Abstract
Solar radiation reduces Cryptosporidium infectivity. Biofilms grown from stream microbial assemblages inoculated with oocysts were exposed to solar radiation. The infectivity of oocysts attached at the biofilm surface and oocysts suspended in water was about half that of oocysts attached at the base of a 32-μm biofilm.
TEXT
Cryptosporidium parvum is a public health concern, infecting thousands of individuals as a result of contaminated water (2, 3, 13, 32). Traditional water treatment targeting Cryptosporidium is difficult (28). Artificial UV radiation has irreversible effects on oocyst infectivity (5, 8, 11, 12, 16, 23, 33) but can be cost-prohibitive, so the use of natural solar radiation to inactivate oocysts is worth investigating. Solar radiation (UV and non-UV wavelengths) has been shown through cell culture infectivity to effectively disinfect C. parvum (6).
Biofilms retain pathogens at high densities, with the potential for release to the water, and protect oocysts from environmental conditions (9, 18, 24, 28, 30, 31); biofilm cells are more resistant to biocides and environmental conditions than planktonic cells of the same species (1, 10, 14, 17, 19, 31).
Oocysts (i) attached to the biofilm surface, (ii) attached at depth in the biofilm, and (iii) attached to a biofilm resuspended in water were exposed to solar radiation to test the hypothesis that biofilms provide protection for oocysts against exposure to solar radiation.
Biofilms and creek water were collected from Monocacy Creek (Bethlehem, PA) (30, 31). Biofilms were scraped from rocks, filtered through 6-μm filter paper, and centrifuged, and the pelleted biofilm cells were resuspended in filter-sterilized creek water (0.22-μm-pore-size filter). Cell concentration was determined by DAPI (4′,6-diamidino-2-phenylindole) staining (19). Aliquots of 5 × 106 cells in sterile creek water with 30% glycerol were frozen (−80°C) until used to inoculate flow chambers.
Single-channel flow chambers (24 mm by 8 mm by 4 mm [length by width by height]) with glass coverslips (Stovall Life Science, Inc., Greensboro, NC) were inoculated with 5 ×106 biofilm cells for 24 h before flow of sterile creek water was started. A 12-channel peristaltic pump (IPC pump; Ismatec, Glattbrugg, Switzerland) maintained constant laminar flow (0.17 ml/min) (4).
Biofilms were grown for 3 days with 2.5 × 106 C. parvum oocysts (Iowa isolate; Waterborne, Inc., New Orleans, LA) seeded into the sterile creek water influent (8.3 × 105 oocysts/liter). Biofilm thickness was measured using a scanning confocal microscope (Zeiss LSM 510 META laser scanning microscope).
Three experiments (Table 1) were performed using four treatments: (i) oocysts attached at the top of a biofilm (the sun-exposed surface), (ii) oocysts attached at the bottom of a 30-μm biofilm (with the biofilm between the oocysts and the sun), (iii) biofilm-associated oocysts scraped from the flow chamber and resuspended in creek water (simulating oocysts that detach from biofilm), and (iv) oocysts in sterile creek water. All experiments included light and dark controls: light controls consisted of oocysts in creek water exposed to sunlight, and dark controls were wrapped in aluminum foil to block sunlight. The three experiments were exposed to solar radiation for 90, 50, and 60 min, respectively. The experimental duration was determined by the total solar radiation (Table 1), which varied as a result of weather and season conditions and was monitored in real time using a calibrated solar radiometer (PUV-500; Biospherical Instruments, Inc., San Diego, CA).
Table 1.
Solar radiation exposure (dose) for each experiment at four wavelengths (305, 320, 340, 380 nm) and the equivalent exposure days for the most biologically effective wavelength of 320 nm
| Experiment | Date | Dose (kJ/m2) |
No. of 320-nm exposure daysa | |||
|---|---|---|---|---|---|---|
| 305 nm | 320 nm | 340 nm | 380 nm | |||
| A: Oocysts on top and bottom of biofilm | July 9 | 0.7 | 4.0 | 8.6 | 10.4 | 0.37 |
| B: Biofilm-associated oocysts resuspended in creek water | July 15 | 0.5 | 3.1 | 6.6 | 8.3 | 0.28 |
| C: Oocysts on top and bottom of biofilm and resuspended biofilm-associated oocysts | August 25 | 0.3 | 2.5 | 5.5 | 6.9 | 0.23 |
A value of 10.9 kJ/m2 represents one 320-nm exposure day or the amount of solar UV at 320 nm received during 1 day of full sunlight (no clouds) at the water surface during summer solstice and average ozone conditions at 41°N latitude (7).
Experiments were performed on a rooftop with no shadows. Temperature was maintained at 2 to 5°C using a water bath, ice bath, and recirculating pump, monitored with iButton temperature sensors (Dallas Semiconductor, Dallas, TX).
Because previous work demonstrated that oocysts attach and remain at the biofilm surface under these flow conditions (30), flow chambers were placed face up for experiments exposing oocysts at the top of the biofilm to direct solar radiation. For treatments with oocysts at the bottom of the biofilm, flow chambers were placed upside down, allowing the solar radiation to pass through the biofilm before reaching the oocysts. For treatments with biofilm-associated oocysts, biofilms were scraped from the flow chamber and resuspended in creek water. This suspension was injected back into the flow chamber for solar exposure. Oocysts with no biofilm association were tested by suspending oocysts in sterile creek water injected into the flow chamber.
After exposure, biofilms were resuspended in sterile creek water, and oocysts were purified by immunomagnetic separation (IMS) using the Virusys IMS kit (Virusys Co., Sykesville, MD) according to the manufacturer's protocol (oocysts were dissociated from beads using 0.05 M HCl).
Oocyst infectivity was determined using in vitro cell culture infection of human ileocecal adenocarcinoma (HCT-8) cells grown in eight-well chamber slides (22, 27). Oocysts were pretreated with 10% sodium hypochlorite, and oocyst concentration was determined by hemocytometry. For each treatment (performed in duplicate), six wells on each chamber slide were infected with 200 oocysts and two wells were left uninfected to monitor the cell monolayer. The infectivity of the oocyst stock (stored at 4°C in the dark) was also determined. Infected chamber slides were incubated (37°C, 5% CO2, 48 h) and stained with Sporo-Glo antibody (Waterborne, Inc., New Orleans, LA) according to the manufacturer's protocol. Infection foci were counted using a Nikon epifluorescence microscope (Nikon, Inc., Melville, NY), and the percentage of infective oocysts was calculated by dividing the number of infection foci by 200 (number of oocysts inoculated into each well).
Transmittance through flow chambers containing (i) creek water, (ii) intact biofilm, and (iii) biofilm scraped from the flow chamber and resuspended in water was measured in the lab using custom spectrophotometer components (USB200 diode array UV-VIS spectrometer [Ocean Optics, Inc., Dunedin, FL] and 50-mm reflectance integrating sphere illuminated with an Ocean Optics PX-2 xenon strobe lamp emitting 250 to 850 nm [Avantes, Inc., Broomfield, CO]). Attenuation by the flow chamber with deionized water was not significant (19% at 320 nm) compared to biofilm attenuation and was used as 100% relative transmittance.
Independent t tests were used to determine if a significant difference existed between oocyst infectivity in all treatments using the Analyze-it add-in (Analyze-it Software, Ltd., Leeds, England) for Microsoft Excel.
Sun-exposed oocysts were less infectious than oocysts in the dark (P < 0.01) (Fig. 1). These data support the observations of others that solar radiation reduces oocyst infectivity (6, 11, 15); however, approximately 20% of the oocysts exposed to the highest levels of solar radiation remained infectious and a potential public health threat.
Fig 1.
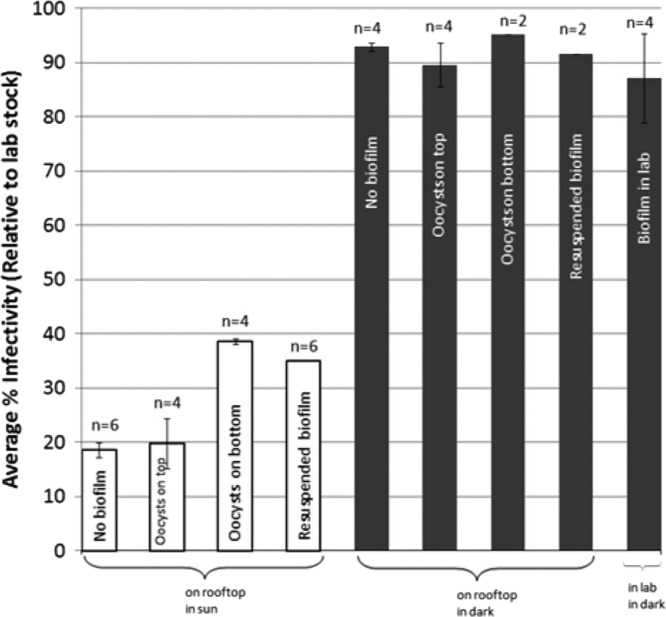
Summary of oocyst infectivity results for solar exposure experiments on July 9, July 15, and August 25. White columns are light-exposed treatments. Gray bars are dark control treatments. Lab stock infectivity for each experiment was 5.8% (July 9), 5.3% (July 15), and 10.4% (August 25). The July 9 and July 15 experiments were performed with the same oocyst lot. n, number of individual flow chambers.
Total solar exposure for each experiment was converted to 320-nm exposure days using a value of 10.9 kJ/m2, the amount of solar UV at 320 nm received during a day of full sunlight during summer solstice and average ozone conditions at 41°N latitude (7). Despite various lengths of exposure time (90, 50, and 60 min for the July 9, July 15, and August 25 experiments, respectively), sky conditions resulted in all three experiments having similar solar exposure (0.37, 0.28, and 0.23 exposure days, respectively) (Table 1), and these exposures were comparable to those in previous experiments (6) (0.33 to 0.38 exposure days), in which infectivity was reduced to 0 to 6% of the stock infectivity (compared to 16 to 20% in these experiments). Differences in infectivity reductions may result from the greater attenuation of radiation by the glass-covered flow chambers used here compared to the quartz dishes used previously (6), variation in oocyst lots (26), and overnight oocyst storage before infectivity processing (6).
The infectivity of oocysts attached at the top of the biofilm (n = 4 individual flow chambers) was significantly less than the infectivity of oocysts on the bottom of the biofilm (n = 4 individual flow chambers) (P < 0.01) (Fig. 1), suggesting that the biofilm provides a protective barrier for oocysts against solar radiation. Others found that shortwave radiation did not reach the bottom of a biofilm, allowing UV-sensitive organisms to survive (20). This conclusion is supported by optical transmittance data that showed that solar radiation was attenuated by the biofilm: less than one percent of the shortwave radiation (less than 300 nm) passed through a biofilm (23 to 40 μm), while up to 82% of longer-wavelength radiation, shown to significantly reduce infectivity (6), was able to pass through the biofilm (Fig. 2). These data suggest that oocysts at depth in a biofilm may be impacted more by longwave radiation as opposed to the shortwave UV radiation commonly used for disinfection.
Fig 2.
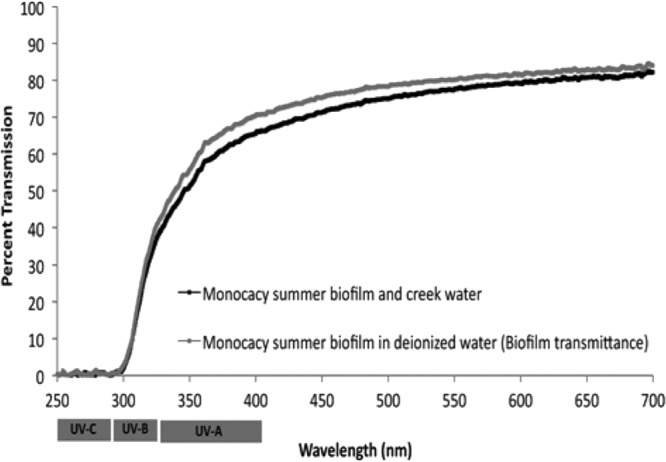
Biofilm transmittance in Monocacy Creek water and deionized water (100% relative transmittance by calibration for clean flow chamber with deionized water). UV-C, 100 to 280 nm; UV-B, 280 to 315 nm; UV-A, 315 to 400 nm.
The ability of the biofilm to absorb UV wavelengths may be a result of microbial production of sunscreen-like compounds as a response to strong solar radiation (20, 25). The biofilm culture used in these experiments was collected in the summer, when production of these compounds should be highest, if present. However, no difference was observed in the transmittance data from biofilms collected in different seasons or other sites with various sunlight exposure (data not shown), suggesting that either these compounds are present in all the sampled biofilms in equal quantities, which is unlikely because of the various environmental conditions under which they were collected, or that the inherent structure of the biofilm prevents the penetration of shortwave radiation.
Although biofilms may provide a protective barrier for oocysts against solar radiation, environmental oocysts are likely to be found suspended in the water column attached to fecal debris or biofilm material from a previous association. Oocysts may remain associated with biofilm material in suspension, providing similar protection to the oocyst as being embedded at depth within the biofilm (Fig. 1). The infectivity of biofilm-associated oocysts resuspended in creek water (n = 6 individual flow chambers) was not significantly different than that of oocysts at the bottom of a biofilm (n = 4 individual flow chambers) (P = 0.19) (Fig. 1). Oocysts have been shown to be protected from environmental conditions following storage in fecal material, a result of mucopolysaccharides inserted into the oocyst wall (21). Biofilm material may similarly embed in the oocyst wall and protect the oocyst from solar radiation through solar radiation absorption.
In the environment, oocysts are likely found attached to biofilm or fecal material from previous association. As shown by these experiments, associations with biofilm material reduce the impacts of detrimental solar radiation.
ACKNOWLEDGMENT
This research was funded by National Science Foundation CAREER award (no. 0545687) to K. L. Jellison.
Footnotes
Published ahead of print 30 March 2012
REFERENCES
- 1. Camper AK, Jones WL, Hayes JT. 1996. Effect of growth conditions and substratum composition in the persistence of coliforms in mixed-population biofilms. Appl. Environ. Microbiol. 62:4014–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Causer LM, et al. 2006. An outbreak of Cryptosporidium hominis infection at an Illinois recreational waterpark. Epidemiol. Infect. 134:147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention 2007. Summary of notifiable diseases—United States, 2005. MMWR Morb. Mortal. Wkly. Rep. 54:2–92 [PubMed] [Google Scholar]
- 4. Christensen BB, et al. 1999. Molecular tools for study of biofilm physiology. Methods Enzymol. 310:20–42 [DOI] [PubMed] [Google Scholar]
- 5. Clancy JL, Marshall MM, Hargy TM, Korich DG. 2004. Susceptibility of five strains of Cryptosporidium parvum oocysts to UV light. J. AWWA 96:84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Connelly SJ, Wolyniak EA, Williamson CE, Jellison KL. 2007. Artificial UV-B and solar radiation reduce in vitro infectivity of the human pathogen Cryptosporidium parvum. Environ. Sci. Technol. 41:7101–7106 [DOI] [PubMed] [Google Scholar]
- 7. Cooke SL, Williamson CE. 2006. Positive effects of UV radiation on a calanoid copepod in a transparent lake: do competition, predation, or food availability play a role? J. Plankton Res. 28:171–179 [Google Scholar]
- 8. Craik SA, Weldon D, Finch GR, Bolton JR, Belosevic M. 2001. Inactivation of Cryptosporidium parvum oocysts using medium- and low-pressure ultraviolet radiation. Water Res. 35:1387–1398 [DOI] [PubMed] [Google Scholar]
- 9. Flood JA, Ashbolt NJ. 2000. Virus-sized particles can be entrapped and concentrated one hundred fold within wetland biofilms. Adv. Environ. Res. 3:403–411 [Google Scholar]
- 10. Ito A, Taniuchi A, May T, Kawata K, Okabe S. 2009. Increased antibiotic resistance of Escherichia coli in mature biofilms. Appl. Environ. Microbiol. 75:4093–4100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Linden KG, Shin GA, Sobsey MD. 2001. Comparative effectiveness of UV wavelengths for the inactivation of Cryptosporidium parvum. Water Sci. Technol. 43:171–174 [PubMed] [Google Scholar]
- 12. Lorenzo-Lorenzo MJ, Ares-Mazas ME, Villacorta-Martinex de Maturana I, Duran-Oreiro D. 1993. Effect of ultraviolet disinfection of drinking water on the viability of Cryptosporidium parvum oocysts. J. Parasitol. 79:67–70 [PubMed] [Google Scholar]
- 13. MacKenzie WR, et al. 1994. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 331:161–167 [DOI] [PubMed] [Google Scholar]
- 14. Martinez LR, Casadevall A. 2007. Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl. Environ. Microbiol. 73:4592–4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Méndez-Hermida F, Castro-Hermida JA, Ares-Mazás E, Kehoe SC, McGugian KG. 2005. Effect of batch-process solar disinfection on survival of Cryptosporidium parvum oocysts in drinking water. Appl. Environ. Microbiol. 71:1653–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morita S, et al. 2002. Efficacy of UV irradiation in inactivating Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 68:5387–5393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nett JE, Guite KM, Ringeisen A, Holoyda KA, Andes DR. 2008. Reduced biocide susceptibility in Candida albicans biofilms. Antimicrob. Agents Chemother. 52:3411–3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Percival SL, Walker JT, Hunter PR. 2000. Microbiological aspects of biofilms and drinking water. CRC Press, New York, NY [Google Scholar]
- 19. Porter KG, Keig YS. 1980. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 25:943–948 [Google Scholar]
- 20. Quesada A, Vincent WF, Lean DRS. 1999. Community and pigment structure of Arctic cyanobacterial assemblages: the occurrence and distribution of UV-absorbing compounds. FEMS Microbiol. Ecol. 28:315–323 [Google Scholar]
- 21. Robertson LJ, Campbell AT, Smith HV. 1992. Survival of Cryptosporidium parvum oocysts under various environmental pressures. Appl. Environ. Microbiol. 58:3494–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rochelle PA, et al. 2002. Comparison of in vitro cell culture and a mouse assay for measuring infectivity of Cryptosporidium parvum. Appl. Environ. Microbiol. 68:3209–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rochelle PA, Upton SJ, Montelone BA, Woods K. 2005. The response of Cryptosporidium parvum to UV light. Trends Parasitol. 21:81–87 [DOI] [PubMed] [Google Scholar]
- 24. Rogers J, Keevil CW. 1995. Survival of Cryptosporidium parvum oocysts in biofilm and planktonic samples in a model system, p 209–213 In Betts WB, Casemore D, Fricker C, Smith H, Watkins J, Protozoan parasites and water. The Royal Society of Chemistry, Cambridge, United Kingdom [Google Scholar]
- 25. Sinha RP, Klisch M, Gröniger A, Häder DP. 2001. Responses of aquatic algae and cyanobacteria to solar UV-B. Plant Ecol. 154:221–236 [Google Scholar]
- 26. Sivaganesan M, Sivaganesan S. 2005. Effect of lot variability on ultraviolet radiation inactivation kinetics of Cryptosporidium parvum oocysts. Environ. Sci. Technol. 39:4166–4171 [DOI] [PubMed] [Google Scholar]
- 27. Slifko TR, Friedman D, Rose JB, Jakubowski W. 1997. An in-vitro method for detecting infectious Cryptosporidium oocysts with cell culture. Appl. Environ. Microbiol. 63:3669–3675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Szewzyk U, Szewzyk R, Manz W, Schleifer KH. 2000. Microbiological safety of drinking water. Annu. Rev. Microbiol. 54:81–127 [DOI] [PubMed] [Google Scholar]
- 29. Théraud M, Bédouin Y, Guiguen C, Gangneux JP. 2004. Efficacy of antiseptics and disinfectants on clinical and environmental yeast isolates in planktonic and biofilm conditions. J. Med. Microbiol. 53:1013–1018 [DOI] [PubMed] [Google Scholar]
- 30. Wolyniak EA, Hargreaves BR, Jellison KL. 2009. Retention and release of Cryptosporidium parvum oocysts by experimental biofilms composed of a natural stream microbial community. Appl. Environ. Microbiol. 75:4624–4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wolyniak EA, Hargreaves BR, Jellison KL. 2010. Seasonal retention and release of Cryptosporidium parvum oocysts by environmental biofilms in the laboratory. Appl. Environ. Microbiol. 76:1021–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu R. 1 November 2005. Crypto lawsuit hits N.Y.; Ohio. (Cryptosporidium outbreak). Aquat. Intl. (Newsletter.) Hanley Wood, Washington, DC [Google Scholar]
- 33. Zimmer JL, Slawson RM, Huck PM. 2003. Inactivation and potential repair of Cryptosporidium parvum following low- and medium-pressure ultraviolet irradiation. Water Res. 37:3517–3523 [DOI] [PubMed] [Google Scholar]


