔCT (20). The mean values of eight different PCR runs are shown in Fig. 2. For both the cld- and the clrA-specific reactions, samples from cultures grown anaerobically with chlorate showed an 8- to 9-fold-larger amount of cDNA than samples from aerobic cultures with oxygen as the only electron acceptor. Student's t test was performed, and the difference between anaerobic and aerobic samples was found to be significant (P < 0.001) for both genes. When samples from aerobic cultures with oxygen and chlorate were compared to those with oxygen as the sole electron acceptor, the relative expression ratios were found to be 1.4 and 1.1 for cld and clrA, respectively (Fig. 2). There was no statistically significant difference between the two aerobic samples for either of the genes (P < 0.001). Together, these results show that transcriptional activation of the cld and clrA genes occur under anaerobic conditions, while the addition of chlorate does not increase transcription under aerobic conditions. The ∼8-fold-larger amount of cld mRNA in anaerobic cells than in aerobic cells can account for the observed increase in enzyme activity of about 5 times. For clrA, however, the increase in enzyme activity is much greater than the increase in the mRNA level: more than 200 compared to 9 times.
Fig 2.
Relative expression ratio of the cld (white) and the clrA (gray) genes for comparison of different growth conditions. (A) Anaerobic compared with aerobic without chlorate. (B) Aerobic with chlorate compared with aerobic without chlorate. The error bars indicate standard errors of the mean (SEM).
DISCUSSION
Transcription of cld and clrA during aerobic and anaerobic conditions.
Dissimilatory (per)chlorate reduction is generally reported to be inhibited by oxygen (14). The reason for this inhibition is not clear, and several mechanisms are possible, e.g., transcriptional or posttranscriptional regulation of the (per)chlorate-metabolizing enzymes or other components of the anaerobic electron transport chain or protein-damaging reactions in an aerobic environment. Only a few transcriptional and no posttranscriptional studies of dissimilatory (per)chlorate reduction have been reported. Upregulation of transcription from the cld and pcrA genes under anaerobic conditions was demonstrated by Northern analyses in D. agitata (5, 7) and by real-time qRT-PCR in Rhodocyclaceae strain JDS4 (16). A basal level of transcription from the cld gene (5), but not for the pcrA gene (7), was detected in aerobically grown cells of D. agitata, whereas it was not clear if expression of any of these genes occurred under aerobic conditions in Rhodocyclaceae strain JDS4 (16). There are no previous reports on mRNA levels in a CRB, but our results show that the cld and clrA genes of I. dechloratans are also upregulated at the transcriptional level during anaerobic growth. In contrast to what was reported for the pcrA gene in D. agitata, we found that the clrA gene of I. dechloratans is transcribed in cells grown with oxygen as the sole terminal electron acceptor. The amount of transcript was about 1/10 of what was found in anaerobically grown cells, and it most likely originated from de novo synthesis, since it was detected in cells that had been in exponential growth under aerobic conditions (with a generation time of 1 h) for 20 h. This result, together with the very low levels of chlorate reductase activity in aerobic growth, indicates that caution should be taken in using mRNA measurements of (per)chlorate reductase as an indicator of the actual (per)chlorate-reducing ability of a microbial community.
Posttranscriptional events influence Clr activity.
The 20-times-greater increase in enzyme activity compared to the increase in the clrA expression level upon transfer of cells from aerobic to anaerobic growth conditions shows that the activity of Clr is dependent on other processes in addition to the transcription of the clrA gene. One such process could be oxygen-dependent deactivation of Clr. While preparation of Cld seems to be insensitive to oxygen (30), loss of (per)chlorate reductase activity in the presence of oxygen is reported for cell extracts or purified protein preparations from I. dechloratans (31) and other species (19, 28, 41). This may or may not mirror the in vivo situation during aerobic growth, depending on the actual intracellular concentration of dissolved oxygen and the presence of other cellular components. There is at least one organism, Pseudomonas sp. strain PDA, that expresses chlorate-reducing capability under aerobic growth conditions (44). We also found low but significant activity of Clr in I. dechloratans after 20 h of exponential growth under aerobic conditions, showing that Clr is not completely inhibited during aerobic growth. Another possible reason for the discrepancy between the enzyme activity and the mRNA level under different growth conditions is that posttranscriptional regulation of Clr occurs. However, further studies are needed to clarify this issue.
Effects of chlorate on Cld and Clr expression.
The observed activation of Cld and Clr under anaerobic growth conditions could be signaled by oxygen/redox and/or chlorate sensing. To investigate this further, we determined enzyme activities and mRNA levels in cells grown aerobically with and without the addition of chlorate. The presence of chlorate had no inducing effect on either enzyme activity or the mRNA level for either of the two enzymes under aerobic conditions. Even if inhibition of Clr by oxygen could have overridden an inducing effect of chlorate, the equal mRNA levels in cells grown in the presence or absence of chlorate suggest that transcriptional regulation of dissimilatory chlorate reduction is sensed by oxygen- or redox-dependent mechanisms in I. dechloratans.
ACKNOWLEDGMENT
We thank Ann Erlandsson for helpful advice concerning the real-time qRT-PCR experiments.
Footnotes
Published ahead of print 6 April 2012
REFERENCES
- 1. Achenbach LA, Bender KS, Sun Y, Coates JD. 2006. The biochemistry and genetics of perchlorate reduction, p 297–310 In Gu B, Coates JD. (ed), Perchlorate: environmental occurrence, interactions, and treatment. Springer, New York, NY [Google Scholar]
- 2. Achenbach LA, Michaelidou U, Bruce RA, Fryman J, Coates JD. 2001. Dechloromonas agitata gen. nov., sp. nov. and Dechlorosoma suillum gen. nov., sp. nov., two novel environmentally dominant (per)chlorate-reducing bacteria and their phylogenetic position. Int. J. Syst. Evol. Microbiol. 51:527–533 [DOI] [PubMed] [Google Scholar]
- 3. Balk M, et al. 2010. (Per)chlorate reduction by an acetogenic bacterium, Sporomusa sp., isolated from an underground gas storage. Appl. Microbiol. Biotechnol. 88:595–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balk M, van Gelder T, Weelink SA, Stams AJM. 2008. (Per)chlorate reduction by the thermophilic bacterium Moorella perchloratireducens sp. nov., isolated from underground gas storage. Appl. Environ. Microbiol. 74:403–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bender KS, O'Connor SM, Chakraborty R, Coates JD, Achenbach LA. 2002. Sequencing and transcriptional analysis of the chlorite dismutase gene of Dechloromonas agitata and its use as a metabolic probe. Appl. Environ. Microbiol. 68:4820–4826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bender KS, Rice MR, Fugate WH, Coates JD, Achenbach LA. 2004. Metabolic primers for detection of (per)chlorate-reducing bacteria in the environment and phylogenetic analysis of cld gene sequences. Appl. Environ. Microbiol. 70:5651–5658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bender KS, et al. 2005. Identification, characterization, and classification of genes encoding perchlorate reductase. J. Bacteriol. 187:5090–5096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergnor E, Germgård U, Kolar JJ, Lindgren BO. 1987. Formation of chlorate in chlorine dioxide bleaching. Cellulose Chem. Technol. 21:307–314 [Google Scholar]
- 9. Brown-Grant K. 1957. The iodide concentrating mechanism of the mammary gland. J. Physiol. 135:644–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bruce RA, Achenbach LA, Coates JD. 1999. Reduction of (per)chlorate by a novel organism isolated from paper mill waste. Environ. Microbiol. 1:319–329 [DOI] [PubMed] [Google Scholar]
- 11. Bustin SA, et al. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55:611–622 [DOI] [PubMed] [Google Scholar]
- 12. Chaudhuri SK, O'Connor SM, Gustavson RL, Achenbach LA, Coates JD. 2002. Environmental factors that control microbial perchlorate reduction. Appl. Environ. Microbiol. 68:4425–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coates JD, Achenbach LA. 2004. Microbial perchlorate reduction: rocket-fuelled metabolism. Nat. Rev. Microbiol. 2:569–580 [DOI] [PubMed] [Google Scholar]
- 14. Coates JD, Achenbach LA. 2006. The microbiology of perchlorate reduction and its bioremediative application, p 279–295 In Gu B, Coates JD. (ed), Perchlorate: environmental occurrence, interactions, and treatment. Springer, New York, NY [Google Scholar]
- 15. Coates JD, et al. 1999. Ubiquity and diversity of dissimilatory (per)chlorate-reducing bacteria. Appl. Environ. Microbiol. 65:5234–5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Long SK, Kinney KA, Kirisits MJ. 2010. qPCR assays to quantify genes and gene expression associated with microbial perchlorate reduction. J. Microbiol. Methods 83:270–274 [DOI] [PubMed] [Google Scholar]
- 17. Dohán O, et al. 2007. The Na+/I− symporter (NIS) mediates electroneutral active transport of the environmental pollutant perchlorate. Proc. Natl. Acad. Sci. U. S. A. 104:20250–20255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giblin T, Frankenberger WT. 2001. Perchlorate and nitrate reductase activity in the perchlorate-respiring bacterium perclace. Microbiol. Res. 156:311–315 [DOI] [PubMed] [Google Scholar]
- 19. Kengen SWM, Rikken GB, Hagen WR, van Ginkel CG, Stams AJM. 1999. Purification and characterization of (per)chlorate reductase from the chlorate-respiring strain GR-1. J. Bacteriol. 181:6706–6711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 21. Logan BE, et al. 2001. Kinetics of perchlorate- and chlorate-respiring bacteria. Appl. Environ. Microbiol. 67:2499–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malmqvist A, et al. 1994. Ideonella dechloratans gen. nov., sp. nov., a new bacterium capable of growing anaerobically with chlorate as an electron acceptor. Syst. Appl. Microbiol. 17:58–64 [Google Scholar]
- 23. Mehboob F, et al. 2009. Purification and characterization of a chlorite dismutase from Pseudomonas chloritidismutans. FEMS Microbiol. Lett. 293:115–121 [DOI] [PubMed] [Google Scholar]
- 24. Rikken GB, Kroon AGM, van Ginkel CG. 1996. Transformation of (per)chlorate into chloride by a newly isolated bacterium: reduction and dismutation. Appl. Microbiol. Biotechnol. 45:420–426 [Google Scholar]
- 25. Rosemarin A, Lehtinen KJ, Notini M, Mattson J. 1994. Effects of pulp mill chlorate on Baltic Sea algae. Environ. Pollution 85:3–13 [DOI] [PubMed] [Google Scholar]
- 26. Song Y, Logan BE. 2004. Effect of O2 exposure on perchlorate reduction by Dechlorosoma sp. KJ. Water Res. 38:1626–1632 [DOI] [PubMed] [Google Scholar]
- 27. Song Y, Logan BE. 2004. Inhibition of aerobic respiration and dissimilatory perchlorate reduction using cyanide. FEMS Microbiol. Lett. 239:229–234 [DOI] [PubMed] [Google Scholar]
- 28. Steinberg LM, Trimble JJ, Logan BE. 2005. Enzymes responsible for chlorate reduction by Pseudomonas sp. are different from those used for perchlorate reduction by Azospira sp. FEMS Microbiol. Lett. 247:153–159 [DOI] [PubMed] [Google Scholar]
- 29. Stenklo K, Thorell HD, Bergius H, Aasa R, Nilsson T. 2001. Chlorite dismutase from Ideonella dechloratans. J. Biol. Inorg. Chem. 6:601–607 [DOI] [PubMed] [Google Scholar]
- 30. Streit BR, DuBois JL. 2008. Chemical and steady-state kinetic analyses of a heterologously expressed heme dependent chlorite dismutase. Biochemistry 47:5271–5280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thorell HD, Stenklo K, Karlsson J, Nilsson T. 2003. A gene cluster for chlorate metabolism in Ideonella dechloratans. Appl. Environ. Microbiol. 69:5585–5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thorneley RNF. 1974. A convenient electrochemical preparation of reduced methyl viologen and a kinetic study of the reaction with oxygen using an anaerobic stopped-flow apparatus. Biochim. Biophys. Acta 333:487–496 [DOI] [PubMed] [Google Scholar]
- 33. Urbansky ET. 1998. Perchlorate chemistry: implications for analysis and remediation. Bioremediation J. 2:81–95 [Google Scholar]
- 34. van Ginkel CG, Plugge CM, Stroo CA. 1995. Reduction of chlorate with various energy substrates and inocula under anaerobic conditions. Chemosphere 31:4057–4066 [Google Scholar]
- 35. van Wijk DJ, Hutchinson TH. 1995. The ecotoxicity of chlorate to aquatic organisms: a critical review. Ecotoxicol. Environ. Safety 32:244–253 [DOI] [PubMed] [Google Scholar]
- 36. Wallace W, Ward T, Breen A, Attaway H. 1996. Identification of an anaerobic bacterium which reduces perchlorate and chlorate as Wolinella succinogenes. J. Ind. Microbiol. Biotechnol. 16:68–72 [Google Scholar]
- 37. Waller AS, Cox EE, Edwards EA. 2004. Perchlorate reducing microorganisms isolated from contaminated sites. Environ. Microbiol. 6:517–527 [DOI] [PubMed] [Google Scholar]
- 38. Wolff J. 1998. Perchlorate and the thyroid gland. Pharmacol. Rev. 50:89–105 [PubMed] [Google Scholar]
- 39. Wolterink A, Jonker AB, Kengen SWM, Stams AJM. 2002. Pseudomonas chloritidismutans sp. nov., a non-denitrifying, chlorate-reducing bacterium. Int. J. Syst. Evol. Microbiol. 52:2183–2190 [DOI] [PubMed] [Google Scholar]
- 40. Wolterink A, et al. 2005. Dechloromonas hortensis sp. nov. and strain ASK-1, two novel (per)chlorate-reducing bacteria, and taxonomic description of strain GR-1. Int. J. Syst. Evol. Microbiol. 55:2063–2068 [DOI] [PubMed] [Google Scholar]
- 41. Wolterink AFWM, et al. 2003. Characterization of the chlorate reductase from Pseudomonas chloritidismutans. J. Bacteriol. 185:3210–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu J, Logan BE. 2003. Measurement of chlorite dismutase activities in perchlorate respiring bacteria. J. Microbiol. Methods 54:239–247 [DOI] [PubMed] [Google Scholar]
- 43. Xu J, Song Y, Min B, Steinberg L, Logan BE. 2003. Microbial degradation of perchlorate: principles and applications. Environ. Eng. Sci. 20:405–422 [Google Scholar]
- 44. Xu J, Trimble JJ, Steinberg L, Logan BE. 2004. Chlorate and nitrate reduction pathways are separately induced in the perchlorate-respiring bacterium Dechlorosoma sp. KJ and the chlorate-respiring bacterium Pseudomonas sp. PDA. Water Res. 38:673–680 [DOI] [PubMed] [Google Scholar]