Abstract
In developing countries, one-third of reactive arthritis (ReA) cases are associated with Salmonella enterocolitis; nevertheless, there is no animal model for studying this pathology. Here we induced a self-limiting Salmonella enterica serovar Enteritidis enterocolitis in mice to analyze the onset of ReA. BALB/c mice received orally 20 μg of streptomycin 24 h before intragastric inoculation of a low dose (3 × 103 to 4 × 103 CFU) of S. Enteritidis. In response to Salmonella infection, a 30-fold increase in the expression of interleukin-17 (IL-17), measured by quantitative PCR, was observed in mesenteric lymph nodes 5 days postinfection. At this time synovitis was already evident, and concomitantly, a significant increase in joint tumor necrosis factor alpha (TNF-α) was detected by enzyme-linked immunosorbent assay (ELISA). The early development of joint lesions was accompanied by an increased expression of IL-17 in inguinal and popliteal lymph nodes. Infection with 107 CFU of an isogenic ΔinvG mutant bearing a defective type III secretion system of Salmonella encoded in the pathogenicity island 1 apparatus (TTSS-1) induced enterocolitis histologically similar to that triggered by the wild-type strain. Interestingly, despite the higher infective dose used, the mutant did not trigger intestinal IL-17. Moreover, no synovitis was observed in mice suffering ΔinvG enterocolitis. Neutralization of IL-17 in mice infected with S. Enteritidis prevented both synovitis and the increment of TNF-α in the joints, suggesting that IL-17 participates in the generation of Salmonella-induced ReA through the induction of TNF-α in the joints.
INTRODUCTION
Reactive arthritis (ReA), an inflammatory arthritic condition that is commonly associated with Salmonella infections, represents a significant health burden, yet it is poorly understood. It can be defined as the development of sterile inflammatory arthritis as a sequel to remote infection, often in the gastrointestinal tract (24). This observation is particularly interesting because there is no anatomic link between these two organs. In some patients, symptoms resolve within months, but in others, they may persist for years. The strong genetic association of spondyloarthritis with human leukocyte antigen (HLA) B27 has been extensively documented (3, 44, 46, 54), although the mechanism by which HLA-B27 mediates inflammation remains unclear (47).
One of the theories trying to explain the cooccurrence of gut and joint inflammation points to trafficking of intestinal lymphocytes or mononuclear cells, particularly macrophages. Lymphocyte migration and homing to specific target tissues are mediated by a variety of adhesion molecules, such as integrins and selectins, and by chemokine receptors. The homing of intestinal lymphocytes is determined by a specific set of adhesion molecules, the β7 integrin subfamily, of which two members are critically involved in lymphocyte homing to the intestine: α4β7 and αeβ7 (6). In early spondyloarthritis patients, activated T cells carrying the α4β7 and αeβ7integrins were enriched in inflamed synovial tissue (10), suggesting an intestinal origin of these T cells.
Th17 cells are critical for pathogenesis in several models of arthritis (22, 31, 41). Because activated Th17 cells produce interleukin-17 (IL-17), this inflammatory cytokine is a likely contributor to the pathogenesis of arthritis. IL-17 can induce cell adhesion molecules and proinflammatory cytokines, like tumor necrosis factor alpha (TNF-α) (49, 58, 64), and therefore could play a critical role in the development of joint inflammation. In fact, IL-17 is considered a key mediator of TNF-α-induced bone loss by closely interacting with IL-1 in blocking bone-protective T-cell responses (67). In rheumatoid arthritis patients, IL-17 is present in the synovium and synovial fluid (7, 34, 65), and it plays a crucial role in the activation of T cells at the sensitization phase in the development of autoimmune arthritis (41). In ReA, IL-17 contributes to the development of joint inflammation (52); in fact, elevated levels of IL-17 have been detected in synovial tissue of these patients (51).
IL-17-producing Th17 cells are present almost exclusively in the small intestinal lamina propria and other mucosal tissues of mice kept under specific-pathogen-free conditions (25). This suggests the involvement of IL-17 in mucosal immune surveillance against the invasion of enteric pathogens such as Salmonella (38). In this regard, it has been recently demonstrated that Salmonella infection triggers early IL-17 production, which is crucial for host defense and is mediated by CD4+ T helper cells (14). Therefore, it is likely that IL-17-mediated joint inflammation during Salmonella ReA is triggered by the initial IL-17 response of the intestine to infection. In support of this hypothesis, a linkage between intestinal IL-17-producing T helper cells and joint inflammation has been recently shown in K/BxN mice (60).
Despite the coexistence of gut and joint inflammation in ReA, very few models exist in which gut and joint inflammation appear simultaneously. One such model is an HLA-B27 transgenic rat, which develops a spondyloarthritis-like phenotype with colitis and arthritic features (26, 27). These rats, however, remain free from disease when kept in germfree conditions, reflecting the interplay between predisposing genes and bacteria (19). Furthermore, T cells are required for the development of colitis as well as arthritis in these rats, as demonstrated in studies with athymic HLA-B27 transgenic rats (2).
Salmonella enterica serovar Enteritidis is the most common bacterial infectious cause of food-borne disease in the United States (37) and—followed by Salmonella enterica serovar Typhimurium—the most frequently isolated from humans worldwide (21). To our knowledge, there is no animal model for studying Salmonella enterocolitis-induced ReA. Here we applied a previously described infection protocol to induce synovitis (43) in streptomycin-pretreated mice to achieve a self-limiting S. Enteritidis enterocolitis model in which gut and joint inflammation coexist.
MATERIALS AND METHODS
Mice.
Female BALB/c mice were obtained from our vivarium, maintained under standard conditions, and provided with food and water ad libitum. At the end of the experiments, mice were killed with carbon dioxide. The Animal Ethics Committee of the University of Buenos Aires approved all experimental protocols used in this work.
Bacterial strains and growth conditions.
The wild-type (wt) strain of Salmonella enterica serovar Enteritidis 5694 and isogenic mutant AC518 (ΔinvG) were used to infect mice. S. Enteritidis 5694 was kindly given by Anne Morris Hooke, Miami University; it was originally from F. Collins's collection, Trudeau Institute, Saranac Lake, NY. Mutant AC518 was constructed in our laboratory as described before (43). InvG protein is a core component of the type III secretion system (TTSS) of Salmonella encoded in the pathogenicity island 1 (SPI-1) apparatus (TTSS-1). Bacteria were cultured in Trypticase soy broth at 37°C at 200 cycles per minute up to an optical density (OD) of 0.6. Bacteria were pelleted by centrifugation and suspended to the appropriate density in saline solution. In all cases the number of bacteria was determined by plating appropriate dilutions on Trypticase soy agar plates.
Salmonella infection and generation of enterocolitis.
Mice were pretreated with 20 mg of streptomycin (Sigma-Aldrich) given by the oral route (20), and 24 h later they received 3 × 103 to 4 × 103 CFU of the wt or 107 CFU of the ΔinvG mutant strain of S. Enteritidis by the same route. For intragastric infection, 0.2 ml of the bacterial suspension was introduced into the stomach with a 21-gauge blunt needle on a 1.0-ml plastic syringe.
Bacterial colonization and persistence.
At the indicated times postinfection, mice were sacrificed and bacterial loads were analyzed. All Peyer's patches located along the large intestine (six to eight), one-third of the spleen, one knee, and both popliteal lymph nodes were removed aseptically from each animal. Samples were homogenized in 1 ml of sterile saline solution, except for spleens, which were processed in 0.33 ml; thus, in these experiments, the number of CFU/ml equals the number of CFU/organ. Homogenates were diluted properly in saline and plated on Salmonella-Shigella (SS) agar. Samples were also cultured for 18 h in selenite broth for enrichment. Salmonella-like colonies appearing on SS plates were grown on triple sugar iron agar slants and tested for somatic antigen O9.
Persistence and colonization of the bacteria were also studied by PCR. Tissues were homogenized in 1% peptone-buffered water (PBW). After 24 h of enrichment at 37°C, DNA extraction was performed using the phenol-chloroform technique (8). A standard PCR of 45 cycles was carried out using selective primers to amplify invA and sopA genes with 285-bp and 113-bp products, respectively. The primers used were as follows: for invA, forward 5′-CTGAAATTATCGCCACGTTCGGGCAA-3′ and reverse 5′-CATCGCACCGTCAAAGGAACC-3′ (8); for sopA, forward 5′-TCCACCGTGAAGTTGATTG-3′ and reverse 3′-GCACTGAGGATGTGCTGGTA-5′ (15). The cycling program was 95°C for 10 s, 55°C for 10 s, and 72°C for 15 s and one cycle of 40°C for 30 s. Aliquots (20 μl) of the reaction mixtures were electrophoresed through a 2.0% agarose gel, and fragments were revealed by staining with ethidium bromide.
Anti-cytokine-blocking experiments.
IL-17 was neutralized using 2.5 mg/kg of body weight of rat anti-mouse IL-17 monoclonal antibody (MAb) (MAB421; R&D Systems, Minneapolis, MN) intraperitoneally. As a control, the same dose of isotype-control Ab was injected. Briefly, 20 mice were treated 24 h before infection and at days 1 and 3 postinfection with an anti-IL-17 MAb or the isotype control Ab. On day 5, animals were sacrificed. Left knees obtained were used for histological analysis, and right knees were utilized for TNF-α detection.
Histological analysis of intestine and joints.
Intestinal samples were fixed in formalin and processed by standard procedures for paraffin embedding. Knee joints were dissected, fixed in formalin for 2 days, decalcified in EDTA for 30 to 40 days, and then embedded in paraffin. Standard sections of 5 μm were prepared and stained with hematoxylin-eosin (HE) using routine histology techniques. An experienced pathologist blinded to the experimental protocol evaluated findings of intestine and joint abnormalities. Synovial alterations were scored as follows: 0, no changes; 1, slight thickening of synovial cell layer (up to 3 layers of synoviocytes) accompanied by congestion and edema of the external membrane; 2, moderate thickening of synovial cell layer (3 to 5 layers of synoviocytes) accompanied by congestion and edema of the external membrane; 3, severe thickening of synovial lining (more than 5 layers) accompanied by congestion and edema of the external membrane.
qPCR.
Total RNA was extracted from the midportion of the cecum (approximately 0.5 cm), knee joints, and popliteal and mesenteric lymph nodes using TRIzol reagent (Life Technologies, Inc., Carlsbad, CA) at different time points according to the experiment. Total RNA (1 μg per sample) was reverse transcribed with oligo(dT) as a primer using Expand reverse transcriptase (Promega Corporation, Madison, WI) according to the manufacturer's protocol. Quantitative real-time reverse transcriptase PCR (qPCR) was performed using a Sybr green PCR kit (Applied Biosystems Inc., Foster City, CA) in an Applied Biosystems 7500 sequence detector. Primer sequences were as follows: for TNF-α, forward 5′-ATGAGCACAGAAAGCATGATC-3′ and reverse 5′-TACAGGCTTGTCACTCGAATT-3′ (23); for IL-17, forward 5′-GCTCCAGAAGGCCCTCAGA-3′ and reverse 5′-AGCTTTCCCTCCGCATTGA-3′ (17). All samples were analyzed in the same run for 18S expression for normalization: forward 5′-AACACGGGAAACCTCACCC-3′ and reverse 5′-CCACCAACTAAGAACGGCCA-3′. PCR parameters were 50°C for 2 min, 94°C for 2 min, and 40 cycles of 94°C for 30 s and 60°C for 1 min. Quantification of gene expression was calculated using the comparative threshold cycle (CT) method, normalized to the 18S control and efficiency of the reverse transcriptase reaction (relative quantity, 2−ΔΔCT). The replicates were then averaged, and the fold induction was determined, with the value in the control group as 1 (23).
Cytokine analysis.
Knee samples were obtained 5 days after oral inoculation with 3 × 103 to 4 × 103 CFU of Salmonella enterica. Tissue homogenates from five animals were pooled and subjected to centrifugation (12,000 rpm, 1 min) to pellet all cell debris prior to concentration using an Amicon Ultra-4 centrifugal filter unit (Merck Millipore). Supernatants were stored at −20°C until further use. Samples were analyzed for TNF-α and IL-17 using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Cytokine levels were expressed as picograms per ml.
Statistical analysis.
Unless otherwise stated, all results are the average ± standard deviation (SD) of results from at least three separate experiments. P values were determined using the Mann-Whitney test for nonparametric values. A P value of <0.05 was considered to be statistically significant.
RESULTS
A Salmonella enterocolitis model useful for studying reactive arthritis.
In developing countries, one-third of reactive arthritis cases are associated with Salmonella enterocolitis (53). Here we describe an experimental model useful for studying the pathogenesis of Salmonella reactive arthritis and its treatment or prevention. Mice received 20 μg of streptomycin 24 h before intragastric infection. The experimental group was inoculated with a low dose (3 × 103 to 4 × 103 CFU) of the wild-type (wt) strain of S. Enteritidis. A second group of mice received 107 CFU of the isogenic ΔinvG mutant unable to secrete SPI-1 effectors. We have shown previously in the typhoid model of infection that a functional SSTT-1 is necessary to trigger joint inflammation (43). A third group of untreated mice were included as a control.
Regardless of the infecting bacterial strain, 48 h after inoculation, all animals showed signs of disease, including diarrhea, rough hair coat, and lethargy. Histology studies revealed that wt S. Enteritidis induces diffuse enterocolitis, characterized by an epithelium diminished in height, mononuclear infiltration of the mucosa and submucosa, and loss of normal villus architecture (Fig. 1B). As seen in Fig. 1C, the enteritis generated by 107 CFU of the ΔinvG mutant is indistinguishable from that induced by 103 CFU of the wt strain. Twenty percent of the animals infected with the wt strain died by day 7 postinoculation (Table 1); the remaining 80% overcame the infection and survived for at least 70 days (data not shown). No deaths occurred among mice infected with the ΔinvG mutant (Table 1).
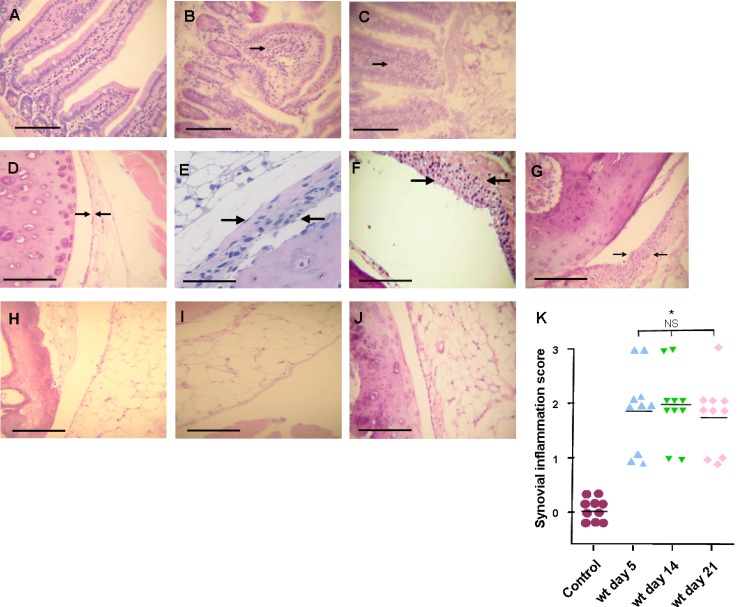
Fig 1.
Histological changes in large intestine and joints after S. Enteritidis enterocolitis. Histology studies after intragastric inoculation with 3 × 103 to 4 × 103 CFU of the wild type (wt) or 107 CFU of the invG mutant of S. Enteritidis (ΔinvG). (A, B, and C) Large intestine. (A) Control animals: normal display of intestinal mucosa; (B) Enterocolitis in wt infected mice, note the loss of normal villi display and the infiltration of mononuclear cells (arrow); (C) enterocolitis in mice inoculated with the ΔinvG strain, changes are similar to those observed in the wt group. HE stain. Bar: 100 μm. (D to J) Joints. (D) Control animals: normal synovial capsule (arrows). (E, F, and G) Mice infected with the wt strain: moderate hyperplasia, with 3 to 5 layers of synoviocytes (arrows) at days 5, 14, and 21, respectively. (H, I, and J) Mice infected with the ΔinvG strain: no differences were found with respect to control mice (D) at any time point studied (days 5, 14, and 21, respectively). HE stain. Bar, 1 mm (A, C, D, E, F and G) or 100 μm (B). (K) Synovial inflammation scores. *, significant differences (P < 0.01) between wt-infected mice and control group mice were found at all time points assessed. NS, no significant differences among wt-infected mice. Data were collected from three independent experiments.
Table 1.
Colonization and persistence of S. Enteritidis after enterocolitis onset
| Dose (CFU) and infecting strain | Days p.i. | CFU/ml (range) |
DNA in knees & lymph nodesa | Survivalc | |
|---|---|---|---|---|---|
| Peyer's patches | Spleen | ||||
| 3 × 103 to 4 × 103, wild type | 2 | 58 (16–85) | 57 (20–97) | Negative | 5/5 |
| 4 | 77 (13–105) | 94 (32–104) | Negative | 5/5 | |
| 14 | Negative | 72 (23–97) | Negative | 4/5 | |
| 21 | Negative | Positiveb | Negative | 4/5 | |
| 107, ΔinvG mutant | 2 | 112 (47–124) | 135 (73–252) | Negative | 5/5 |
| 4 | 97 (38–112) | 126 (57–139) | Negative | 5/5 | |
| 14 | Negative | Negative | Negative | 5/5 | |
| 21 | Negative | Negative | Negative | 5/5 | |
Negative, no bacterial DNA was detected by PCR assay.
Detected by PCR assay only.
Number of survivors/total number of mice. On day 7 two animals died, one from the group to be sacrificed at day 14 and one from the group to be sacrificed at day 21. Survival rate was 80% for mice infected with the wild-type strain and 100% for animals receiving the invG mutant.
Colonization and persistence of Salmonella strains after enterocolitis onset were analyzed at days 2, 4, 14, and 21 postinfection. The presence of bacteria was assessed in Peyer's patches, spleen, knee joints, and popliteal lymph nodes (Table 1). Bacterial loads recovered from Peyer's patches of animals infected with the ΔinvG mutant were higher than those found in mice infected with the wt strain, although these differences were not statistically significant. Cultures were positive for at least 4 days. By day 14, the wt and the mutant strains were cleared from Peyer's patches. The spleen was also colonized by both strains; again, at days 2 and 4 bacterial loads of the ΔinvG mutant were higher than those of the wt strain but not significantly different. At day 14 only the wt strain could be quantified in spleen, and by day 21 the wt strain was detected in spleen homogenates only by PCR. Neither live Salmonella bacteria nor their DNA were detected in the joints or draining lymph nodes at any time tested for any of the bacterial strains studied.
Clinical studies showed that the median delay between intestinal infection and the onset of reactive arthritis symptoms is about 15 days (39). With this in mind, we investigated whether joint inflammation was evident early upon Salmonella infection. To this purpose, animals were sacrificed at days 5, 14, and 21 after infection to evaluate joint histological changes. We found that alterations of the synovial membrane were already present at day 5 postinfection with the wt strain. The synovial membrane presented moderate hyperplasia with mononuclear infiltration (Fig. 1E); in addition, the external membrane showed congestion and edema (not shown). As shown in Fig. 1F and G, the observed lesions were similar at all time points assessed and persisted for at least 21 days. The average score of these lesions was 2 according to our scale (Fig. 1K). On the other hand, mice infected with the ΔinvG mutant did not present synovial changes at any of the time points assessed (Fig. 1H to J).
Different intestinal TNF-α and IL-17 expression between animals infected with the wild type or the ΔinvG mutant of S. Enteritidis.
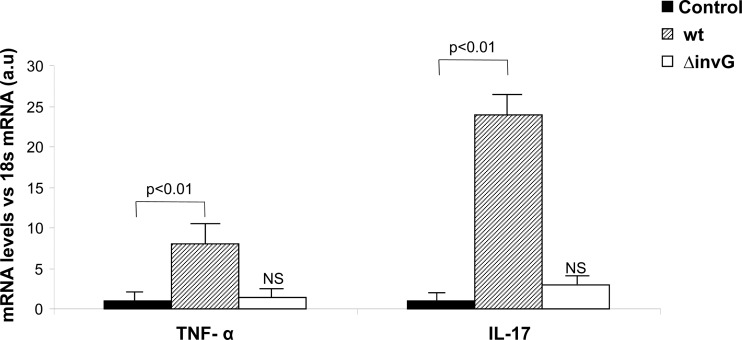
Gut inflammation plays a crucial role in the development of reactive arthritis following intestinal infection (28). TNF-α and IL-17 are among the most prominent cytokines induced soon after Salmonella infection (17, 35), and they are also linked to the generation of arthritis (39). With this in mind, we analyzed the expression of TNF-α and IL-17 in the large intestine by qPCR 48 h after Salmonella inoculation. The results are depicted in Fig. 2. Soon after infection with 3 × 103 to 4 × 103 CFU of wt Salmonella, mice showed an 8-fold increase in the intestinal expression of TNF-α with respect to the control group (P < 0.01). IL-17 expression was also significantly elevated in mice infected with the wt strain. A 24-fold increase in the expression of this inflammatory cytokine was detected in mice suffering enterocolitis compared to that in untreated animals (P < 0.01). In contrast, in mice infected with 107 CFU of the ΔinvG mutant, neither the expression of TNF-α nor that of IL-17 was augmented (Fig. 2). Altogether, our results suggest a relation between intestinal inflammatory cytokines induced by Salmonella with a functional SSTT-1 and the appearance of synovitis.
Fig 2.
Cytokine expression in the large intestine after S. Enteritidis enterocolitis. TNF-α and IL-17 expression was evaluated by qPCR 48 h after intragastric inoculation with 3 × 103 to 4 × 103 CFU of the wild-type (wt) or 107 CFU of the invG mutant of S. Enteritidis (ΔinvG). Results are expressed as mean ± SD (n = 7). a.u, arbitrary units; NS, no significant differences compared to the control group. Representative data from three independent experiments.
Blocking IL-17 prevents synovitis induced by Salmonella enterocolitis.
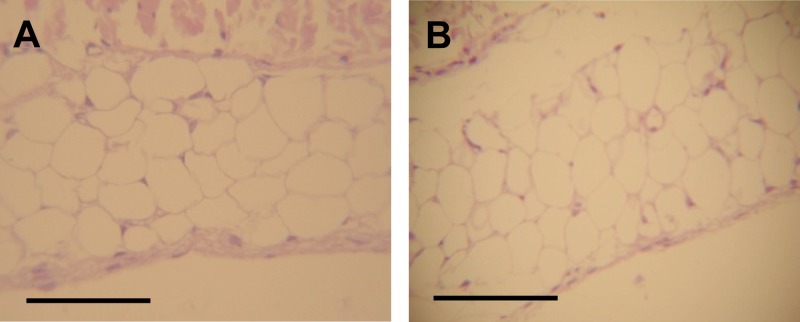
In order to investigate the involvement of IL-17 in the Salmonella-induced synovitis, blocking experiments were conducted in mice infected with the wt strain of S. Enteritidis by neutralizing IL-17 in vivo. Remarkably, blockade of IL-17 successfully suppressed synovitis in knee joints analyzed on day 5 postinfection (Fig. 3). These findings support the essential role of IL-17 in Salmonella enterocolitis-induced ReA.
Fig 3.
Neutralization of IL-17 suppresses Salmonella-induced synovitis. Histology of knee joints in mice treated with anti-IL-17 MAb at day 5 after intragastric inoculation with 3 × 103 to 4 × 103 CFU of wild-type S. Enteritidis. (A) Control animals: normal display of the synovial layer. (B) Mice received anti-IL-17 MAb intraperitoneally 24 h before and at day 1 and 3 after infection. Histology was similar to that in the uninfected animals. HE stain. Bar, 1 mm.
TNF-α and IL-17 in the joints during Salmonella enterocolitis-induced synovitis.
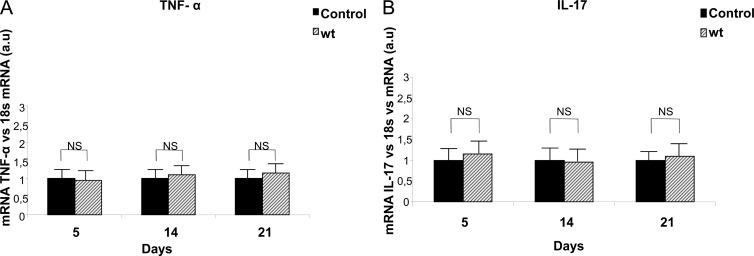
Next, we analyzed whether synovitis observed in mice infected with the wt strain of Salmonella was accompanied by the local expression of TNF-α and IL-17. To this purpose, qPCR was assessed in joint homogenates at days 5, 14, and 21 postinfection. No significant differences were found in the expression of articular TNF-α of mice suffering Salmonella enterocolitis compared to that of control animals for any time point evaluated (Fig. 4). Likewise, no significant differences in the relative amounts of IL-17 mRNA were found between infected and untreated mice (Fig. 4).
Fig 4.
Cytokine expression in joints after S. Enteritidis enterocolitis. TNF-α (A) and IL-17 (B) expression were evaluated by qPCR at different time points after infection of mice with 3 × 103 to 4 × 103 CFU of wild-type S. Enteritidis (wt). Results are expressed as mean ± SD (n = 5). a.u, arbitrary units. Representative data from three independent experiments. NS, no significant differences.
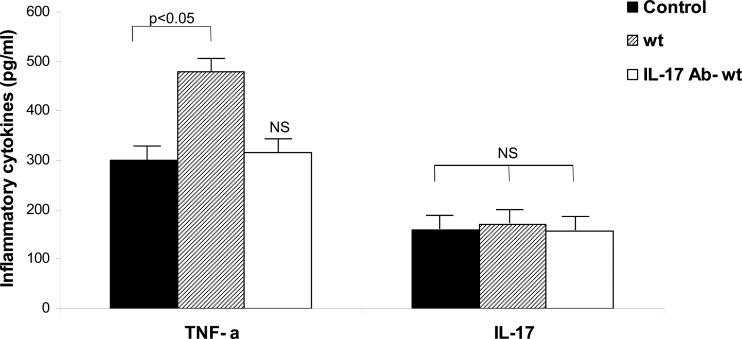
In view of these results, and keeping in mind that the number of cytokine-producing cells in the synovial tissue may be low (55), we decided to investigate the presence of TNF-α and IL-17 in 5× concentrated joint homogenates. The production of these cytokines was measured by ELISA (Fig. 5). Five days after the onset of Salmonella enterocolitis, the amount of local TNF-α was significantly higher (P < 0.05) than that in untreated animals. As shown in Fig. 5, the increase in TNF-α did not go along with an increase in local IL-17. Additional experiments showed that blocking of IL-17 prevents the increment in local TNF-α observed in infected mice (Fig. 5). These results suggest that local production of TNF-α (induced by IL-17) may be responsible for the early inflammation of the synovial membrane triggered by Salmonella enterocolitis.
Fig 5.
TNF-α and IL-17 in the inflamed joints early after S. Enteritidis enterocolitis. The presence of TNF-α and IL-17 was evaluated by ELISA 5 days after oral infection with 3 × 103 to 4 × 103 CFU of wild-type S. Enteritidis. wt, mice received only the pathogen. IL-17 Ab- wt, Mice received anti-IL-17 MAb intraperitoneally 24 h before and at day 1 and 3 after infection. Results are expressed as mean ± SD (n = 5). Data were collected from three to five independent experiments. NS, no significant differences with the control group.
Cytokine profile resemblance between joint and intestinal draining lymph nodes during enterocolitis induced by S. Enteritidis.
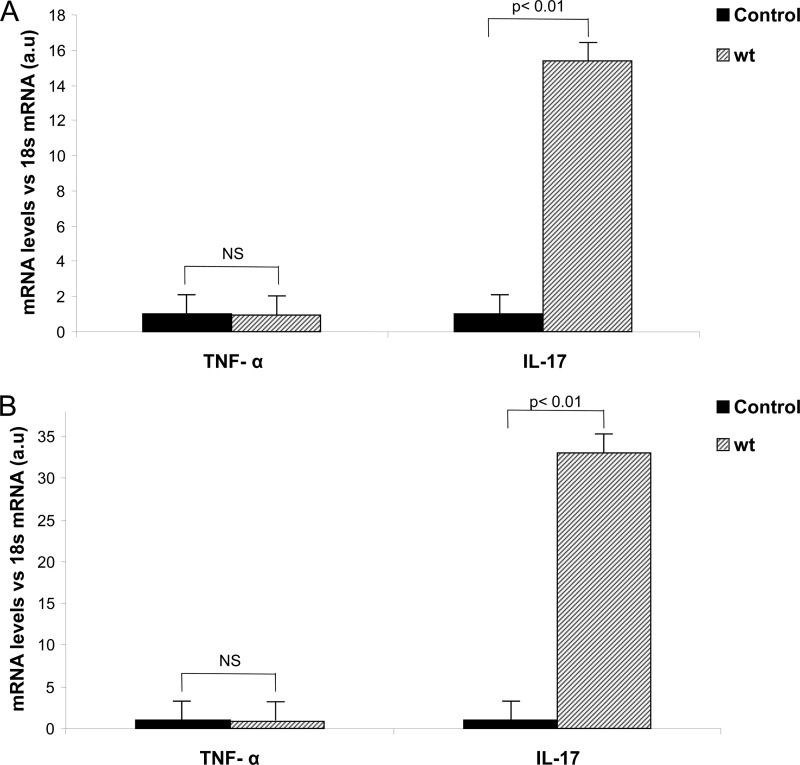
The involvement of lymph nodes in the pathogenesis of arthritis was proposed decades ago; moreover, it was shown that during acute inflammatory arthritis, Th17 cell differentiation is initiated in draining lymph nodes (9). Therefore, we decided to investigate whether the early onset of synovitis triggered by Salmonella enterocolitis is accompanied by an increase in the expression of cytokines in the joint-draining lymph nodes. To this end, qPCR was performed on RNA collected from popliteal and inguinal lymph nodes of mice infected with S. Enteritidis. We found that whereas the expression of TNF-α was not significantly augmented 5 days after infection, the expression of IL-17 showed a 15-fold increase (P < 0.01) compared to that of the control (Fig. 6A). Concomitantly, a similar cytokine profile was found in mesenteric lymph nodes. As shown in Fig. 6B, a significant 30-fold increase in the expression of IL-17 (P < 0.01) compared to that in controls was observed at day 5 postinfection. On the other hand, the expression of TNF-α in mesenteric lymph nodes of infected mice was not different from that found in the control group (Fig. 6B). These results show that S. Enteritidis enterocolitis triggers a similar IL-17 expression profile in both intestinal and joint-draining lymph nodes.
Fig 6.
Cytokine profile resemblance between joint and intestinal draining lymph nodes early after S. Enteritidis enterocolitis. The expression of IL-17 was evaluated in popliteal and inguinal lymph nodes (A) and mesenteric lymph nodes (B) by qPCR 5 days after oral infection with 3 × 103 to 4 × 103 CFU of wild-type S. Enteritidis (wt). Results are expressed as mean ± SD (n = 4). a.u, arbitrary units. Data were collected from three independent experiments. NS, no significant differences.
On the whole, our studies demonstrate that Salmonella enterocolitis triggers early synovitis, increased expression of IL-17 in intestinal and joint-draining lymph nodes, and an augmented amount of TNF-α in the inflamed joints.
DISCUSSION
Oral Salmonella infection of streptomycin-pretreated mice has been widely utilized to study the early stages of enterocolitis (20), yet the high dose of the pathogen used renders the model unsuitable for long-term experiments. Here we show that a reduction of the bacterial burden induces self-limiting enterocolitis in 80% of the animals pretreated with the antibiotic. An oral dose of circa 103 CFU of S. Enteritidis was sufficient to increase expression of IL-17 in intestinal and joint-draining lymph nodes, to augment TNF-α in the inflamed joints, and to trigger early synovitis.
A recent clinical study reported that the median delay between enterocolitis and the beginning of ReA symptoms is about 15 days (39). These data suggest that joint damage triggered by intestinal infection could be an early event. In fact, taking advantage of our animal model, we demonstrated here that Salmonella induces aseptic joint inflammation within 5 days after enterocolitis onset. Altogether, our results indicate that the animal model used in this work is useful for studying the pathogenesis of ReA induced by Salmonella enterocolitis.
In a previous work, we demonstrated that a functional TTSS-1 in the infecting Salmonella strain is essential for the development of synovitis; it was suggested that proinflammatory signaling mediated by Salmonella TTSS-1 in the gut is required for the induction of histological changes in the joints (43). In this work, the observation that the ΔinvG mutant is unable to induce IL-17 expression in the large intestine allowed us to speculate that this cytokine is crucial in the generation of Salmonella-induced synovitis. This hypothesis was further confirmed by the neutralization of IL-17 in vivo.
Clinical evidence indicates that Th17 cells may play a role in the induction and maintenance of gut inflammation in human diseases such as Crohn's disease and ulcerative colitis. Indeed, IL-17A and IL-17F are highly expressed in the gut of these patients. Analogously, high expression of IL-17 was found in the synovial fluids of spondyloarthritis-affected patients, and an increased number of circulating Th17 memory-like T cells has been recently reported in these patients (29, 59). In our model, while the neutralization of IL-17 prevented joint inflammation, neither IL-17 protein nor IL-17 expression could be detected in inflamed joints. These results are in line with some clinical and experimental findings. A detailed work by Steiner and coworkers (55) showed that although important in the generation of synovitis, the number of cytokine-expressing cells in the synovium of arthritis patients is low, about 0.1 to 0.3% of all T cells investigated. In concordance, T-cell cytokines have proven difficult to detect in synovial samples of arthritis patients (66). Moreover, it was reported that in experimental adjuvant arthritis, the expression of synovial IL-17 is transient and restricted to day 13 after the induction of the disease (4). Therefore, we cannot rule out the presence of IL-17 or the expression of IL-17 mRNA in the joints of mice suffering S. Enteritidis enterocolitis.
Conflicting data have been reported on the production of cytokines in ReA patients. Some studies revealed low levels of Th1 cytokines in peripheral blood and synovium (1, 62, 63); the impaired production of these cytokines has been suggested as being part of the pathogenesis of ReA (5) by causing the failure of bacterial elimination at the early stages of the disease. On the other hand, it was reported that in T-cell clones derived from synovial fluid of ReA patients, antigens from disease-related bacteria induce Th1 cytokine secretion (36, 48, 50) and the presence of Th17 cytokines in synovial fluid of ReA patients was also reported (51, 52). We found that in S. Enteritidis enterocolitis, early synovitis is accompanied by a moderate increase in the amount of TNF-α in the inflamed joints. This result could be in agreement with the slight increment in circulating TNF-α observed in ReA patients compared to that in healthy controls (56). Whether ReA patients experience slight or substantial increases in synovial TNF-α, compared to that in controls, is uncertain because of the ethical restriction to obtain synovial samples from healthy individuals. This fact certainly stresses the importance of developing animal models to study ReA. Live Salmonella can induce TNF-α production in synovial fibroblasts, as shown in vitro (40). It is unlikely, however, that this is the source of synovial TNF-α in our animal model because neither live Salmonella bacteria nor their DNA was found in the inflamed joints. Many attempts to isolate living pathogens from synovial fluid during ReA failed, although the presence of Salmonella-related antigens has been detected in the joints of these patients (18). Therefore, it is possible that recirculation of antigen-loaded macrophages may provide the antigenic stimulus necessary to sustain T cell activation and inflammation of the joints.
Increasing evidence points to IL-17 as a key mediator of TNF-α-induced joint damage in arthritis (32, 67). Our results support this proposition; in addition to preventing synovitis, the neutralization of IL-17 reduces the amount of TNF-α in the joints of S. Enteritidis-infected mice. A study on the mechanism of joint damage during collagen-induced arthritis suggests that IL-17, produced by T cells, acts on monocytes, resulting in TNF-α production; in turn, TNF-α can induce the differentiation of monocytes into mature osteoclasts (61). The induction of TNF-α by IL-17 could also be explained because synoviocytes and fibroblasts express receptors for this interleukin (30), which, once stimulated, induce the production of inflammatory molecules (13, 45), including TNF-α (33). Moreover, a reciprocal stimulation of synoviocytes and IL-17-producing cells has been proposed to explain the persistent inflammatory cytokine milieu (i.e., TNF-α, IL-6, IL-15) in the joints of rheumatoid arthritis patients (12). Furthermore, IL-17 and TNF-α have additive effects on the expression of synoviolin, a novel E3 ubiquitin ligase implicated in the pathogenesis of rheumatoid arthritis. It has been demonstrated that the induction of synoviolin by IL-17 prolongs the survival of fibroblast-like synoviocytes contributing to synovial hyperplasia (57).
Early works on adjuvant arthritis suggested the role of draining lymph nodes in the development of joint lesions (4, 42). More recently, investigations conducted in the B27-transgenic rat model for spondyloarthritis showed that Th17 cells contribute to both intestinal and joint inflammation; IL-17-producing cells were expanded in mesenteric as well as in popliteal lymph nodes (16). In agreement with this, we found early expression of IL-17 in popliteal and inguinal lymph nodes of mice undergoing S. Enteritidis enterocolitis. Our findings are also in line with data obtained from an IL-17-dependent model of acute inflammatory arthritis. It was found that following intra-articular inoculation of bovine serum albumin, Th17 cell differentiation is initiated in draining lymph nodes (9).
Our results, together with previously published data (43), provide evidence in favor of the gut-joint axis proposed to explain the pathogenesis of ReA (11, 28). Several lines of evidence indicate that ReA may originate from the relocation to the joints of the immune process primarily induced in the gut (11). The transfer of the intestinal inflammatory process into the joints implies that immune cells activated in the gut-draining lymph nodes can localize, at a certain point of the intestinal disease, either into the gut or into the joints (11). The concept that immune cells, activated in the inflamed gut and migrating into the joints, may be able to reproduce in this tissue a similar immune response is sustained by the observation that common immunological processes operate at both these sites. Moreover, a linkage between intestinal IL-17-producing T helper cells and joint inflammation has been recently shown in K/BxN mice (60). In agreement with these results, we found that—in S. Enteritidis enterocolitis—the inflammatory cytokine profile of mesenteric lymph nodes resembles that of popliteal and inguinal lymph nodes.
In summary, a low dose of S. Enteritidis inoculated to streptomycin-pretreated mice generates a self-limiting enterocolitis model useful for studying ReA. Soon after infection, animals develop synovitis with the presence of TNF-α in the joints and the expression of IL-17 in the large intestine and in mesenteric, inguinal, and popliteal lymph nodes. Neutralization of IL-17 abrogated synovitis as well as the increase of TNF-α in the joints. To our knowledge, this is the first report showing a rapid response to S. Enteritidis enterocolitis in an extraintestinal tissue. Altogether, the mouse model presented here closely resembles human Salmonella ReA.
ACKNOWLEDGMENTS
We thank María Isabel Bernal, Lida Suligoy, and Roberto Caccuri for their superb technical assistance.
This work was supported in part by grants from Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina (PIP-O992/08), and Secretaría de Ciencia y Técnica de la Universidad de Buenos Aires, Argentina (UBACyT M009 and 20020100100541).
Footnotes
Published ahead of print 9 April 2012
REFERENCES
- 1. Braun J, et al. 1999. Low secretion of tumor necrosis factor alpha, but no other Th1 or Th2 cytokines, by peripheral blood mononuclear cells correlates with chronicity in reactive arthritis. Arthritis Rheum. 42:2039–2044 [DOI] [PubMed] [Google Scholar]
- 2. Breban M, et al. 1996. T cells, but not thymic exposure to HLA-B27, are required for the inflammatory disease of HLA-B27 transgenic rats. J. Immunol. 156:794–803 [PubMed] [Google Scholar]
- 3. Brewerton DA, et al. 1973. Ankylosing spondylitis and HL-A 27. Lancet i:904–907 [DOI] [PubMed] [Google Scholar]
- 4. Bush KA, Walker JS, Lee CS, Kirkham BW. 2001. Cytokine expression and synovial pathology in the initiation and spontaneous resolution phases of adjuvant arthritis: interleukin-17 expression is upregulated in early disease. Clin. Exp. Immunol. 123:487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butrimiene I, et al. 2004. Different cytokine profiles in patients with chronic and acute reactive arthritis. Rheumatology 10:1300–1304 [DOI] [PubMed] [Google Scholar]
- 6. Cepek KL, et al. 1994. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature 372:190–193 [DOI] [PubMed] [Google Scholar]
- 7. Chabaud M, et al. 1999. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 42:963–970 [DOI] [PubMed] [Google Scholar]
- 8. dos Santos LR, et al. 2001. Polymerase chain reaction (PCR) for the detection of Salmonella in artificially inoculated chicken meat. Rev. Inst. Med. Trop. Sao Paulo 5:247–250 [DOI] [PubMed] [Google Scholar]
- 9. Egan PJ, van Nieuwenhuijze A, Campbell IK, Wicks IP. 2008. Promotion of the local differentiation of murine Th17 cells by synovial macrophages during acute inflammatory arthritis. Arthritis Rheum. 58:3720–3729 [DOI] [PubMed] [Google Scholar]
- 10. Elewaut D, et al. 1998. Enrichment of T cells carrying beta7 integrins in inflamed synovial tissue from patients with early spondyloarthropathy, compared to rheumatoid arthritis. J. Rheumatol. 25:1932–1937 [PubMed] [Google Scholar]
- 11. Fantini MC, Pallone F, Monteleone G. 2009. Common immunologic mechanisms in inflammatory bowel disease and spondylarthropathies. World J. Gastroenterol. 15:2472–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferraccioli G, Zizzo G. 2011. The potential role of Th17 in mediating the transition from acute to chronic autoimmune inflammation: rheumatoid arthritis as a model. Discov. Med. 60:413–424 [PubMed] [Google Scholar]
- 13. Fossiez F, et al. 1996. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 6:2593–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geddes K, et al. 2011. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat. Med. 17:837–844 [DOI] [PubMed] [Google Scholar]
- 15. Giacomodonato MN, et al. 2009. Impaired synthesis and secretion of SopA in Salmonella Typhimurium dam mutants. FEMS Microbiol. Lett. 1:71–77 [DOI] [PubMed] [Google Scholar]
- 16. Glatigny S, et al. 2012. Proinflammatory Th17 cells are expanded and induced by dendritic cells in spondylarthritis-prone HLA-B27-transgenic rats. Arthritis Rheum. 1:110–120 [DOI] [PubMed] [Google Scholar]
- 17. Godinez I, et al. 2009. Interleukin-23 orchestrates mucosal responses to Salmonella enterica serotype Typhimurium in the intestine. Infect. Immun. 77:387–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Granfors K, et al. 1990. Salmonella lipopolysaccharide in synovial cells from patients with reactive arthritis. Lancet 335:685–688 [DOI] [PubMed] [Google Scholar]
- 19. Hammer RE, Maika SD, Richardson JA, Tang JP, Taurog JD. 1990. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell 63:1099–1112 [DOI] [PubMed] [Google Scholar]
- 20. Hapfelmeier S, Hardt WD. 2005. A mouse model for S. Typhimurium-induced enterocolitis. Trends Microbiol. 13:497–503 [DOI] [PubMed] [Google Scholar]
- 21. Hendriksen RS, et al. 2011. Global monitoring of Salmonella serovar distribution from the World Health Organization Global Foodborne Infections Network Country Data Bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog. Dis. 8:887–900 [DOI] [PubMed] [Google Scholar]
- 22. Hickman-Brecks CL, Racz JL, Meyer DM, LaBranche TP, Allen PM. 2011. Th17 cells can provide B cell help in autoantibody induced arthritis. J. Autoimmun. 36:65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hovsepian E, Penas F, Goren NB. 2010. 15-Deoxy-Δprostaglandin GJ2 but not rosiglitazone regulates metalloproteinase 9, Nos-2, and ciclooxygenase 2 expression and functions by peroxisome proliferators-activated receptor γ-dependent and independent mechanisms in cardiac cells. Shock 34:60–67 [DOI] [PubMed] [Google Scholar]
- 24. Inman RD, Johnston ME, Hodge M, Falk J, Helewa A. 1988. Postdysenteric reactive arthritis. A clinical and immunogenetic study following an outbreak of salmonellosis. Arthritis Rheum. 31:1377e–1383e [DOI] [PubMed] [Google Scholar]
- 25. Ivanov II, et al. 2006. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126:1121–1133 [DOI] [PubMed] [Google Scholar]
- 26. Jacques P, Mielants H, Coppieters K, De Vos M, Elewaut D. 2007. The intimate relationship between gut and joint in spondyloarthropathies. Curr. Opin. Rheumatol. 19:353–357 [DOI] [PubMed] [Google Scholar]
- 27. Jacques P, Elewaut D. 2008. Joint expedition: linking gut inflammation to arthritis. Mucosal Immunol. 1:364–371 [DOI] [PubMed] [Google Scholar]
- 28. Jacques P, Elewaut D, Mielants H. 2010. Interactions between gut inflammation and arthritis/spondylitis. Curr. Opin. Rheumatol. 4:368–374 [DOI] [PubMed] [Google Scholar]
- 29. Jandus C, et al. 2008. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 58:2307–2317 [DOI] [PubMed] [Google Scholar]
- 30. Kehlen A, Thiele K, Riemann D, Langner J. 2002. Expression, modulation and signalling of IL-17 receptor in fibroblast-like synoviocytes of patients with rheumatoid arthritis. Clin. Exp. Immunol. 3:539–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koenders MI, et al. 2005. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am. J. Pathol. 167:141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koenders MI, et al. 2011. Tumor necrosis factor-interleukin-17 interplay induces S100A8, interleukin-1β, and matrix metalloproteinases, and drives irreversible cartilage destruction in murine arthritis: rationale for combination treatment during arthritis. Arthritis Rheum. 8:2329–2339 [DOI] [PubMed] [Google Scholar]
- 33. Kontny E, et al. 2012. Comparison of rheumatoid articular adipose and synovial tissue reactivity to proinflammatory stimuli: contribution to adipocytokine network. Ann. Rheum. Dis. 2:262–267 [DOI] [PubMed] [Google Scholar]
- 34. Kotake S, et al. 1999. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 103:1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kum WW, Lo BC, Yu HB, Finlay BB. 2011. Protective role of Akt2 in Salmonella enterica serovar Typhimurium-induced gastroenterocolitis. Infect. Immun. 7:2554–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lahesmaa R, et al. 1992. Yersinia enterocolitica activates a T helper type 1-like T cell subset in reactive arthritis. J. Immunol. 148:3079–3085 [PubMed] [Google Scholar]
- 37. Linam WM, Gerber MA. 2007. Changing epidemiology and prevention of Salmonella infections. Pediatr. Infect. Dis. J. 8:747–748 [DOI] [PubMed] [Google Scholar]
- 38. Mayuzumi H, Inagaki-Ohara K, Uyttenhove C, Okamoto Y, Matsuzaki G. 2010. Interleukin-17A is required to suppress invasion of Salmonella enterica serovar Typhimurium to enteric mucosa. Immunology 131:377–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meyer A, et al. 2011. Safety and efficacy of anti-tumor necrosis factor α therapy in ten patients with recent-onset refractory reactive arthritis. Arthritis Rheum. 5:1274–1280 [DOI] [PubMed] [Google Scholar]
- 40. Meyer-Bahlburg A, et al. 2004. Yersinia enterocolitica leads to transient induction of TNF-alpha and activates NF-kappaB in synovial fibroblasts. Clin. Exp. Rheumatol. 22:278–284 [PubMed] [Google Scholar]
- 41. Nakae S, et al. 2003. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. U. S. A. 100:5986–5990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Neubould BB. 1964. Role of lymph nodes in adjuvant-induced arthritis in rats. Ann. Rheum. Dis. 23:392–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Noto Llana M, et al. 2009. Sublethal infection with Salmonella Enteritidis by the natural route induces intestinal and joint inflammation in mice. Microbes Infect. 11:74–82 [DOI] [PubMed] [Google Scholar]
- 44. Palm O, Moum B, Ongre A, Gran JT. 2002. Prevalence of ankylosing spondylitis and other spondyloarthropathies among patients with inflammatory bowel disease: a population study (the IBSEN study). J. Rheumatol. 29:511–515 [PubMed] [Google Scholar]
- 45. Park H, et al. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 11:1133–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Purrmann J, et al. 1988. HLA antigens in ankylosing spondylitis associated with Crohn's disease. Increased frequency of the HLA phenotype B27,B44. J. Rheumatol. 15:1658–1661 [PubMed] [Google Scholar]
- 47. Rohekar S, et al. 2008. Symptomatic acute reactive arthritis after an outbreak of Salmonella. J. Rheumatol. 35:1599–1602 [PubMed] [Google Scholar]
- 48. Schlaak J, et al. 1992. Predominance of Th1-type T cells in synovial fluid of patients with Yersinia-induced reactive arthritis. Eur. J. Immunol. 22:2771–2776 [DOI] [PubMed] [Google Scholar]
- 49. Shin EW, et al. 1999. A case of Salmonella-triggered reactive arthritis in a child, initially presented as juvenile rheumatoid arthritis. Pediatr. Allergy Respir. Dis. 9:320–326 [Google Scholar]
- 50. Simon AK, et al. 1993. Analysis of cytokine profiles in synovial T cell clones from chlamydial reactive arthritis patients: predominance of the Th1 subset. Clin. Exp. Immunol. 94:122–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Singh AK, Misra R, Aggarwal A. 2011. Th-17 associated cytokines in patients with reactive arthritis/undifferentiated spondyloarthropathy. Clin. Rheumatol. 6:771–776 [DOI] [PubMed] [Google Scholar]
- 52. Singh R, Aggarwal A, Misra R. 2007. Th1/Th17 cytokine profiles in patients with reactive arthritis/ undifferentiated spondyloarthropathy. J. Rheumatol. 34:2285–2290 [PubMed] [Google Scholar]
- 53. Sinha R, Aggarwal A, Prasad K, Misra R. 2003. Sporadic enteric reactive arthritis and undifferentiated spondyloarthropathy: evidence for involvement of Salmonella typhimurium. J. Rheumatol. 30:105–113 [PubMed] [Google Scholar]
- 54. Steer S, et al. 2003. Low back pain, sacroiliitis, and the relationship with HLA-B27 in Crohn's disease. J. Rheumatol. 30:518–522 [PubMed] [Google Scholar]
- 55. Steiner G, et al. 1999. Cytokine production by synovial T cells in rheumatoid arthritis. Rheumatology 3:202–213 [DOI] [PubMed] [Google Scholar]
- 56. Straub RH, Paimela L, Peltomaa R, Schölmerich J, Leirisalo-Repo M. 2002. Inadequately low serum levels of steroid hormones in relation to interleukin-6 and tumor necrosis factor in untreated patients with early rheumatoid arthritis and reactive arthritis. Arthritis Rheum. 3:654–662 [DOI] [PubMed] [Google Scholar]
- 57. Toh ML, et al. 2010. Role of interleukin 17 in arthritis chronicity through survival of synoviocytes via regulation of synoviolin expression. PLoS One 10:e13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Trentham DE, Townes AS, Kang AH. 1977. Autoimmunity to type II collagen an experimental model of arthritis. J. Exp. Med. 146:857–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wendling D, Cedoz JP, Racadot E, Dumoulin G. 2007. Serum IL-17, BMP-7, and bone turnover markers in patients with ankylosing spondylitis. Joint Bone Spine 74:304–305 [DOI] [PubMed] [Google Scholar]
- 60. Wu HJ, et al. 2010. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 6:815–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yago T, et al. 2007. IL-23 induces human osteoclastogenesis via IL-17 in vitro, and anti-IL-23 antibody attenuates collagen-induced arthritis in rats. Arthritis Res. Ther. 9:R96 http://arthritis-research.com/content/9/5/R96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yin Z, et al. 1997. Crucial role of interleukin-10/interleukin-12 balance in the regulation of the type 2 T helper cytokine response in reactive arthritis. Arthritis Rheum. 40:1788–1797 [DOI] [PubMed] [Google Scholar]
- 63. Yin Z, et al. 1999. The elevated ratio of interferon gamma-/interleukin-4-positive T cells found in synovial fluid and synovial membrane of rheumatoid arthritis patients can be changed by interleukin-4 but not by interleukin-10 or transforming growth factor beta. J. Rheumatol. 38:1058–1067 [DOI] [PubMed] [Google Scholar]
- 64. Zanelli E, Gonzalez-Gay MA, David CS. 1995. Could HLA-DRB1 be the protective locus in rheumatoid arthritis? Immunol. Today 16:274–278 [DOI] [PubMed] [Google Scholar]
- 65. Ziolkowska M, et al. 2000. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J. Immunol. 164:2832–2838 [DOI] [PubMed] [Google Scholar]
- 66. Zizzo G, et al. 2011. Synovial fluid-derived T helper 17 cells correlate with inflammatory activity in arthritis, irrespectively of diagnosis. Clin. Immunol. 138:107–116 [DOI] [PubMed] [Google Scholar]
- 67. Zwerina K, et al. 2012. Anti IL-17A therapy inhibits bone loss in TNF-α mediated murine arthritis by modulation of the T-cell balance. Eur. J. Immunol. 42:413–423 [DOI] [PubMed] [Google Scholar]