Abstract
The genus Cryptosporidium is a group of waterborne protozoan parasites that have been implicated in significant outbreaks of gastrointestinal infections throughout the world. Biofilms trap these pathogens and can contaminate water supplies through subsequent release. Biofilm microbial assemblages were collected seasonally from three streams in eastern Pennsylvania and used to grow biofilms in laboratory microcosms. Daily oocyst counts in the influx and efflux flow allowed the calculation of daily oocyst retention in the biofilm. Following the removal of oocysts from the influx water, oocyst attachment to the biofilm declined to an equilibrium state within 5 days that was sustained for at least 25 days. Varying the oocyst loading rate for the system showed that biofilm retention could be saturated, suggesting that discrete binding sites determined the maximum number of oocysts retained. Oocyst retention varied seasonally but was consistent across all three sites; however, seasonal oocyst retention was not consistent across years at the same site. No correlation between oocyst attachment and any measured water quality parameter was found. However, oocyst retention was strongly correlated with biofilm surface roughness and roughness varied among seasons and across years. We hypothesize that biofilm roughness and oocyst retention are dependent on environmentally driven changes in the biofilm community rather than directly on water quality conditions. It is important to understand oocyst transport dynamics to reduce risks of human infection. Better understanding of factors controlling biofilm retention of oocysts should improve our understanding of oocyst transport at different scales.
INTRODUCTION
The waterborne protozoan parasite Cryptosporidium parvum has been implicated in large outbreaks of gastrointestinal infections as a result of fecal contamination of drinking and recreational waters affecting thousands of individuals (46). Oocysts are consistently found in surface waters and drinking water supplies throughout the world, including the United States, Canada, and Japan, especially in water supplies with waste discharges (18, 28, 29, 30, 42, 43). Sporadic outbreaks of infection occur despite water suppliers' compliance with regulations because of the difficulty in treating water for C. parvum as a result of the oocysts' small size (4 to 8 μm) and resistance to chlorine (46, 53). Because of these factors, Cryptosporidium has been recognized as a major water quality management issue in developed countries and treatment has come to include a focus on source water management (1). Knowledge of the Cryptosporidium population in source waters aids in creating water treatment and watershed management plans.
Biofilms are ubiquitous microbial communities that grow attached to surfaces in aquatic environments and are enclosed in a self-produced polymeric matrix that contains bacteria, cyanobacteria, microalgae, protozoa, fungi, and invertebrate larvae (1, 8, 26, 47). Previous work showed that bacteria and viruses attach to biofilms in very high concentrations and remain attached for extended periods of time (16, 40). Similarly, biofilms can contain concentrated reservoirs of pathogens, including Cryptosporidium, which may not be detected until these pathogens are released from the biofilm through detachment events (26, 36, 47).
Little information linking outbreaks of waterborne disease to biofilms is available (36). Biofilms have the potential to hold large quantities of oocysts that may not be represented in water samples, potentially resulting in contamination of source waters that may be classified as oocyst free and safe for human exposure. Oocysts found in water samples may be a result of oocysts released from biofilms that were captured during a previous contamination event and are not necessarily evidence of a new contamination event. Risk management approaches to reduce outbreaks of waterborne infections include a catchment-to-tap approach; however, biofilms are not considered to be a contribution to oocyst loading, although little is known about oocyst interaction with biofilms, including how long oocysts may persist after a contamination event (1). In addition, biofilms provide a more stable environment and protection for attached organisms as the extrapolymeric substances protect cells against changing environmental conditions (11, 25, 26). This protection has been shown for bacterial biofilm cells in response to both environmental pressures and antimicrobial agents and may allow pathogens to survive longer than they would in the planktonic stage (8, 15, 36).
The dynamics of oocyst attachment to a biofilm (number attached and attachment duration) has not been extensively studied. The percentage of 1-μm beads that attached to a biofilm was shown to be proportional to the standard deviation of biofilm thickness measurements, as an estimate of surface roughness (13). Searcy et al. (44) suggested that oocyst attachment to biofilms is controlled by biofilm architecture and surface-chemical interactions dependent on water chemistry. The characteristics of a specific biofilm, including composition, thickness, and morphology, are dependent on the number and diversity of organisms in the water, the concentration and nature of biodegradable organic matter, and the characteristics of the support material (36).
Little previous work has been done to directly observe oocyst attachment to biofilms (1). Searcy et al. (44) demonstrated greater attachment of oocysts to a monoculture Pseudomonas aeruginosa biofilm than to a clean surface. Similar results were found with mixed-culture biofilms created from reservoir and drinking water in which oocysts attached to the densest locations within the biofilm structure and remained viable for at least 15 days, with oocysts released into the water column for 2 to 6 weeks (41, 52). This intermittent release is similar to the situation in Lancashire, England, during an outbreak of cryptosporidiosis in 2000, in which biofilm-associated oocysts were detected in tap water for up to 19 days after the water source was changed and the system was flushed (19).
This study provides information about (i) the number of oocysts that may accumulate within a biofilm over short and long time scales through a full mass balance analysis and (ii) how variation in the biofilm community affects oocyst attachment. Previous work presented by Wolyniak et al. (54, 55) demonstrated that (i) a large percentage of oocysts attached to laboratory biofilms during a 3-day dosing period, (ii) some of the attached oocysts sloughed from the biofilm at the end of an 8-day experiment, and (iii) oocyst attachment varied seasonally with the biofilm community. In this study, we tested the hypotheses that (i) oocyst attachment varies seasonally when testing biofilms collected within a limited geographic area from streams with different water chemistry and (ii) this seasonal variation in oocyst attachment is a result of seasonal changes in the microbial community.
MATERIALS AND METHODS
Stream sites.
Biofilms were collected from three stream sites in eastern Pennsylvania: Monocacy Creek (Bethlehem, PA), Pocono Creek (Tannersville, PA), and Cranberry Creek (Tannersville, PA). These three sites were chosen for variation in their water quality (Table 1). Specifically, Cranberry Creek has high levels of dissolved organic carbon (DOC) because it drains a bog and low conductivity as a result of bedrock formations. Pocono Creek is similar to Cranberry Creek in that it also has low conductivity, but it has low levels of DOC. Monocacy Creek has low DOC levels, but it has high conductivity because of limestone bedrock.
Table 1.
Average water quality values for the three biofilm collection sites
| Parameter | Monocacy Creek (n = 6) | Pocono Creek (n = 4) | Cranberry Creek (n = 4) |
|---|---|---|---|
| Na (ppb) | 2.5 × 104 | 2.4 × 104 | 1.3 × 104 |
| Mg (ppb) | 2.6 × 104 | 2.2 × 103 | 1.9 × 103 |
| Si (ppb) | 3.7 × 103 | 2.0 × 103 | 2.9 × 103 |
| K (ppb) | 3.9 × 103 | 6.8 × 102 | 4.3 × 102 |
| Ca (ppb) | 4.3 × 104 | 6.9 × 103 | 8.0 × 103 |
| Collection temp (°C) | 10.7 | 9.53 | 11.3 |
| pH | 8.26 | 7.51 | 7.09 |
| Conductivity (μS) | 581 | 190 | 138 |
| DOC (mg/liter) | 1.46 | 2.10 | 11.64 |
| Nitrogen (mg/liter) | 0.04 | 0.06 | 0.07 |
| Phosphorus (mg/liter) | 6.95 | 3.08 | 3.59 |
Preparation of biofilm inoculum.
Biofilms were collected from the three stream sites, processed, and stored as described previously (54, 55). Briefly, biofilms were gently scraped from stream rocks into 500 ml stream water, which was vacuum filtered through 6-μm-pore-size cellulose filter paper to remove algae, large particles and debris, and grazers that could all disturb the biofilm or clog the flow chamber system tubing. Cell concentrations were quantified by 4′,6-diamidino-2-phenylindole (DAPI) staining (39), and aliquots of 5 × 106 cells each were stored in cryovials at −80°C with 30% glycerol. Monocacy Creek (Bethlehem, PA) biofilms were collected in January 2007, July 2008, October 2008, January 2009, April 2009, July 2009, and April 2010. Biofilms were collected from Cranberry and Pocono Creeks (Tannersville, PA) in November 2008, February 2009, May 2009, and July 2009. All biofilm collection was done within a 2-week period in each season.
Water quality parameters.
Creek water was collected at the same time as the biofilms, filter sterilized (0.22-μm-pore-size filter), and stored at 4°C as described by Wolyniak et al. (54, 55). Water quality, including creek temperature, DOC concentration, pH, and conductivity, was measured as described previously (55). In addition, nitrogen and phosphorus were measured using a Hach DR/4000 spectrophotometer by Hach method 10071 (persulfate digestion method for total nitrogen) and Hach method 8048 (using powder pillows for reactive phosphorus). Major cations were measured by inductively coupled plasma mass spectrometry (ICP-MS) with pneumatic nebulization (Thermo X-Series CCT, Winsford, United Kingdom).
Oocysts.
Live, mouse-shed C. parvum oocysts (Iowa isolate purified with sucrose and Percoll gradients and stored in phosphate-buffered saline with antibiotics and 0.01% Tween 20) were obtained from Waterborne, Inc. (New Orleans, LA), and used within 3 weeks of shedding.
Experimental setup.
Single-channel convertible flow chambers (24 by 40 by 8 mm [length by width by height]) with glass coverslips (Stovall Life Science, Inc., Greensboro, NC) as described previously (6, 54, 55) were used for all of the experiments in this study. Flow chambers were inoculated with 5 × 106 cells of the biofilm culture to be tested 24 h before the flow started. A 12-channel peristaltic pump (IPC Pump; Ismatec, Glattbrugg, Switzerland) was used to maintain a constant laminar flow of 0.17 ml/min for all experiments (6). All of the seasonal biofilms for each collection site were grown in the laboratory at room temperature (20 to 25°C). All experimental treatments were performed in duplicate with two individual and separate flow chambers inoculated with the same biofilm culture.
Mass balance analyses.
Mass balance analyses were performed as described by Wolyniak et al. (55). Briefly, 1 × 104 C. parvum oocysts (except for the loading rate experiments described below) were added to 500 ml constantly stirred influent water each day for 3 days, followed by 5 days of filtered creek water flow without oocyst addition. An additional long-term (25-day) experiment using Monocacy Creek biofilm collected in April 2010 was performed under the same oocyst loading conditions but with 22 days of oocyst-free water.
Influent water was replaced each day, and oocysts were recovered from the remaining influent and effluent water and quantified at the end of each 24-h period as described below. The numbers of oocysts remaining in the influent and effluent were determined by direct hemacytometer counting. The total number of oocysts that passed through the flow chamber system was calculated by subtracting the number of oocysts remaining in the influent water after 24 h from the total added to the reservoir each day. The percentage of oocysts in the biofilm after each 24-h period was calculated from the difference between the total number of oocysts that passed through the flow chamber system (100%) and the number of oocysts in the effluent after each 24-h period.
Oocyst recovery from flow system.
Oocysts were recovered from the remaining influent and effluent waters after each 24-h period using membrane filtration through a 3-μm-pore-size filter (33) and immunomagnetic separation (IMS) as described previously (54, 55). IMS of the material retained on the membrane filter was performed with the Virusys IMS kit (Virusys Co., Sykesville, MD) according to the manufacturer's recommendations.
Biofilm thickness was measured at the end of each experiment by using bright-field differential interference contrast transmission (DICT) settings on a scanning confocal microscope (Zeiss LSM 510 META laser scanning microscope). Oocysts were recovered from the biofilm by scraping the biofilm from the flow chambers and then using membrane filtration and IMS as described previously (54, 55). The IMS products of all samples were counted on a hemacytometer.
Loading rate experiment.
To assess the maximum number of oocysts capable of attaching to biofilms, mass balance experiments were performed as described above but with modified oocyst loading rates of 1 × 105 and 1 × 103 oocysts in 500 ml sterile creek water for 3 days. The results of these experiments were compared to those of the previous experiment with a 1 × 104 loading rate to determine if there was a maximum number of oocysts the biofilm could retain. The loading rate experiments were all performed using the biofilm culture collected from Cranberry Creek in July 2009.
Biofilm surface roughness.
To assess differences in the microbial communities, each biofilm culture was grown in duplicate and 100 thickness measurements of each biofilm were made in a grid pattern using the scanning confocal microscope in bright-field DICT settings. Biofilm roughness (R) was defined by the following equation:
where Lf is the mean thickness, Lfi is the ith individual thickness, and N is the number of thickness measurements (44).
Oocyst surface density.
The average density of oocysts on the biofilm surface was calculated using the average number of oocysts attached to the biofilms divided by the growth area in the flow chamber (4 by 2.4 cm; 9.6 cm2). The maximum surface density was calculated by dividing the maximum number of attached oocysts (Pocono spring 2009 biofilm) by the growth area in the flow chamber.
Data reporting.
Some of the Monocacy Creek data presented here were also presented in references 54 (January 2007 and July 2008 data) and 55 (fall 2008; winter and spring 2009; 4°C experiment). These data sets are included again here for comparison with the results obtained with the Pocono and Cranberry Creek biofilms.
Statistical analyses.
Mann-Whitney tests were performed to determine if a significant difference in oocyst attachment exists between (i) days 8 and 25 and (ii) day 8 with 104 and 105 loading rates. Spearman correlations were performed to determine if any correlation exists between oocyst attachment and (i) water temperature at the time of biofilm collection, (ii) pH, (iii) conductivity, or the concentration of (iv) DOC, (v) nitrogen, (vi) phosphorus, (vii) sodium, (viii) magnesium, (ix) silica, (x) potassium, or (xi) calcium. Kruskal-Wallis tests were performed to determine if a significant difference exists between oocyst attachment at days 3 and 8 for (i) all winter biofilms, (ii) all spring biofilms, (iii) all summer biofilms, and (iv) all fall biofilms collected within the same year and across all of the years. All statistical analyses were performed with the Analyze-it add-in (Analyze-it Software, Ltd., Leeds, England) for Microsoft Excel.
RESULTS
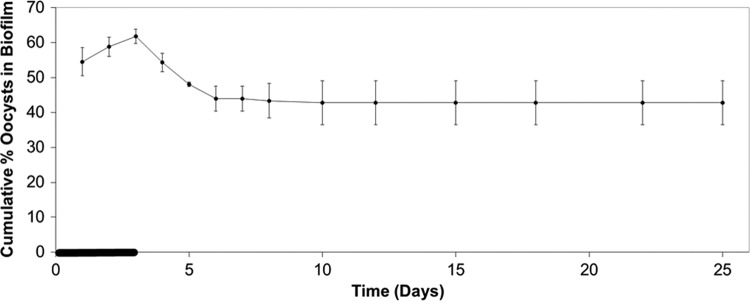
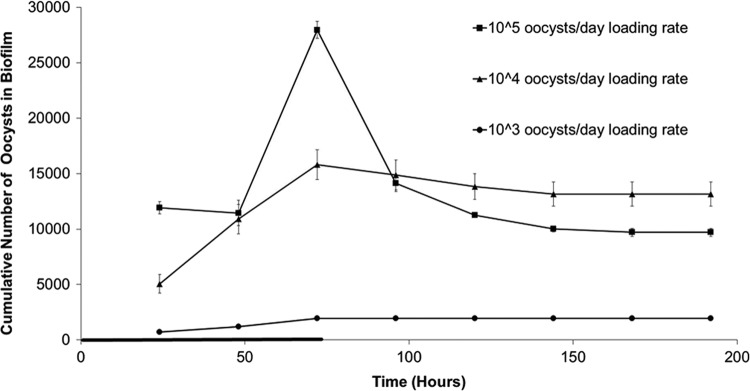
In all microcosm tests with different biofilms, oocyst retention in biofilms rapidly reached a steady state during oocyst dosing (within 3 days) and steadily decreased to an equilibrium level following removal of the oocyst supply (Fig. 1). Biofilms maintained this final equilibrium level of oocysts for at least an additional 20 days (Fig. 2). Varying the oocyst loading rates showed that oocyst retention can be saturated at high loading rates, as no increase in the number of oocysts attached to the biofilm was observed when the loading rate was increased from 104 to 105 oocysts/day (Fig. 3).
Fig 1.
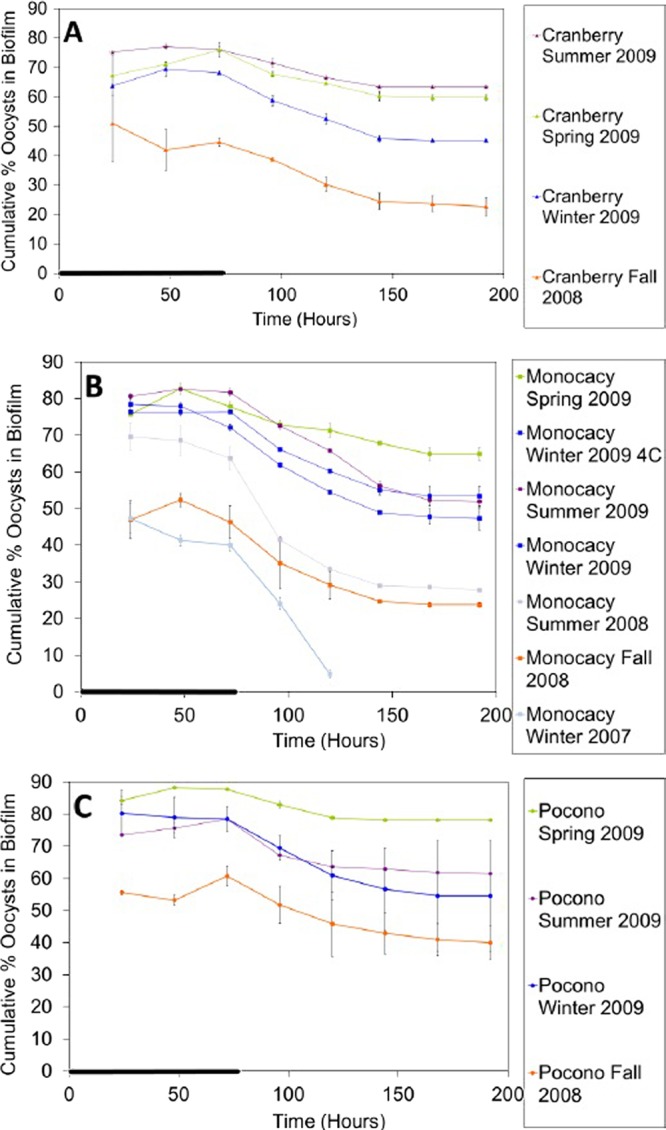
Oocyst retention by biofilms collected seasonally from three streams: Cranberry Creek (A), Monocacy Creek (B), and Pocono Creek (C). The cumulative percentage of oocysts associated with the biofilm over time for each culture was determined. Error bars show the percent standard error (n = 2) and are smaller than the symbols where not visible. The thick line on the time axis indicates the period of oocyst dosing (0 to 72 h). The legend is presented in the order of the most oocysts retained at day 8 to the least oocysts retained at day 8.
Fig 2.
Long-term retention of oocysts by biofilm. The cumulative percentage of oocysts associated with the biofilm (Monocacy Creek biofilm collected in April 2010) over 25 days was determined. Error bars indicate the percent standard error (n = 2) and are smaller than the symbols where not visible. The thick line on the time axis indicates the period of oocyst dosing (0 to 72 h).
Fig 3.
Effect of oocyst loading rate on oocyst retention by biofilm. The cumulative number of oocysts associated with a biofilm (Cranberry Creek biofilm collected in July 2009) over time under different oocyst loading rate conditions was determined. Error bars indicate the standard error (n = 2) and are smaller than the symbols where not visible. The thick line on the time axis indicates the period of oocyst dosing (0 to 72 h).
Within a given season and geographical region, biofilms showed similar oocyst retention (Fig. 1). There was no significant difference in oocyst retention by biofilms from each collection site within each season, both for the same collection year and across all of the collection years (P > 0.05 at days 3 and 8 for all biofilms). The oocyst attachment data from all three of the sites from the fall of 2008 through the summer of 2009 showed similar seasonal patterns (Fig. 4), with some differences between years (Fig. 4, January 2007 and July 2008 outlier points).
Fig 4.
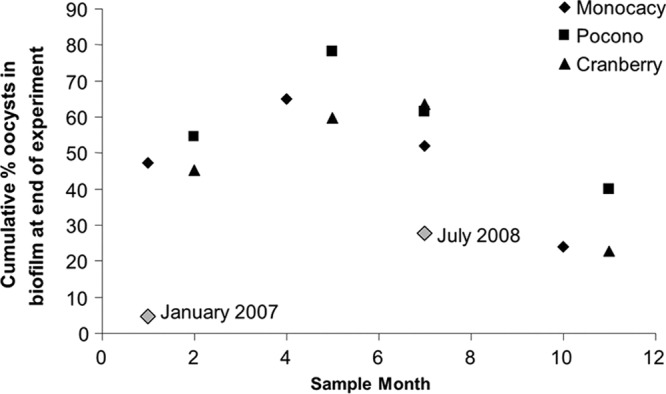
The percentage of oocysts attached to the biofilm at the end of the experiment versus the month in which the biofilm was collected. Black symbols indicate biofilms collected from the fall of 2008 through the summer of 2009. Gray symbols indicate biofilms collected from Monocacy Creek in January 2007 and July 2008, and the biofilm collection dates are shown.
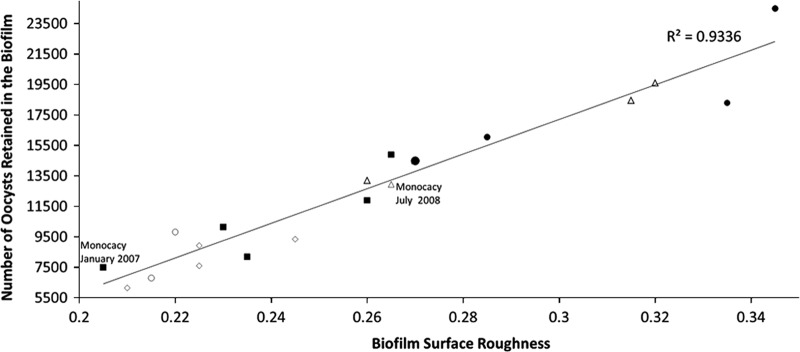
Variation in percent oocyst retention in the biofilm among months, years, and stream sites was strongly correlated with biofilm roughness (R2 = 0.9336; Fig. 5). Water quality parameters (stream collection temperature, conductivity, pH, and concentrations of DOC, nitrogen, phosphorus, sodium, magnesium, silica, potassium, and calcium) showed no correlation with variations in percent oocyst retention and no seasonal trends common to all three stream sites that would explain the similar variations in oocyst retention across all three sites.
Fig 5.
Number of oocysts retained in the biofilm at the end of the experiment as a function of biofilm surface roughness for all of the biofilms sampled. The data points represent biofilms collected from the following sites and dates at various experiment durations: (i) Cranberry Creek 8-day experiments in November 2008, February 2009, May 2009, and July 2009; (ii) Pocono Creek 3- and 8-day experiments in November 2008, February 2009, May 2009, and July 2009; (iii) Monocacy Creek 3-day experiment in April 2010, 8-day experiments in January 2007, July 2008, October 2008, January 2009, April 2009, and July 2009, and 25-day experiment in April 2010. Squares are winter biofilm cultures, diamonds are fall biofilm cultures, circles are spring biofilm cultures, and triangles are summer biofilm cultures.
DISCUSSION
The results of this study confirm previous laboratory observations that oocysts in the water column attach to biofilms (54, 55) and the biofilms can maintain a portion of these oocyst populations for at least 25 days in an equilibrium condition that is established within 8 days. Also, the loading rate experiments described here confirm that experiments performed previously using a 104 oocyst loading rate (54, 55) represent the maximum oocyst attachment to the biofilms.
In this study, oocysts attached to environmental biofilms at an average surface density of 2,100/cm2 (average of all biofilms tested) and a maximum surface density of 3,342/cm2 (Pocono Creek biofilm collected in spring 2009). These concentrations of attached oocysts are similar to the 1,400 oocysts/cm2 reported by Rogers and Keevil (41) but significantly lower than the 14,000 oocysts/cm2 reported by Keevil (23); however, no biofilm roughness measurements were reported for either of these potable-water biofilms. These oocysts remained viable at these concentrations in the biofilm for several months (23, 41). Together, these results indicate that biofilms can accumulate low numbers of viable oocysts for long periods of time, creating a potential public health risk if these oocysts are released back into the water column. The fate of biofilm-attached oocysts may be different in natural waters, as the results presented here describe the interactions of oocysts with environmental biofilms in an isolated environment without the potential disturbances of invertebrates, particularly through biofilm grazing or scraping by stream macroinvertebrates.
Two factors that can potentially control oocyst retention in biofilms are (i) water chemistry and (ii) the characteristics of the aquatic microbial community and resulting biofilm. The water quality data from previous work (55) were not conclusive because of the small data range, so the three biofilm collection sites used in this study were chosen for varying water quality parameters (Table 1). Measured water quality parameters did not show any correlation with oocyst attachment patterns (all Spearman correlation values were >0.05). The results showed seasonal variability in oocyst attachment to biofilms but not variability between biofilm collection sites, suggesting that the variable quality of the water from the three sites did not significantly impact oocyst attachment to the biofilms.
Water quality may impact the net oocyst surface charge, which mediates adhesion (4, 48). Several specific water quality parameters have been shown to affect the oocyst surface charge, including pH, DOC concentration, calcium ion concentration, and conductivity. These water quality factors impact the glycoprotein layer on the oocyst surface that extends into solution and imposes repulsive forces between oocysts and surfaces (7, 10, 27, 48). However, any change in the oocyst surface charge did not create any significant difference in oocyst attachment to biofilms in these experiments.
No relationship between oocyst attachment and pH was found. Oocysts have a pH-dependent surface charge that is negative above pH 3, and increased oocyst removal has been observed in lower-pH solutions (7, 9, 10, 13, 48, 56). However, the variation in the surface charge in the range of pH values of the sample creeks is quite small (12) and probably did not have a significant impact on oocyst interaction with biofilms.
No relationship between oocyst attachment and DOC or calcium ion concentration was found. The protein layer on the oocyst surface collapses in the presence of DOC and calcium ions, resulting in more oocyst adhesion (56). The oocyst surface charge has been shown to become more negative as the DOC concentration increased to as much as 5.9 mg/liter (56). Based on these results, the oocysts should have experienced a surface charge change when exposed to the Cranberry Creek water (DOC concentration range, 6 to 17 mg/liter) compared to water from the other two stream sites (DOC concentration range, 0.9 to 3 mg/liter). Similarly, the high calcium concentration in Monocacy Creek (range, 2.9 × 104 to 5.0 × 104 ppb) compared to that in the other two streams (5.7 × 103 to 1.0 × 104 ppb) may also generate a change in the oocyst surface charge. However, oocyst attachment to Cranberry Creek biofilms or Monocacy Creek biofilms was not different from the attachment to biofilms from the other two streams in any season, as would be expected based on surface charge effects due to stream water quality.
The surface charge also becomes more negative with increasing conductivity (20). In this study, the conductivity of Monocacy Creek water (range, 517 to 664 μS) was higher than that of water from the other two creek sites (range, 118 to 211 μS); however, the variations in conductivity seen in the creek waters used here did not yield a difference in oocyst attachment to biofilms.
This lack of an observable relationship between oocyst attachment and water chemistry may be a result of the oocyst purification method used. Although the impact of purification methods on the oocyst surface charge is largely unknown, Butkus et al. (5) and Dai et al. (10) reported that oocysts maintain a neutral surface charge after phosphate-buffered saline washes, suggesting that the oocysts used in these experiments may have a neutral surface charge. In addition, oocysts purified by Percoll-sucrose (as used by Waterborne, Inc.) have been shown to have altered oocyst wall characteristics that may impact interaction with surfaces (21).
The observed seasonal variability in oocyst attachment, as opposed to variation with the biofilm collection site (despite varying water quality), suggests that the characteristics of the biofilm control the oocyst attachment patterns. This corroborates previous findings that suggest that the biofilm structure is more important in oocyst attachment than is water quality (55).
All of the biofilm cultures used in these experiments showed a strong correlation between oocyst attachment and surface roughness (used in this study to characterize biofilms), even the January 2007 and July 2008 Monocacy Creek biofilms that did not follow oocyst attachment patterns in the same season from other years (Fig. 5; R2 = 0.9336).
Although the oocyst retention data showed a seasonal trend, this trend does not follow from year to year as stream conditions change, resulting in variation in the biofilm community (Fig. 4). Seasonal variation in biofilm communities has been reported by others; both composition and function can change in response to environmental conditions. Microbial biofilm communities shift in composition and biomass with seasonal environmental changes in the stream but are stable within seasonal limits (3, 14, 17, 31, 35). Seasonal analysis of biofilm communities showed dramatic seasonal differences but consistent biofilm communities throughout each season, controlled by temperature, nutrient availability, carbon source, discharge, and light (22, 24, 31, 35). However, temperature and rainfall were the primary factors responsible for the transition between seasonal communities (31). Abnormal seasonal temperature and precipitation patterns were observed in this study and may have changed oocyst retention patterns in the Monocacy Creek biofilms collected in the winter of 2007 (average January 2007 temperature, 1.8°C [range, −9.4 to 17°C]; average January temperature, −2.5°C) and the summer of 2008 (July 2008 precipitation, 16.6 cm, with several very large rainfalls of >5 cm; average July precipitation, 12.7 cm), in which we observed high levels of oocyst attachment followed by a large oocyst release, which was not seen in the other biofilm cultures (Fig. 1). These unusual, and often sudden, changes in weather patterns may change the planktonic microbial communities and their ability to form biofilms, resulting in more unstable biofilm structures (2, 31, 38).
Similarly, Okabe et al. (34) showed that the dynamics of inert 1-μm-diameter beads within biofilms were strongly influenced by biofilm structure and growth patterns, which vary seasonally. Increased surface roughness significantly influenced bead distribution, as a rough surface provides more surface area for attachment. The rate of bead release from the biofilm was dependent on biofilm properties, as well as shear stress and substrate loading rates. These results showed that biofilm structure and growth patterns are important factors in determining particle transport in biofilms, and from the results presented here, this appears to be true for oocysts and environmental biofilms as well.
A role for biofilm surface roughness in oocyst retention is consistent with the results of the oocyst loading rate experiments (Fig. 3). Roughly the same number of oocysts attached to the biofilm under 104 and 105 loading rate conditions, indicating that the biofilm has a maximum number of oocysts that can be retained. The number of oocysts attached to the biofilm was controlled by the biofilm's surface roughness.
The results presented here suggest that as the bacterial community composition changes with the season, biofilm roughness changes as a result. Shear stress and biofilm growth rates may also affect biofilm surface roughness. In these studies, the water flow was constant and laminar for all of the biofilms, limiting the effects of shear stress across the biofilm cultures. However, growth rate variations in the microbial communities present in each biofilm culture may have contributed to the seasonal variation in biofilm surface roughness that controlled oocyst attachment. In slow-growing biofilms or under high-shear conditions, the biofilm becomes smoother and less susceptible to shear forces (32, 45, 49, 51). In fast-growing biofilms or under low-shear conditions, biofilm outgrowths will proceed rapidly until the biofilm becomes unstable and a sloughing event occurs (37, 38, 49, 50).
These results show the importance of biofilms in Cryptosporidium oocyst transport. Waters that are considered clean and safe may contain biofilms that are holding oocyst reservoirs. Monitoring of the biofilms in water supplies may aid in determining how large these reservoirs are and how great the risk of oocyst resuspension from these biofilm reservoirs is.
ACKNOWLEDGMENTS
This research was funded by National Science Foundation CAREER Award 0545687 to K. L. Jellison.
ICP-MS analysis was performed by Stephen Peters at Lehigh University.
Footnotes
Published ahead of print 6 April 2012
REFERENCES
- 1. Angles ML, Chandy JP, Cox PT, Fisher IH, Warnecke WR. 2007. Implications of biofilm-associated waterborne Cryptosporidium oocysts for the water industry. Trends Parasitol. 23:352–356 [DOI] [PubMed] [Google Scholar]
- 2. Araya R, Tani K, Takagi T, Yamaguchi N, Nasu M. 2003. Bacterial activity and community composition in stream water and biofilm from an urban river determined by fluorescent in situ hybridization and DGGE analysis. FEMS Microbiol. Ecol. 43:111–119 [DOI] [PubMed] [Google Scholar]
- 3. Brümmer IHM, Fehr W, Wagner-Döbler I. 2000. Biofilm community structure in polluted rivers: abundance of dominant phylogenetic groups over a complete annual cycle. Appl. Environ. Microbiol. 66:3078–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brush CF, Walter MF, Anguish LJ, Ghiorse WC. 1998. Influence of pretreatment and experimental conditions on electrophoretic mobility and hydrophobicity of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 64:4439–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butkus MA, Bays JT, Labare MP. 2003. Influence of surface characteristics on the stability of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 69:3819–3825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christensen BB, et al. 1999. Molecular tools for study of biofilm physiology. Methods Enzymol. 310:20–42 [DOI] [PubMed] [Google Scholar]
- 7. Considine RF, Dixon DR, Drummond CJ. 2002. Oocysts of Cryptosporidium parvum and model sand surfaces in aqueous solutions: an atomic force microscope (AFM) study. Water Res. 36:3421–3428 [DOI] [PubMed] [Google Scholar]
- 8. Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 9. Dai X, Hozalski RM. 2002. Effect of NOM and biofilm on the removal of Cryptosporidium parvum oocysts in rapid filters. Water Res. 36:3523–3532 [DOI] [PubMed] [Google Scholar]
- 10. Dai X, Boll Hayes JME, Aston DE. 2004. Adhesion of Cryptosporidium parvum and Giardia lamblia to solid surfaces: the role of surface charge and hydrophobicity. Colloid Surf. B Biointerfaces 34:259–263 [DOI] [PubMed] [Google Scholar]
- 11. Davey ME, O'Toole GA. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drozd C, Schwartzbrod J. 1996. Hydrophobic and electrostatic cell surface properties of Cryptosporidium parvum. Appl. Environ. Microbiol. 62:1227–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drury WJ, Characklis WG, Stewart PS. 1993. Interactions of 1-μm latex particles with Pseudomonas aeruginosa biofilms. Water Res. 27:1119–1126 [DOI] [PubMed] [Google Scholar]
- 14. Findlay SEG, Sinsabaugh RL, Sobczak WV, Hoostal M. 2003. Metabolic and structural response of hyporheic microbial communities to variations in supply of dissolved organic matter. Limnol. Oceanogr. 48:1608–1617 [Google Scholar]
- 15. Fletcher M. 1991. The physiological activity of bacteria attached to solid surfaces. Adv. Microb. Physiol. 32:53–85 [DOI] [PubMed] [Google Scholar]
- 16. Flood JA, Ashbolt NJ. 2000. Virus-sized particles can be entrapped and concentrated one hundred fold within wetland biofilms. Adv. Environ. Res. 3:403–411 [Google Scholar]
- 17. Gao X, Olapade OA, Leff LG. 2005. Comparison of benthic bacterial community composition in nine streams. Aquat. Microb. Ecol. 40:51–60 [Google Scholar]
- 18. Hashimoto A, Kunikane S, Hirata T. 2002. Prevalence of Cryptosporidium oocysts and Giardia cysts in the drinking water supply in Japan. Water Res. 36:519–526 [DOI] [PubMed] [Google Scholar]
- 19. Howe AD, et al. 2002. Cryptosporidium oocysts in a water supply associated with a cryptosporidiosis outbreak. Emerg. Infect. Dis. 8:619–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hsu BM, Huang C. 2002. Influence of ionic strength and pH on hydrophobicity and zeta potential of Giardia and Cryptosporidium. Colloid Surf. A Physicochem. Eng. Asp. 201:201–206 [Google Scholar]
- 21. Jenkins MB, et al. 2010. Significance of wall structure, macromolecular composition, and surface polymers to the survival and transport of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 76:1926–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Judd KE, Crump BC, Kling GW. 2006. Variation in dissolved organic matter controls bacterial production and community composition. Ecology 87:2068–2079 [DOI] [PubMed] [Google Scholar]
- 23. Keevil CW. 2003. Rapid detection of biofilms and adherent pathogens using scanning confocal laser microscopy and episcopic differential interference contrast microscopy. Water Sci. Technol. 47(5):105–116 [PubMed] [Google Scholar]
- 24. Keith-Roach MJ, Bryan ND, Bardgett RD, Livens FR. 2002. Seasonal changes in the microbial community of a salt marsh, measured by phospholipid fatty acid analysis. Biogeochemistry 60:77–96 [Google Scholar]
- 25. King BJ, Monis PT. 2007. Critical processes affecting Cryptosporidium oocyst survival in the environment. Parasitology 134:309–323 [DOI] [PubMed] [Google Scholar]
- 26. Köster M, Meyer-Reil LA. 2002. Ecology of marine microbial biofilms, p 1081–1089 In Britton G. (ed), Encyclopedia of environmental microbiology. Wiley, New York, NY [Google Scholar]
- 27. Kuznar KA, Elimelech M. 2005. Role of surface proteins in the deposition kinetics of Cryptosporidium parvum oocysts. Langmuir 21:710–716 [DOI] [PubMed] [Google Scholar]
- 28. LeChevallier MW, Norton WD, Lee RG. 1991. Giardia and Cryptosporidium spp. in filtered drinking water supplies. Appl. Environ. Microbiol. 57:2617–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. LeChevallier MW, Norton WD, Lee RG. 1991. Occurrence of Giardia and Cryptosporidium spp. in surface water supplies. Appl. Environ. Microbiol. 57:2610–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. LeChevallier MW, Norton WD. 1995. Giardia and Cryptosporidium in raw and finished water. J. Am. Water Works Assoc. 87:54–68 [Google Scholar]
- 31. Moss JA, Nocker A, Lepo JE, Snyder RA. 2006. Stability and change in estuarine biofilm bacterial community diversity. Appl. Environ. Microbiol. 72:5679–5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nam TK, Timmons MB, Montemagno CD, Tsukuda SM. 2000. Biofilm characteristics as affected by sand size and location in fluidized bed vessels. Aquacult. Eng. 22:213–224 [Google Scholar]
- 33. Oda T, et al. 2000. Size selective continuous flow filtration method for detection of Cryptosporidium and Giardia. Water Res. 34:4477–4481 [Google Scholar]
- 34. Okabe S, Yasuda T, Watanabe Y. 1997. Uptake and release of inert fluorescence particles by mixed population biofilms. Biotechnol. Bioeng. 53:459–469 [DOI] [PubMed] [Google Scholar]
- 35. Olapade OA, Leff LG. 2005. Seasonal response of stream biofilm communities to dissolved organic matter and nutrient enrichments. Appl. Environ. Microbiol. 71:2278–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Percival SL, Walker JT. 1999. Potable water and biofilms: a review of the public health implications. Biofouling 14:99–115 [Google Scholar]
- 37. Picioreanu C, van Loosdrecht MCM, Heijnen JJ. 1999. Discrete-differential modeling of biofilm structure. Water Sci. Technol. 39:115–122 [Google Scholar]
- 38. Picioreanu C, van Loosdrecht MCM, Heijnen JJ. 2001. Two-dimensional model of biofilm detachment caused by internal stress from liquid flow. Biotechnol. Bioeng. 72:205–218 [PubMed] [Google Scholar]
- 39. Porter KG, Keig YS. 1980. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 25:943–948 [Google Scholar]
- 40. Quignon F, Kiene L, Levi Y, Sardin M, Schwartzbrod L. 1997. Virus behaviour within a distribution system. Water Sci. Technol. 35:311–318 [Google Scholar]
- 41. Rogers J, Keevil CW. 1995. Survival of Cryptosporidium parvum oocysts in biofilm and planktonic samples in a model system, p 209–213 In Betts WB, et al. (ed), Protozoan parasites and water. The Royal Society of Chemistry, Cambridge, United Kingdom [Google Scholar]
- 42. Rose JB. 1988. Occurrence and significance of Cryptosporidium in water. J. Am. Water Works Assoc. 80:53–58 [Google Scholar]
- 43. Rose JB, Gerba CP, Jakubowski W. 1991. Survey of potable water supplies for Cryptosporidium and Giardia. Environ. Sci. Technol. 25:1393–1400 [Google Scholar]
- 44. Searcy KE, Packman AI, Atwill ER, Harter T. 2006. Capture and retention of Cryptosporidium parvum oocysts by Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 72:6242–6247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shen L, Ganczarczyk J. 2005. Seasonal changes in the structure of RBC heterotrophic biofilms. Environ. Technol. 26:55–63 [DOI] [PubMed] [Google Scholar]
- 46. Solo-Gabriele H, Neumeister S. 1996. US Outbreaks of Cryptosporidiosis. J. Am. Water Works Assoc. 88:76–86 [Google Scholar]
- 47. Szewzyk U, Szewzyk R, Manz W, Schleifer KH. 2000. Microbiological safety of drinking water. Annu. Rev. Microbiol. 54:81–127 [DOI] [PubMed] [Google Scholar]
- 48. Tufenkji N, Dixon DR, Considine R, Drummond CJ. 2006. Multi-scale Cryptosporidium/sand interactions in water treatment. Water Res. 40:3315–3331 [DOI] [PubMed] [Google Scholar]
- 49. van Loosdrecht MCM, et al. 1995. Biofilm structures. Water Sci. Technol. 32:35–43 [Google Scholar]
- 50. van Loosdrecht MCM, Picioreanu C, Heijnen JJ. 1997. A more unifying hypothesis for biofilm structures. FEMS Microbiol. Ecol. 24:181–183 [Google Scholar]
- 51. van Loosdrecht MCM, Heijnen JJ, Eberl H, Kreft J, Picioreanu C. 2002. Mathematical modeling of biofilm structures. Antonie Van Leeuwenhoek 81:245–256 [DOI] [PubMed] [Google Scholar]
- 52. Warnecke M. 2006. Cryptosporidium oocyst interactions with drinking water pipe biofilms. Research report 5 The Cooperative Research Center for Water Quality and Treatment, Salisbury, South Australia, Australia [Google Scholar]
- 53. Weir SC, Pokorny NJ, Carreno RA, Trevors JT, Lee H. 2002. Efficacy of common laboratory disinfectants on the infectivity of Cryptosporidium parvum oocysts in cell culture. Appl. Environ. Microbiol. 68:2576–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wolyniak EA, Hargreaves BR, Jellison KL. 2009. Retention and release of Cryptosporidium parvum oocysts by experimental biofilms composed of a natural stream microbial community. Appl. Environ. Microbiol. 75:4624–4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wolyniak EA, Hargreaves BR, Jellison KL. 2010. Seasonal retention and release of Cryptosporidium parvum oocysts by environmental biofilms in the laboratory. Appl. Environ. Microbiol. 76:1021–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xagoraraki I, Harrington GW. 2004. Zeta potential, dissolved organic carbon, and removal of Cryptosporidium oocysts by coagulation and sedimentation. J. Environ. Eng. 130:1424–1432 [Google Scholar]