Abstract
Highly pathogenic avian influenza A viruses of subtypes H5 and H7 are the causative agents of fowl plague in poultry. Influenza A viruses of subtype H5N1 also caused severe respiratory disease in humans in Hong Kong in 1997 and 2003, including at least seven fatal cases, posing a serious human pandemic threat. Between the end of February and the end of May 2003, a fowl plague outbreak occurred in The Netherlands. A highly pathogenic avian influenza A virus of subtype H7N7, closely related to low pathogenic virus isolates obtained from wild ducks, was isolated from chickens. The same virus was detected subsequently in 86 humans who handled affected poultry and in three of their family members. Of these 89 patients, 78 presented with conjunctivitis, 5 presented with conjunctivitis and influenza-like illness, 2 presented with influenza-like illness, and 4 did not fit the case definitions. Influenza-like illnesses were generally mild, but a fatal case of pneumonia in combination with acute respiratory distress syndrome occurred also. Most virus isolates obtained from humans, including probable secondary cases, had not accumulated significant mutations. However, the virus isolated from the fatal case displayed 14 amino acid substitutions, some of which may be associated with enhanced disease in this case. Because H7N7 viruses have caused disease in mammals, including horses, seals, and humans, on several occasions in the past, they may be unusual in their zoonotic potential and, thus, form a pandemic threat to humans.
Migratory birds and waterfowl are thought to be the reservoir for influenza A viruses in nature (1, 2). To date, influenza A viruses representing 15 hemagglutinin (HA) and 9 neuraminidase (NA) subtypes have been described in wild birds and poultry throughout the world (3). Viruses belonging to the antigenic subtypes H5 and H7, in contrast to viruses possessing other HA subtypes, may become highly pathogenic when transmitted from wild birds to poultry and, thus, cause fowl plagues (4).
Avian influenza A viruses (AIV) pose the threat of initiating new pandemics in humans because the human population is serologically naive toward most HA and NA subtypes. Until recently, it was thought that pigs were required as an intermediate host for transmission of AIV to humans (5, 6). AIV, in general, do not replicate efficiently or cause disease in humans (7). However, in Hong Kong in 1997 and 2003, highly pathogenic avian influenza (HPAI) of subtype H5N1 was transmitted from birds to humans, of whom at least seven died (8–13). The only other reports on natural infections of humans by HPAI viruses were cases of conjunctivitis associated with avian H7N7 viruses, transmitted either directly from birds to humans or by seals (14, 15). Low pathogenic AIV may also be transmitted to humans, either directly or on transmission to pigs; the pandemics of 1918 (H1N1), 1957 (H2N2), and 1968 (H3N2) were caused by influenza viruses harboring HA and NA genes of avian or swine origin (1, 2). Because of the recent increase in awareness of AIV zoonoses and the increase in the intensity of surveillance studies, sporadic zoonotic infections with H9N2 AIV in China have been detected also (16). The transmission of AIV from birds to humans, therefore, continues to be a threat to public health.
Here, we describe the characterization of virus isolates obtained from poultry and humans during an outbreak of HPAI that started in February 2003 in The Netherlands and spread subsequently to poultry in Germany and Belgium (13).
Methods
Subjects. Case finding of H7N7 infections in humans was initiated under the auspices of the Dutch Ministry of Public Health, Welfare, and Sport (The Hague, The Netherlands). From individuals in contact with H7 AIV and suffering from conjunctivitis and/or influenza-like illness, throat, nose, and conjunctiva swabs were collected in virus transport media for diagnostic testing, subtyping, and further characterization (M.K., B. Wilbrink, M. Conyn, G. Natrop, H. van der Nat, H. Vennema, A. Meijer, J. van Steenbergen, R.A.M.F., A.D.M.E.O., and A.B., unpublished data).
Virus Isolation. Samples from birds and humans were inoculated in the allantoic cavity of 11-day-old specific pathogen-free embryonated chicken eggs (17). All RT-PCR-positive samples from humans were inoculated in tertiary monkey kidney cells or Madine–Darby canine kidney cells (18). Isolation of H7N7 virus was successful for 47 samples, and samples from which no virus could be isolated generally had low “virus load” [high threshold cycle (Ct) values in RT-PCR assays]. AIV was detected by hemagglutination assays using turkey erythrocytes (18).
RT-PCR. RNA isolation and RT-PCR for the detection of AIV, as described in ref. 17, and a real-time RT-PCR using RNA isolated on a MagnaPure Light Cycler system were performed independently for all human samples in two laboratories. An H7-specific TaqMan assay was designed based on the HA gene of A/Chicken/Netherlands/1/03 by using the primers 5′-GGCAACAGGAATGAAGAATGTTCC-3′ and 5′-AATCAGACCTTCCCATCCATTTTC-3′ and the probe 5′-f luorescein -AGAGGCCTATTGGTGCTATAGCGGGTTTCAT-tetramethylrhodamine-3′. A real-time RT-PCR assay for the detection of the HA gene of human H3N2 influenza A viruses was used also (19). Samples were run on an ABI 7700 machine with the EZ recombinant thermus thermophilus kit (Applied Biosystems). Cycling conditions were as follows: 2 min at 50°C, 30 min at 60°C, and 5 min at 95°C (one time), and 0.15 sec at 95°C and 1 min at 62°C (40 times).
Sequence Analyses and Phylogenetic Trees. RT-PCR specific for the conserved noncoding regions of AIV was described (20). PCR products were purified by using the QIAquick gel extraction kit (Qiagen, Leusden, The Netherlands) and sequenced directly or when cloned in pCR2.1 (Invitrogen). Sequencing was performed by using the Big Dye Terminator sequencing kit, version 3.0 (Amersham Biosciences) and an ABI Prism 3100 genetic analyzer (Applied Biosystems). Primer sequences are available on request. Nucleotide sequences are available from GenBank.
Nucleotide sequences were aligned by using bioedit 5.0.9, and maximum likelihood trees were generated with PHYLIP 3.6 (21) by using 100 bootstraps and 3 jumbles. The consensus tree was used as a user tree in dnaml to recalculate branch lengths, and trees were rerooted at midpoint. The following reference sequences, representing the known genetic lineages of influenza A virus, were used for phylogenic trees: American avian (AF457697-AF457701 and AF457703), Eurasian avian (AF144300-AF144303, AF144306, and AF144307), gull (M80959, M73525, M26088, M25933, M27521, and M63539), equine H3N8 (M73526, M30758, M63529, M80973, M26082, and M25929), equine H7N7 (M63533, M30750, M73520, M25928, M26087, and M80954), swine H3N2 (M80960 and AF251390-AF251394), swine H1N1 (M55481, M55482, M55473, M55469, M55472, and M22578), human H1N1 (ISDN13419-ISDN13421, ISDN13423, ISDN13425, and ISDN13426), human H2N2 (M81576–M81581), and human H3N2 (AF348170, AF348173, AF348175, AF348180, AF348188, and AF348198).
Serological Assays. The Directigen Flu A+B immunoassay (Beckton Dickinson) was used for the detection of AIV antigen (22). Hemagglutination inhibition assays were performed as described (18). Rabbit sera raised against purified HA and NA of A/Seal/Massachussets/1/80 (H7N7) and A/Equine/Prague/1/54 (H7N7) were used for identification of H7 viruses. Human sera were tested against A/Mallard/Netherlands/12/00 (H7N3) or A/Chicken/Netherlands/1/03 (H7N7) (18).
Histological and Immunohistochemical Examinations. Histological and immunohistochemical examinations were performed as described (23). Briefly, samples for histological examination were stained with hematoxylin and eosin or with an avidin–biotin complex immunoperoxidase method, by using an mAb to the nucleoprotein of influenza virus A as a primary antibody. Positive control sections from the wattles of a chicken infected with H7N7 AIV were tested concurrently. The following tissues from the fatal case were examined by light microscopy for the presence of lesions and for detection of influenza viral antigen: adrenal gland, epiglottis, heart, liver, lung, lymph node, kidney, pancreas, and spleen.
Results
Characterization of the Virus Causing the Outbreak in Poultry. On March 1, 2003, five 70-week-old layer chickens at a farm in Scherpenzeel, The Netherlands, suffering from respiratory problems, diarrhea, yawning, swollen heads, and swollen combs were culled. In autopsy, gross lesions included marked s.c. edema of head, comb and wattles, diffuse pulmonary edema, and multifocal hemorrhage in various tissues, consistent with HPAI. Cloacal and tracheal swabs from four animals showed a weak reaction in the Directigen Flu A+B test. RT-PCR specific for the matrix gene showed that all five animals were positive (data not shown). The HA and NA genes were sequenced, providing clear evidence for the viral subtype H7N7. A virus isolate, A/Chicken/Netherlands/1/03, was characterized by hemagglutination inhibition assays as an H7 AIV. The HA and NA genes displayed high homology to those of A/Mallard/Netherlands/12/00 (H7N3) (Fig. 1) and A/Mallard/Netherlands/2/00 (H10N7), respectively (Fig. 2), two viruses isolated from mallard ducks (Anas platyrhynchos) within our AIV surveillance studies in migratory birds. The HA of the H7N7 AIV contains a protease cleavage site consisting of multiple basic amino acids, PEIPKRRRR*GLF, which is in agreement with this virus being classified as a HPAI virus. This basic cleavage site is distinct from those seen in other recent H7 AIV outbreaks and is lacking in H7 of the Mallard isolate (PEIPKGR*GLF) (24, 25).
Fig. 1.
Amino acid sequence alignment of the HA ORFs of selected isolates. The HA genes of A/Mallard/Netherlands/12/00 (H7N3), A/Chicken/Netherlands/1/03 (H7N7), and A/Netherlands/219/03 (H7N7) are shown. The sequence of the chicken isolate is identical to that of A/Netherlands/33/02 (H7N7). Gaps are indicated by dashes, and identical residues to the mallard isolate are indicated by periods. Boxes indicate the potential N-linked glycosylation sites (N-X-T/S), and the shaded residues represent the cleavage site in the HA precursor protein.
Fig. 2.
Amino acid sequence alignment of the NA ORFs of selected isolates. The NA genes of A/Mallard/Netherlands/2/00 (H10N7), A/Chicken/Netherlands/1/03 (H7N7), and A/Netherlands/219/03 (H7N7) are shown. The sequence of the chicken isolate is identical to that of A/Netherlands/33/02 (H7N7). Gaps are indicated by dashes, and identical residues to the mallard isolate are indicated by periods. Boxes indicate the potential N-linked glycosylation sites (N-X-T/S).
Characterization of the Virus Causing Disease in Humans. The first confirmed case of human conjunctivitis caused by H7N7 AIV was in a veterinarian who visited several farms with infected flocks. The symptoms in the first eye started 30 h after his last farm visit, and similar problems occurred in the other eye within 24 h. Swabs collected from both eyes revealed the presence of AIV by RT-PCR. A virus isolate (A/Netherlands/33/03) was obtained in chicken eggs and tertiary monkey kidney cells, and it was characterized as an H7 AIV (data not shown). The HA and NA genes were identical to those of the chicken isolate (Figs. 1 and 2).
After this initial detection, other persons in contact with infected poultry presented with similar symptoms and active case finding was initiated (M.K., B. Wilbrink, M. Conyn, G. Natrop, H. van der Nat, H. Vennema, A. Meijer, J. van Steenbergen, R.A.M.F., A.D.M.E.O., and A.B., unpublished work). A case of conjunctivitis was defined as possible contact with H7 AIV and two or more of the following symptoms: red, tearful, itching, painful, burning eyes; purulent fluid in eyes; or sensitivity to light. A case of influenza-like illness was defined as an acute onset of symptoms (prodromal phase maximal 4 days), fever (≥38.5°C), and at least one of the following symptoms: cough, rhinorrhoea, sore throat, myalgia, or headache.
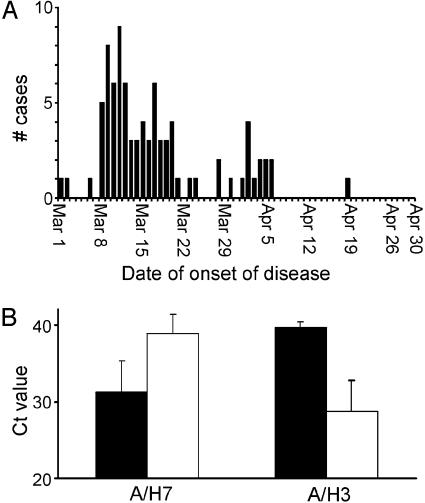
Between March 1 and May 16, 89 confirmed cases of human H7N7 infection were detected (Fig. 3A). Of these cases, 78 presented with conjunctivitis, 5 presented with conjunctivitis and influenza-like illness, 2 presented with influenza-like illness, and 4 did not fit the case definitions. Six of seven cases of influenza-like illness were mild, with patients reporting subjective (unrecorded) fever (n = 7), cough (n = 4), runny nose (n = 3), headache (n = 3), muscle pain (n = 3), sore throat (n = 2), and fatigue (n = 1). Red eyes were reported most frequently (94%) in cases of conjunctivitis, followed by tearful (77%), burning (64%), and itching (57%) eyes, and by pus in eyes (48%), photophobia (33%), and pain in eyes (31%). H7N7 AIV was detected in three individuals who had not been in contact with infected poultry, but who were family members of individuals with H7N7 conjunctivitis, indicating that transmission of virus from human to human had occurred.
Fig. 3.
Detection of H7N7 influenza A virus in humans. (A) Numbers of confirmed cases of H7N7 infection in individuals presenting with conjunctivitis or influenza-like illness in The Netherlands between March 1 and April 30, 2003, as determined by RT-PCR. (B) Comparison of the Ct values, the first TaqMan RT-PCR cycle in which matrix gene of influenza A virus was detected in throat/nose (open bars) or conjunctiva (filled bars) swabs in H7N7 and H3N2 influenza A virus-infected individuals, identified in the same group of individuals in the same time period.
As a measure of virus load in AIV-positive individuals, we compared the first TaqMan RT-PCR cycle in which virus was detected (Ct value) in samples collected from the conjunctivae and the respiratory tract (Fig. 3B). In 91 pairs of samples collected from the 89 H7N7-positive individuals (from two individuals, samples collected 3 days apart were positive), average virus load was higher in conjunctiva swabs as compared with throat/nose swabs (mean Ct values of 31.3 and 38.9, respectively). For nine pairs of samples from eight H3N2-positive individuals, virus load was lower in conjunctiva swabs as compared with throat/nose swabs (mean Ct of 39.7 and 28.8, respectively). The high virus load in conjunctiva swabs collected from H7N7-infected individuals suggests active replication in human cells rather than spillover of virus from the environment. No double infections with H7N7 and H3N2 viruses were detected.
A Fatal Case of Acute Respiratory Distress Syndrome. A 57-year-old veterinarian who visited a poultry farm affected by HPAI H7N7 infection in Teeffelen, The Netherlands, died on April 17, 2003, as the result of pneumonia followed by acute respiratory distress syndrome and related complications in a hospital in's-Hertogenbosch, The Netherlands.
On April 4, the veterinarian suffered from high fever and severe headache. When he first visited his family doctor for persisting fever and headache 4 days later, no signs of respiratory disease or conjunctivitis were observed, and the man, therefore, did not receive medication. Because of his farm visit on April 2, throat and eye swabs were collected on April 9. RT-PCR tests performed in two laboratories were negative. On April 11, the man was admitted to hospital, and interstitial opacities in the lower right lobe were detected (Fig. 4). Treatment with oxygen and i.v. antibiotics (750 mg of cefuroxime three times daily and 1,000 mg of erythromycin four times daily) was started. He was moved to the intensive care unit 2 days later with deteriorating conditions and was put on mechanical ventilation. X-thorax, performed on April 13 when his condition worsened, revealed a bilateral pneumonia, and a bronchoalveolar lavage was performed for diagnostic purposes. Throat swab samples collected on April 11 and April 13 indicated the presence of herpes simplex virus 1, for which he was treated with aciclovir. On April 14, his renal function decreased and dialysis was started. On April 17, his pulmonary condition worsened further and he died of respiratory insufficiency. Laboratory testing for specific diseases (Q fever, brucellosis, psittacosis, Legionnaire's disease, Pneumocystis carinii pneumonia, and tuberculosis) and pathogens [pneumococci, mycoplasms, Chlamydia, respiratory syncytial virus, influenza B virus, human metapneumovirus, cytomegalovirus, herpes simplex virus 2, HIV, Puumala hantavirus, and severe acute respiratory syndrome (SARS)–coronavirus] were all negative.
Fig. 4.
Chest x-ray taken on admission on April 9, 2003, of the veterinarian who developed acute respiratory distress syndrome and died on April 17, 2003. The x-ray reveals extensive infiltrates in the lower right lobe without pleural effusion. The left lung is normal. The label (L) indicates the point of reference on the upper left arm.
The bronchoalveolar lavage revealed the presence of H7 AIV by using real-time PCR assays. PCR analysis and virus isolation from postmortem specimens of the right and left lung confirmed the presence of H7 AIV (A/Netherlands/219/03). A serum sample collected on April 17 displayed very weak, inconclusive reactivity with H7 AIV in hemagglutination inhibition assays. It is unclear why the respiratory sample collected from the veterinarian on April 9 remained negative when RT-PCR and virus isolation were performed.
Pathology. Autopsy revealed generalized edema with 1,500 ml of pleural fluid on both sides and 1,000 ml of serous fluid in the abdominal cavity. The weights of the right and left lungs were about three times the normal weight. On cut-surface, the lungs were oedematous, emphysematous, and firm, with serous fluid in bronchi and bronchioli.
Histologically, there was severe diffuse alveolar damage, characterized by serosanguineous fluid in alveolar lumina, multifocally admixed with fibrin and neutrophils in the lower pulmonary lobes. Alveolar lumina were distended with occasional rupture of alveolar septa. Alveolar septa were thickened by dilatation of capillaries and infiltration with a few lymphocytes and neutrophils. There was evidence of epithelial regeneration, characterized by scattered atypical pneumocytes and ciliated epithelial cells lining the alveolar septa and bronchiolar walls, respectively. These atypical cells were unusually large and had large nuclei, coarse chromatin, and prominent nucleoli. Influenza virus antigen could not be detected in lungs or other tissues by immunohistochemistry. There were no significant lesions in any organs, other than those of the respiratory tract, by gross or histologic examination.
Sequence Analyses of H7N7 Isolates. The entire genomes of A/Chicken/Netherlands/1/03, A/Netherlands/33/03, and A/Netherlands/219/03 were sequenced. All gene segments from these isolates were most similar to Eurasian AIV (Figs. 1, 2, and 5). A/Chicken/Netherlands/1/03 and A/Netherlands/33/03 were almost identical, with two silent nucleotide changes in the basic polymerase 2 (PB2) gene and one amino acid substitution in NS1 (Table 1).
Fig. 5.
Phylogenetic trees representing the internal genes of influenza A viruses. Trees were constructed based on a 2,302-nt fragment of gene segment 1 (PB2), a 2,285-nt fragment of gene segment 2 (PB1), a 2,151-nt fragment of gene segment 3 (PA), a 1,498-nt fragment of gene segment 5 (NP), a 979-nt fragment of gene segment 7 (MA), and a 885-nt fragment of gene segment 8 (NS). Sequences obtained from influenza virus A/Chicken/Netherlands/1/03 were aligned with those of reference strains available from GenBank, representing the known genetic lineages of influenza A virus. Scale bars represent ≈10% of nucleotide changes between close relatives.
Table 1. Comparison of nucleotide and amino acid sequences of influenza viruses A/Netherlands/33/03 and A/Netherlands/219/03 with A/Chicken/Netherlands/1/03.
| No. of substitutions in A/NL/33/03*
|
NO. of substitutions in A/NL/219/03*
|
|||
|---|---|---|---|---|
| Virus gene segment | nt | aa | nt | aa |
| 1 (PB2) | 2 | 0 | 8 | 5 (S79I, V297I, R355K, Q563R, and E627K) |
| 2 (PB1) | 0 | 0 | 2 | 0 |
| 3 (PA) | 0 | 0 | 5 | 1 (F666L) |
| 4 (HA) | 0 | 0 | 4 | 3 (I13S, A143T, and K416R) |
| 5 (NP) | 0 | 0 | 1 | 0 |
| 6 (NA) | 0 | 0 | 5 | 4 (N308S, A346V, T442A, and P458S) |
| 7 (MA) | 0 | 0 | 0 | 0 |
| 8 (NS) | 1 | 1 (K126R)† | 1 | 1 (V137I)† |
nt, nucleotide; aa, amino acid; NP, nucleoprotein; MA, matrix.
Sequences were compared with those of A/Chicken/Netherlands/1/03, and amino acid substitutions are indicated with residue 1 being the start codon in the open reading frame.
Amino acid substitutions are in the NS1 open reading frame of the NS gene segment.
A 343-nt fragment of the HA gene, spanning the basic cleavage site, was obtained from 20 additional cases of human conjunctivitis, and it revealed a single-nucleotide substitution in two virus isolates of which one resulted in an amino acid substitution (D264N in A/Chicken/Netherlands/1/03). In four of these patients, virus was detected both in samples collected from the eyes and in samples collected from nose/throat, revealing identical sequences. We also sequenced parts of the viral genome [616-nt fragment of PB2; 641-nt fragment of PB1; 592-nt fragment of acidic polymerase (PA); 1,675-nt fragment of HA; 388-nt fragment of NA; 972-nt fragment of matrix; and 587-nt fragment of nonstructural gene (NS)] of virus isolates obtained from two patients, the wife and daughter of a poultry worker, who were putative secondary cases of H7N7 infection. With the exception of one silent mutation in the HA gene, all sequences were identical, indicating that the virus did not accumulate significant mutations on human transmission. The genome of A/Netherlands/219/03, isolated from the fatal case, revealed 26 nucleotide substitutions of which 14 resulted in amino acid substitutions. Five amino acid substitutions were found in the PB2 ORF, four were found in NA, three were found in HA, one was found in PA, and one was found in NS1 (Figs. 1 and 2 and Table 1).
Discussion
Here, we describe the characterization of the AIV that caused an outbreak of HPAI that started in February 2003 in The Netherlands and spread subsequently to poultry in Germany and Belgium. This outbreak probably started with the introduction of a low pathogenic virus from wild ducks to poultry in which it evolved to its pathogenic phenotype. This assumption is supported by the fact that no H7 and N7 sequences were more closely related to A/Chicken/Netherlands/1/03 than those from virus isolates obtained from Dutch mallards (25, 26).
In contrast to this H7N7 outbreak, no cases of bird-to-human transmission were reported during the H7N1 and H7N3 episodes in Italy. It may be of interest to identify the determinants of host range for these H7 viruses. Although the HA genes of these recent H7N7, H7N1, and H7N3 are quite similar, they may still play a significant role. Alternatively, the NA or internal genes of these AIV may determine their capacity to replicate and cause disease in humans. H7N7 viruses have caused disease on several occasions in mammals. They have been endemic in horses for a long time (27), and they have caused outbreaks in seals and conjunctivitis in humans when transmitted from seals or birds (14, 15). Therefore, H7N7 viruses may be unusual in their zoonotic potential.
Despite their potential to cross the species barrier, the Dutch H7N7 viruses did not appear to replicate well in the respiratory tract of humans because H3N2-infected individuals had higher virus loads in the respiratory tract than H7N7-infected individuals. These data suggest that although the avian H7N7 virus may replicate well in cells in or close to the human eye, it generally does not replicate to high titers in the respiratory tract.
Coinfections with human and avian viruses could have resulted in the generation of reassortant strains, with enhanced replication properties in humans. We did not detect dual infections nor nonavian gene segments in the H7N7 AIV analyzed, suggesting that reassortment had not occurred. The human AIV isolates, with the exception of A/Netherlands/219/03, also revealed remarkably few mutations even when transmitted from human to human.
The pathologic changes in the lungs of the deceased veterinarian are consistent with a viral pneumonia together with the sequelae of high-pressure ventilation, and they are similar to the pathologic changes in the lungs found in H5N1-infected humans (9, 28) and macaques (23, 29). Acute respiratory distress syndrome and multiple organ dysfunction associated with severe bronchointerstitial pneumonia were detected frequently in fatal cases of H5N1 infection and also in the deceased veterinarian. The failure to detect influenza virus antigen in affected lung tissue, despite the positive results of RT-PCR and virus isolation, may be due to the protracted course of disease. Pathologic examination revealed no preexisting disease to explain this fatal course of H7N7 infection.
One important question that remains is why the veterinarian presented with such severe symptoms and died. The veterinarian was not immunocompromised and did not suffer from any significant underlying disease. He was a smoker, had mild recurrent labial herpes, and was treated for glaucoma, a recurrent mild allergic blepharitis, and conjunctivitis. He did not receive an influenza vaccination. A possible explanation for the severe symptoms could be the accumulation of mutations that may result in more pathogenic properties in humans, for instance, because of enhanced replication in human lungs. One mutation in PB2 (E627K) is of particular interest because this mutation was shown to affect the virulence of H5N1 AIV in mice (30). Mutations in HA and NA may be important also, because these genes are determinants of the host range of AIV. The addition of a potential N-linked glycosylation site at position 141 in HA may be of importance because changes near the receptor-binding site or at the tip of HA have been implicated in determining the pathogenic properties of H7N7 AIV (31, 32).
As a consequence of our findings, the World Health Organization Global Influenza Surveillance Network has initiated the development of test kits and candidate vaccines. In accordance with the World Health Organization pandemic preparedness plan, surveillance and diagnosis should be enhanced in humans and in susceptible animals in countries where cases of H7N7 infection are detected.
Acknowledgments
We thank R. van Beek, C. Baas, J. Guldemeester, and T. Bestebroer for technical assistance and Jan de Jong for critically reading the manuscript. This work was supported in part by grants from the Dutch Ministry of Agriculture and the European Union. R.A.M.F. is a fellow of the Royal Dutch Academy of Arts and Sciences.
Abbreviations: Ct, threshold cycle; HA, hemagglutinin; NA, neuraminidase; AIV, avian influenza A viruses; HPAI, highly pathogenic avian influenza; PBn, basic polymerase n; PA, acidic polymerase; NS, nonstructural gene.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AY338455–AY338462, AY340074 –AY340091, and AY342410–AY342427).
References
- 1.Webster, R. G., Bean, W. J., Gorman, O. T., Chambers, T. M. & Kawaoka, Y. (1992) Microbiol. Rev. 56, 152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy, B. R. & Webster, R. G. (1996) in Virology, eds. Fields, B. N., Knipe, D. M. & Howley, P. M. (Lippincott, Philadelphia), Vol. 1, pp. 1397–1445. [Google Scholar]
- 3.Rohm, C., Zhou, N., Suss, J., Mackenzie, J. & Webster, R. G. (1996) Virology 217, 508–516. [DOI] [PubMed] [Google Scholar]
- 4.Senne, D. A., Panigrahy, B., Kawaoka, Y., Pearson, J. E., Suss, J., Lipkind, M., Kida, H. & Webster, R. G. (1996) Avian. Dis. 40, 425–437. [PubMed] [Google Scholar]
- 5.Gammelin, M., Mandler, J. & Scholtissek, C. (1989) Virology 170, 71–80. [DOI] [PubMed] [Google Scholar]
- 6.Yasuda, J., Shortridge, K. F., Shimizu, Y. & Kida, H. (1991) J. Gen. Virol. 72, 2007–2010. [DOI] [PubMed] [Google Scholar]
- 7.Beare, A. S. & Webster, R. G. (1991) Arch. Virol. 119, 37–42. [DOI] [PubMed] [Google Scholar]
- 8.de Jong, J. C., Claas, E. C., Osterhaus, A. D., Webster, R. G. & Lim, W. L. (1997) Nature 389, 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claas, E. C., de Jong, J. C., van Beek, R., Rimmelzwaan, G. F. & Osterhaus, A. D. (1998) Vaccine 16, 977–978. [DOI] [PubMed] [Google Scholar]
- 10.Shortridge, K. F., Zhou, N. N., Guan, Y., Gao, P., Ito, T., Kawaoka, Y., Kodihalli, S., Krauss, S., Markwell, D., Murti, K. G., et al. (1998) Virology 252, 331–342. [DOI] [PubMed] [Google Scholar]
- 11.Subbarao, K., Klimov, A., Katz, J., Regnery, H., Lim, W., Hall, H., Perdue, M., Swayne, D., Bender, C., Huang, J., et al. (1998) Science 279, 393–396. [DOI] [PubMed] [Google Scholar]
- 12.Suarez, D. L., Perdue, M. L., Cox, N., Rowe, T., Bender, C., Huang, J. & Swayne, D. E. (1998) J. Virol. 72, 6678–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(2003) MMWR Morb. Mortal. Wkly. Rep. 52, 516–521. [PubMed] [Google Scholar]
- 14.Lang, G., Gagnon, A. & Geraci, J. R. (1981) Arch. Virol. 68, 189–195. [DOI] [PubMed] [Google Scholar]
- 15.Banks, J., Speidel, E. & Alexander, D. J. (1998) Arch. Virol. 143, 781–787. [DOI] [PubMed] [Google Scholar]
- 16.Peiris, M., Yuen, K. Y., Leung, C. W., Chan, K. H., Ip, P. L., Lai, R. W., Orr, W. K. & Shortridge, K. F. (1999) Lancet 354, 916–917. [DOI] [PubMed] [Google Scholar]
- 17.Fouchier, R. A., Bestebroer, T. M., Herfst, S., Van Der Kemp, L., Rimmelzwaan, G. F. & Osterhaus, A. D. (2000) J. Clin. Microbiol. 38, 4096–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rimmelzwaan, G. F., Baars, M., Claas, E. C. & Osterhaus, A. D. (1998) J. Virol. Methods 74, 57–66. [DOI] [PubMed] [Google Scholar]
- 19.Schweiger, B., Zadow, I., Heckler, R., Timm, H. & Pauli, G. (2000) J. Clin. Microbiol. 38, 1552–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann, E., Stech, J., Guan, Y., Webster, R. G. & Perez, D. R. (2001) Arch. Virol. 146, 2275–2289. [DOI] [PubMed] [Google Scholar]
- 21.Felsenstein, J. (1989) Cladistics 5, 164–166. [Google Scholar]
- 22.Chan, K. H., Maldeis, N., Pope, W., Yup, A., Ozinskas, A., Gill, J., Seto, W. H., Shortridge, K. F. & Peiris, J. S. (2002) J. Clin. Microbiol. 40, 1675–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rimmelzwaan, G. F., Kuiken, T., van Amerongen, G., Bestebroer, T. M., Fouchier, R. A. & Osterhaus, A. D. (2001) J. Virol. 75, 6687–6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banks, J., Speidel, E. C., McCauley, J. W. & Alexander, D. J. (2000) Arch. Virol. 145, 1047–1058. [DOI] [PubMed] [Google Scholar]
- 25.Banks, J., Speidel, E. S., Moore, E., Plowright, L., Piccirillo, A., Capua, I., Cordioli, P., Fioretti, A. & Alexander, D. J. (2001) Arch. Virol. 146, 963–973. [DOI] [PubMed] [Google Scholar]
- 26.Capua, I. & Marangon, S. (2000) Avian Pathol. 29, 289–294. [DOI] [PubMed] [Google Scholar]
- 27.Webster, R. G. (1993) Equine Vet. J. 25, 537–538. [DOI] [PubMed] [Google Scholar]
- 28.Yuen, K. Y., Chan, P. K., Peiris, M., Tsang, D. N., Que, T. L., Shortridge, K. F., Cheung, P. T., To, W. K., Ho, E. T., Sung, R. & Cheng, A. F. (1998) Lancet 351, 467–471. [DOI] [PubMed] [Google Scholar]
- 29.Kuiken, T., Rimmelzwaan, G. F., Van Amerongen, G. & Osterhaus, A. D. (2003) Vet. Pathol. 40, 304–310. [DOI] [PubMed] [Google Scholar]
- 30.Hatta, M., Gao, P., Halfmann, P. & Kawaoka, Y. (2001) Science 293, 1840–1842. [DOI] [PubMed] [Google Scholar]
- 31.Naeve, C. W. & Webster, R. G. (1983) Virology 129, 298–308. [DOI] [PubMed] [Google Scholar]
- 32.Perdue, M. L., Latimer, J. W. & Crawford, J. M. (1995) Virology 213, 276–281. [DOI] [PubMed] [Google Scholar]