Abstract
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous Gr1+ CD11b+ population of immature cells containing granulocytic and monocytic progenitors, which expand under nearly all inflammatory conditions and are potent repressors of T-cell responses. Studies of MDSCs during inflammatory responses, including sepsis, suggest they can protect or injure. Here, we investigated MDSCs during early and late sepsis. To do this, we used our published murine model of cecal ligation and puncture (CLP)-induced polymicrobial sepsis, which transitions from an early proinflammatory phase to a late anti-inflammatory and immunosuppressive phase. We confirmed that Gr1+ CD11b+ MDSCs gradually increase after CLP, reaching ∼88% of the bone marrow myeloid series in late sepsis. Adoptive transfer of early (day 3) MDSCs from septic mice into naive mice after CLP increased proinflammatory cytokine production, decreased peritoneal bacterial growth, and increased early mortality. Conversely, transfer of late (day 12) MDSCs from septic mice had the opposite effects. Early and late MDSCs studied ex vivo also differed in their inflammatory phenotypes. Early MDSCs expressed nitric oxide and proinflammatory cytokines, whereas late MDSCs expressed arginase activity and anti-inflammatory interleukin 10 (IL-10) and transforming growth factor β (TGF-β). Late MDSCs had more immature CD31+ myeloid progenitors and, when treated ex vivo with granulocyte-macrophage colony-stimulating factor (GM-CSF), generated fewer macrophages and dendritic cells than early MDSCs. We conclude that as the sepsis inflammatory process progresses, the heterogeneous MDSCs shift to a more immature state and from being proinflammatory to anti-inflammatory.
INTRODUCTION
Sepsis involves a shift from an early systemic proinflammatory response to a late anti-inflammatory and immunosuppressive state as the process progresses (15, 28, 38). This shift in the sepsis inflammatory profile profoundly alters both innate and adaptive immunity. Important alterations after early sepsis include a reduced capacity of septic monocytes/macrophages and neutrophils to respond to bacterial toxins and to clear microbial infections (3, 4, 11, 16), increased apoptosis of immune effector cells (22, 28, 40), increased T cell suppression, Th2 polarization, and defective myelopoiesis (10, 14). These perturbations in the inflammatory and immune phenotypes may impair the ability to clear primary and secondary (opportunistic) infections and to resolve inflammation.
Recent studies have identified a heterogeneous population of immature myeloid cells, termed myeloid-derived suppressor cells (MDSCs), which have immunosuppressive properties and express the myeloid differentiation markers Gr1 and CD11b (5, 9, 13, 29). Although MDSCs were first recognized for their role in attenuating immune surveillance and antitumor immune response in human and tumor-bearing animals (13, 29), their expansion occurs under most acute and chronic inflammatory conditions (29). The heterogeneous MDSC population consists of precursors of granulocytes, macrophages, and dendritic cells, with a potent ability to suppress T cell responses through production of large amounts of nitric oxide (NO) and reactive oxygen species (ROS) (13, 32). MDSCs may express increased levels of inducible nitric oxide synthase (iNOS), arginase, and ROS, all of which are important for innate immunity effector cell functions (13, 29). They also can produce a range of pro- and anti-inflammatory cytokines (10). Thus, Gr+ CD11b+ MDSCs are a confusing mixture of myeloid phenotypes. Moreover, MDSCs morphologically and functionally differ in various tissues under different inflammatory conditions (9). Even within the same inflammatory process, phenotypic differences in MDSCs occur over time (10). Taken together, data support that the phenotypes within MDSCs change as the inflammatory process progresses (10); this might account for their diverse immunological activities under different inflammatory conditions.
Because early sepsis is proinflammatory and late sepsis is anti-inflammatory with impaired immunoreactivity of innate and adaptive immune effector cells, we hypothesized that the temporal shifts in the inflammatory process during sepsis may accompany shifts in MDSCs. To test this, we used a murine model of polymicrobial sepsis that develops into early and late phases and therefore can be followed over a long period of time (7). We provide confirming evidence that the population of Gr1+ CD11b+ MDSCs expands dramatically in the bone marrow in late sepsis (9). Using adoptive transfer into naive mice, we also show that early and late septic MDSCs differentially modify sepsis responses in vivo and that early and late MDSCs can be further distinguished ex vivo. We conclude that important phenotypic shifts evolve in the myeloid population during sepsis, which may differentially impact clinical outcomes.
MATERIALS AND METHODS
Mice.
Male BALB/c mice, 8 weeks old (Harlan Sprague Dawley, Indianapolis, IN), were used for all experiments. The mice were housed in a pathogen-free temperature-controlled room and were acclimated to the new environment for at least 3 days before surgery. All experiments were conducted in accordance with National Institutes of Health Guidelines and were approved by the East State Tennessee University Animal Care and Use Committee.
CLP.
Polymicrobial sepsis, which emulates general peritonitis occurring in humans (2, 30, 33), was induced by cecal ligation and puncture (CLP) as described previously (7). This model creates a prolonged infection with ∼90% mortality over 4 weeks. Briefly, mice were anesthetized via inhalation with 2.5% isoflurane (Abbott Laboratories, Abbott Park, IL). A midline abdominal incision was made, and the cecum was exteriorized, ligated distal to the ileocecal valve, and then punctured twice. A small amount of feces was extruded into the abdominal cavity. The abdominal wall and skin were sutured in layers with 3-0 silk. Sham-operated mice were treated identically except that the cecum was neither ligated nor punctured. Mice received (intraperitoneally [i.p.]) 1 ml lactated Ringer's solution plus 5% dextrose for fluid resuscitation. To create the late sepsis phenotype, mice were subcutaneously administered antibiotic (imipenem; 25 mg/kg body weight) or saline and 1 ml lactated Ringer's solution plus 5% dextrose or an equivalent volume of 0.9% saline. To establish intra-abdominal infection and approximate the clinical situation of early human sepsis where there is a delay between the onset of sepsis and the delivery of therapy (24), injections of imipenem were given at 8 h after CLP and repeated once for a total of two doses over a 24-h period. Based on our experience, these levels of injury and manipulation create prolonged infections with high mortality (∼60 to 70%) during the late/chronic phase (7).
The presence of early sepsis was confirmed by transient systemic bacteremia and elevated cytokine levels in the first 5 days after CLP. Late/chronic sepsis (after day 5) was confirmed by enhanced peritoneal bacterial overgrowth and reduced circulating proinflammatory cytokines.
For MDSC transfer, survival was reported for 21 days after CLP. Moribund mice, those suffering deep hypothermia (<34°C), lethargy, and weight loss (more than 30%), were sacrificed and analyzed. Mice that were found dead before sacrifice were not included in the analysis.
Isolation and adoptive transfer of MDSCs.
Bone marrow Gr1+ CD11b+ MDSCs were harvested from day 3 and day 12 septic mice (a separate group of mice that had undergone CLP-induced sepsis) and purified using magnetically assisted cell sorting according to the manufacturer's instructions (Miltenyi Biotech, Auburn, CA). Note that blood and peritoneal lavage specimens were also collected from these mice to confirm the presence of early (i.e., day 3) and late (i.e., day 12) sepsis as described above. Briefly, the bone marrow cells were flushed out of the femurs with RPMI 1640 medium (without fetal bovine serum [FBS]) under aseptic conditions (7). A single cell suspension was made by repeated pipetting and filtering through a 70-μm nylon strainer followed by erythrocyte lysis. To purify total Gr1+ CD11b+ MDSCs, bone marrow cells were incubated with biotinylated mouse anti-Gr1 (clone RB6-8C5) (eBioscience, San Diego, CA) for 15 min at 4°C. After washing, cells were incubated with antibiotin magnetic beads (Miltenyi) for 20 min at 4°C and subsequently applied to a mass spectrometry (MS) column for positive selection of Gr1+ CD11b+cells.
To enrich for CD31+ MDSCs (for differentiation experiments), purified Gr1+ cells were incubated with anti-CD31 antibody (eBioscience) and then selected with magnetic beads (Milteny), after the beads used for Gr1+ selection were washed way with a special buffer. Cell purity was determined by flow cytometry and was higher than 95%. Cells were then washed and resuspended in sterile saline.
For the adoptive transfer, mice were injected intravenously (i.v.) (via tail vein) with 4 × 106 total Gr1+ MDSCs or the equivalent (100 μl) of saline immediately after CLP. Mice were followed for 3 weeks after cell transfer.
Blood.
Blood was collected via cardiac puncture from mice that were sacrificed at days 2 to 4 (representing early sepsis) and days 14 to 16 (representing late sepsis) after MDSC transfer. Mice were subjected to deep anesthesia, blood was collected, and sera were separated and used for cytokine measurements. Also, small volumes (20 μl) of blood were collected in heparinized tubes and used for bacterial culture as described below.
Blood and peritoneal bacterial culture.
Immediately after mice were sacrificed, the peritoneal cavity was lavaged with 5 ml phosphate-buffered saline (PBS). The lavage fluid was cleared by centrifugation, diluted 6- to 8-fold, and plated on 5% sheep blood agar plates with Trypticase soy agar base (BD Biosciences, Sparks, MD). The plates were incubated for 24 h at 37°C under aerobic conditions. The plates were read by a microbiologist, and the CFU were determined and multiplied by the dilution factor.
Ex vivo differentiation of MDSCs.
Total or CD31+-enriched Gr1+ CD11b+ cells were cultured with complete RPMI 1640 medium in the presence of 10 ng/ml GM-CSF (PeproTech Inc., Rocky Hill, NJ) and 10 ng/ml recombinant IL-4 (rIL-4) (eBioscience). The cell phenotypes were analyzed by flow cytometry.
Flow cytometry.
Total and differentiated MDSCs were analyzed by flow cytometry. Bone marrow cells or differentiated Gr1+ CD11b+ MDSCs were labeled by incubation for 30 min on ice in staining buffer (PBS plus 2% FBS) with appropriate fluorochrome-conjugated antibodies. After washing, the samples were analyzed by using a FACSCaliber flow cytometer (BD Biosciences, Sparks, MD). About 15,000 cells were analyzed using the CellQuest Pro software program (BD Biosciences). The following antibodies were used: anti-Gr1 conjugated to fluorescein isothiocyanate (FITC), anti-CD11b conjugated to phycoerythrin (PE), anti-F4/80 conjugated to allophycocyanin (APC), CD11c conjugated to PE, anti-major histocompatibility complex class II (MHC-II) conjugated to FITC, and anti-CD31 conjugated to PE57 (eBioscience, San Diego, CA). An appropriate isotype-matched control was used for each antibody.
Cytokine analysis.
Sera were isolated from blood samples as described above. Levels of tumor necrosis factor alpha (TNF-α), IL-6, IL-10, and transforming growth factor β (TGF-β) were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits (eBioscience) according to the manufacturer's instructions. Each sample was run in duplicate.
Nitrite assay.
Bone marrow MDSCs were harvested from early (day 3) and late (day 12) septic mice (a separate group of mice that had undergone CLP-induced sepsis). Gr1+ MDSCs were cultured in RPMI 1640 medium that was supplemented with 10% FBS, 2 mM l-glutamine, and 1× penicillin-streptomycin (HyClone, Logan, UT). The cultures were incubated at 37°C in a 5% CO2-humidified atmosphere. Cells were stimulated with 1 μg/ml lipolysaccharide (LPS) (Escherichia coli; 0111:B4; Sigma, St. Louis, MO) for 24 h. NO production was measured in the culture supernatants using Griess reagent according to the manufacturer's protocol (Molecular Probes, Eugene, OR). Briefly, Griess reagent was made immediately before use by mixing equal volumes of N-(1-naphthyl)ethylenediamine and sulfanilic acid. Samples and sodium nitrite standards (150 μl) were mixed with 20 μl Griess reagent and 130 μl distilled water (dH2O) and then incubated for 30 min at room temperature in a 96-well microplate. Absorbance was read at 548 nm by a spectrophotometer. The sample nitrite concentration was calculated from the NaNO2 standard curve.
Arginase assay.
After the culture supernatants were collected for the nitrite assay, Gr1+ CD11b+ MDSCs were harvested and arginase activity was measured in the cell lysate using an arginase assay kit (Abnova, Walnut, CA). Briefly, ∼1 × 106 cells were lysed in 100 μl of 10 mM Tris-HCl (pH 7.4) containing 1× protease inhibitor cocktail and 0.4% Triton X-100. Lysates were cleared by centrifugation for 10 min at 15,000 rpm. Samples (40 μl) were mixed with 10 μl of 5× substrate buffer (containing L-arginine) in a 96-well plate and incubated at 37°C for 2 h. The reaction was stopped by adding 200 μl urea reagent to all wells, including those containing the urea standard. The plate was incubated at room temperature for 20 min, and urea production was determined by measuring the absorbance at 520 nm. One unit of arginase converts 1 μmol of l-arginine to ornithine and urea per minute at pH 9.5 and 37°C.
Statistical analysis.
The Kaplan-Meier survival curve was plotted using the GraphPad Prism software program, version 5.0 (GraphPad Software), and survival significance was determined by using a log-rank test. Other data were analyzed using the software program Microsoft Excel 3.0, and statistical significance was determined by the unpaired Student t test for two-group comparison or one-way analysis of variance (ANOVA) for multiple comparisons. All values are expressed as means ± standard deviations (SD). Statistical significance was set at a P value of <0.05.
RESULTS
Late sepsis induces dramatic expansion in bone marrow Gr1+ CD11b+ MDSCs.
To study the kinetics and immunological properties of MDSCs in sepsis, we induced a sepsis response that develops in early and late phases. The early/acute phase of sepsis in mice is defined as the first 5 days after CLP (17, 31). Our previous studies showed that the mortality rate in this model is ∼20 to 30% during the early phase (days 1 to 5) of sepsis, and mice surviving the early phase develop late/prolonged bacterial infections and an immunosuppressive inflammatory phenotype, typical of late/chronic sepsis (7).
Using this sensitive model of early and late sepsis, we first measured the numbers of Gr1+ CD11b+ immature myeloid cells in the bone marrow. Because the sepsis response involves a shift from a Th1 to a Th2 immune phenotype (15), which characterizes late sepsis (15, 28), we used the Th2-skewed BALB/c mice to limit the impact, if any, of such a shift on the expansion or immunoreactivity of MDSCs.
Mice MDSCs coexpress the myeloid lineage differentiation antigens Gr1 and CD11b (13) and accumulate in the bone marrow and secondary lymphoid tissue during infection (10, 13). We sorted Gr1+ CD11b+ cells from the bone marrow at various times after CLP to reflect early and late sepsis. The presence of early sepsis was confirmed by detecting elevated circulating levels of the proinflammatory cytokines TNF-α and IL-6 and the presence of bacteria in blood and peritoneal fluid. Late sepsis was confirmed by lower levels of TNF-α and IL-6 and increased peritoneal bacterial growth (data not shown).
Flow cytometry analysis of bone marrow cells at intervals after CLP revealed that Gr1+ CD11b+ cells were detected in naive (day 0) mice, but their numbers expanded dramatically after induction of sepsis, increasing from ∼40% at day 3 to ∼88% by day 12 (Fig. 1A and B). Mice sampled between day 16 and day 28 after CLP also had similar (83 to 90%) proportions (data not shown). Interestingly, cytospin preparations have revealed that Gr1+ CD11b+ are heterogeneous phenotypes with ring-shaped nuclei, characteristic of immature myeloid cells (i.e., MDSCs) (9). We also observed that the numbers of cells with ring-shaped nuclei were increased from 28% at day 3 to 55% at day 6 and reached 68% of the GR1+ CD11b+ cells by day 12 after CLP (Fig. 1C). These confirmatory results demonstrate that late sepsis in our model is associated with dramatic accumulation of Gr1+ CD11b+ MDSCs, with immature phenotypes.
Fig 1.
Accumulation of Gr1+ CD11b+ cells in bone marrow of septic mice at intervals after CLP. Bone marrow cells were harvested and stained with anti-Gr1 and anti-CD11b antibodies (Abs). (A) Example of flow cytometry of bone marrow cells harvested at intervals and gated on Gr1+ CD11b+ staining. Numbers denote doubly positive cells within the gated cell population and represent their percentages in bone marrow. (B) Quantitative analysis of Gr1+ CD11b+ cell percentages. Values represent the means ±SD for 5 to 7 mice per time point. *, P < 0.05. (C) Photograph of cytospin preparation of purified Gr1+ CD11b+ cells stained with Step I Wright-Giemsa stain. Note the increase in the numbers of ring-shaped nuclei, characteristic of immature myeloid cells (MDSCs), for late (days 6 and 12) septic mice. Approximately 400 Gr1+ CD11b+ cells in each field were counted (n = 4 mice), and the percentage of cells with ring-shaped nuclei was calculated (see Results).
Adoptive transfer of Gr1+ CD11b+ MDSCs from early and late septic mice differentially affects survival in early sepsis.
The suppressive functions of Gr1+ CD11b+ MDSCs may owe their immunomodulatory properties to temporal or environmental influences (9, 13, 29). Because Gr1+ CD11b+ cells from early and late septic mice differed in their morphology, we hypothesized that they might have different inflammatory phenotypes, and we therefore, examined their effects on the immune response to sepsis. To do this, we adoptively transferred Gr1+ CD11b+ cells sorted from the bone marrow of day 3 and day 12 septic mice, representing early and late sepsis, respectively. We chose bone marrow because MDSCs in spleen might be modified by the T cell microenvironment (9). About 4 × 106 sorted Gr1+ CD11b+ cells were injected i.v. into naive mice immediately after CLP surgery. As shown in Fig. 2, Gr1+ CD11b+ cells derived from day 3 septic mice significantly reduced survival during early sepsis (between days 2 and 6). By day 5 after CLP, 40% (n = 12) of mice survived versus 60% (n = 12) of CLP controls (P = 0.002). In contrast, Gr1+ CD11b+ cells derived from day 12 septic mice significantly improved early sepsis (between days 3 and 6) survival (86% [n = 30] survived versus 60% [n = 12] of CLP controls by day 5; P = 0.001). Interestingly, 7 days after cell transfer, no significant impact on survival was observed whether mice received Gr1+ CD11b+ cells from day 3 or day 12 septic mice.
Fig 2.

Adaptive transfer of Gr1+CD11b+ cells recovered from early septic mice exaggerate, whereas those from late septic mice attenuate, the early systemic inflammatory response of sepsis. Sepsis was induced by CLP. Bone marrow cells were harvested at day3 (D3) and day 12 (D12) after CLP from a separate group of mice. Gr1+ CD11b+ cells were purified by magnetic cell sorting and injected (4 × 106 cells) i.v. into mice that had just undergone CLP. Control mice received 100 μl saline. Survival was recorded for 21 days. There were no significant differences in survival after day 7.
Adoptive transfer of Gr1+ CD11b+ MDSCs from early versus late septic mice differentially affects the in vivo inflammatory response.
The early/acute phase of sepsis is dominated by excessive inflammation mediated by systemic production of autotoxic proinflammatory cytokines (7, 15, 35). In addition, mortality during the early phase of CLP-induced sepsis is due mainly to this hyperinflammation (33, 34). Because we observed differences (Fig. 2) in early deaths (i.e., the first week after CLP) between mice that received Gr1+ CD11b+ cells from day 3 and day 12 septic mice, we measured the circulating levels of the proinflammatory TNF-α and IL-6 and anti-inflammatory IL-10 and TGF-β cytokines in the moribund mice sacrificed at days 2 to 4 after CLP (i.e., representing early sepsis). Levels of TNF-α and IL-6 were significantly increased and decreased, respectively, in CLP mice that received Gr1+ CD11b+ cells from day 3 and day 12 compared with CLP control (saline) mice (Fig. 3, left panels). Conversely, IL-10 and TGF-β levels were significantly higher for CLP mice that received Gr1+ CD11b+ cells from day 12 septic mice than for mice that received saline or cells from day 3 septic mice (Fig. 3, lower panels). In addition, levels of TNF-α and IL-6 were diminished for mice (from all groups) that were sacrificed at days 10 to 17 (i.e., representing late sepsis), whereas levels of IL-10 and TGF-β elevated further compared with those for all mice sacrificed at days 2 to 4 (Fig. 3, right panels). There were no significant differences among groups whether mice received saline or Gr1+ CD11b+ cells from day 3 or day 12 septic mice. Thus, adoptive transfer of Gr1+ CD11b+ MDSCs from early septic (day 3) mice increases the proinflammatory cytokines, whereas cells from late septic (day 12) mice increase the anti-inflammatory cytokines during the early sepsis response. The transferred cells had no additive effects once the endogenous Gr1+ CD11b+ cells had expanded (i.e., during late sepsis).
Fig 3.

Levels of circulating cytokines in CLP mice after adaptive transfer of Gr1+ CD11b+ MDSCs. Mice were subjected to CLP and received Gr1+ CD11b+ MDSCs or saline as described in the legend for Fig. 2. Sera were collected from moribund mice that were sacrificed at days 2 to 4 (representing early sepsis) and days 10 to 17 after CLP (representing late sepsis). Cytokine levels were measured by ELISA. During early sepsis, levels of TNF-α and IL-6 were elevated in mice that received MDSCs harvested from day 3 (D3) septic mice, whereas IL-10 and TGF-β levels were elevated in mice that received MDSCs harvested from day12 (D12) septic mice. During late sepsis, there were no significant differences among groups. Values represent the means ± SD for 6 mice for the group receiving saline, 8 mice for the group receiving MDSCs from D3, and 4 mice for the group receiving MDSCs from D12. Late sepsis cytokine measurement included 6 to 7 mice per group. Data from 4 experiments were pooled. *, P < 0.05.
Adoptive transfer of early and late septic Gr1+ CD11b+ MDSCs differentially affects local bacterial growth in early sepsis.
Defects in the innate immune response to CLP-induced sepsis may impair bacterial clearance and thus contribute to sepsis-related mortality (2, 15). We determined blood and peritoneal bacterial numbers for CLP mice that were sacrificed at days 2 to 4 and days 14 to 16 after early and late Gr1+ CD11b+ cell transfer. All mice that were sacrificed at days 2 to 4 had low numbers of blood bacteria, which were not significantly different whether mice received saline or Gr1+ CD11b+ cells (data not shown). However, these mice exhibited marked differences in the numbers of peritoneal bacteria. Mice that received Gr1+ CD11b+ cells from day 3 and day 12 septic mice had significantly lower (P = 0.0325) and higher (P = 0.0158) numbers of peritoneal bacteria, respectively, compared with those for control (saline) CLP mice (Fig. 4). In addition, peritoneal bacterial numbers were significantly higher (P = 0.0005) for mice that received Gr1+ CD11b+ cells from day 12 septic mice than for mice that received cells from day 3. In addition, all mice sacrificed at days 14 to 16 still had high numbers of peritoneal bacteria, but there were no significant differences between any animal group (data not shown). These results indicate that adoptive transfer of Gr1+ CD11b+ MDSCs from early (day 3) and late (day 12) septic mice differentially affect local bacterial growth but only during the early sepsis response.
Fig 4.

Adaptive transfer of Gr1+ CD11b+ MDSCs from day 3 septic mice reduces peritoneal bacteria in early sepsis. Peritoneal lavage fluid was collected from moribund mice that were sacrificed at days 2 to 5 after CLP and cultured on 5% sheep blood agar plates. The bacterial CFU were determined after 24 h. The levels of bacteria were lower in mice that received MDSCs harvested from day 3 (D3) septic mice. Values represent the means for 7 to 10 mice per group (pooled from 4 experiments).
Gr1+ CD11b+ MDSCs from early septic mice produce increased amounts of nitric oxide (NO), whereas those from late septic mice predominantly express higher arginase activity.
The data presented above suggested that Gr1+ CD11b+ cells can suppress or enhance the early hyperinflammatory phase of sepsis, depending on when they were harvested. The immune-suppressive phenotypic properties of Gr1+ CD11b+ MDSCs have been attributed to their upregulated expression of inflammatory factors, including NO, reactive oxygen species, and arginase activity (13, 29). We hypothesized that Gr1+ CD11b+ cells from early and late septic mice differ in overall phenotype and that this might account for the differences seen during the early sepsis response. To test this, we measured NO and arginase activities in ex vivo cultures from early versus late sepsis-derived Gr1+ CD11b+ cells. Cells were sorted from day 3 and day 12 septic mice and cultured with or without LPS. NO production was measured by determining the nitrite levels in the culture supernatants and arginase activity was assayed in the cell lysate. Gr1+ CD11b+ cells from day 3 septic mice produced significantly higher levels of NO than cells from day 12 (Fig. 5A). In contrast, Gr1+ CD11b+ cells from day 12 had significantly higher arginase activity than cells from day 3. In addition, all unstimulated cultures (medium alone) exhibited various background levels of both mediators (data not shown). Both cell types produced increased amounts of superoxide (H2O2) after stimulation with PMA, but levels were significantly higher with Gr1+ CD11b+ cells from day 3 (data not shown). In addition, Gr1+ CD11b+ cells from day 3 septic mice produced significantly higher levels of the proinflammatory cytokines TNF-α and IL-6 than cells from day 12, which produced significantly higher levels of the anti-inflammatory IL-10 and TGF-β (Fig. 5B). Thus, Gr1+ CD11b+ MDSCs from early and late sepsis have different inflammatory activities.
Fig 5.

Gr1+ CD11b+ MDSCs harvested from day 3 (D3) septic mice produce more NO and exhibit lower arginase activity than MDSCs harvested from day 12 (D12) septic mice. Gr1+ CD11b+ MDSCs were purified from the bone marrow of a separate group of mice at day 3 and day 12 after CLP. Cells (2 × 106) were cultured with 1 μg/ml LPS for 24 h. (A) Supernatants were collected and analyzed for NO levels (measured as nitrite). The cells were lysed and analyzed for arginase activity. (B) Levels of TNF-α, IL-6, IL-10, and TGF-β were also measured in the culture supernatants. Values represent the means ± SD for 7 mice per group. *, P < 0.05.
Gr1+ CD11b+ MDSCs from late septic mice lack the potential to differentiate ex vivo.
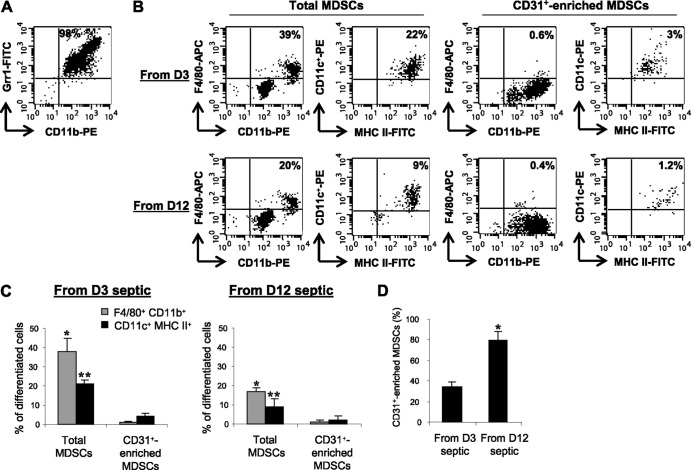
Our data thus far suggested that differences in the immunomodulatory properties of Gr1+ CD11b+ cells might align with differences in maturity. We determined whether Gr1+ CD11b+ cells from early and late septic mice are at the same developmental stage. We postulated that early MDSCs were more mature than late MDSCs. Purified Gr1+ CD11b+ cells were analyzed for the expression of the CD31 marker, which is expressed on very immature stages of myeloid cells and is lost during maturation (23). The number of CD31-positive Gr1+ CD11b+ cells (i.e., the CD31+ subset of total MDSCs) increased from 34% for day 3 septic mice to nearly 79% at day 12 (Fig. 6D). Next, we assessed the differentiation potentials of early and late septic MDSC mixtures, as well as CD3l+-enriched Gr1+ CD11b+ cells. CD31+ cells were enriched from Gr1+ CD11b+ cells that were 98% purified (Fig. 6A). Both total and CD31+-enriched Gr1+ CD11b+ cells were cultured for 6 days with GM-CSF, and cells were then stained for the markers of macrophage (F4/80) and dendritic cell (CD11c) development. About 38% and 21%, respectively, of total Gr1+ CD11b+ cells from day 3 septic mice differentiated into macrophages and dendritic cells, compared with 17% and 9% of Gr1+ CD11b+ cells derived from day 12 septic mice (Fig. 6B and C, left panels). Interestingly, CD31+-enriched Gr1+ CD11b+ cells from both early and late sepsis had markedly reduced differentiation capacity. Only 1% and 4% of cells from day 3 septic mice differentiated into macrophages and dendritic cells, respectively, compared with 1% and 2% of cells from day12 (right panels). These results support the hypothesis that as the septic inflammatory response progresses, a shift occurs toward a more immature stage of Gr1+ CD11b+ MDSCs, which lack the potential to differentiate into immunocompetent innate effector cells. This further indicates an interruption or arrest of myeloid differentiation.
Fig 6.
Differentiation of Gr1+ CD11b+ MDSCs ex vivo. Total Gr1+ CD11b+ MDSCs were harvested from the bone marrows of a separate group of mice at day 3 (D3) and day 12 (D12) after CLP. (A) Flow cytometry of sorted GR1+ CD11b+ MDSCs recovered from septic mice at day 12 after CLP. Sorted Gr1+ CD11b+ MDSCs were enriched for CD31+ cells by positive selection. Equal numbers of total and CD31+-enriched (CD31+ MDSCs) MDSCs were cultured (1 × 106 cell/ml) for 6 days with 10 ng/ml GM-CSF and IL-4. Cells were stained for the monocyte/macrophage marker F4/80 or the dendritic cell marker CD11c and phenotypically analyzed. (B) Example of flow cytometry of differentiated MDSCs gated on F4/80+ CD11b+ or CD11c+ MHC II+ staining. CD31+ subset of GR1+ CD11b+ MDSCs markedly lost their differentiation ex vivo. (C) Percentages of differentiated MDSCs. Values represent the means ± SD for 7 mice per group. * and **, comparison of percentages of macrophages and dendritic cells, respectively, in mice that received total MDSCs with mice that received CD31+ cell-enriched MDSCs (P < 0.05). (D) Percentage of CD31+ Gr1+ CD11b+ MDSCs. Purified Gr1+ CD11b+ cells from day 3 and day 12 septic mice were enriched for the CD31+ subset using a special bead washing buffer followed by positive selection with CD31 antibody. Selected cells were analyzed by flow cytometry (n = 5 mice per group). *, P < 0.05.
DISCUSSION
Postsepsis immune suppression is most evident in chronically septic humans and late septic animals and involves dysregulated innate and adaptive immune responses, which result in sustained primary infection with a predisposition to secondary infections and increased mortality (15, 25, 28). It is now recognized that a shift in early sepsis from a proinflammatory to an anti-inflammatory response underlies and sustains a postsepsis immunosuppressed state, which correlates with the development and distribution of MDSCs (15, 28). This study used adoptive transfer of the bone marrow-derived Gr1+ CD11b+ MDSCs to show that a heterogeneous population of these cells both expands and shifts the overall phenotype from proinflammatory to anti-inflammatory/immunosuppressive as sepsis progresses from early to late stages. These changes differentially affect clinical phenotypes during earlier and later sepsis from peritonitis. The initial phenotype increases mortality but improves local bacterial clearance. Conversely, the later immunosuppressive phenotype reduces mortality but limits clearance of local bacteria. Together, these results emphasize the complexity of the effects of sepsis on bone marrow progenitor cells and provide insight into effects of sepsis on the bone marrow.
Our results with the late MDSC phenotype agree with the findings of Delano et al. (10), who described marked expansion of GR1+ CD11b+ cells in bone marrow, spleen, and lymph nodes during murine polymicrobial sepsis and showed that depletion of MDSCs prevented sepsis-induced T cell suppression and Th2 cell polarization. That study did not report animal survival. We also confirmed the dramatic expansion of GR1+ CD11b+ cells during late sepsis. Further, we used the CD31 marker of myeloid progenitors to identify a more immature subpopulation that increased from 34% in early sepsis to 79% in late sepsis. The CD31+ subpopulation of Gr1+ CD11b+ cells had less capacity to differentiate, as supported by more cells from day 3 septic mice differentiating ex vivo to macrophages and dendritic cells than did those from day 12 septic mice. This supports that the dramatic accumulation of GR1+ CD11b+ cells in late sepsis is not due simply to their expansion but reflects an early disruption of maturation. This arrest of differentiation may explain why the number of mature macrophages and dendritic cells declines during accumulation of GR1+ CD11b+ cells in sepsis and other inflammatory processes (9, 40, 41).
In investigating MDSC differentiation, Kusmartsev et al. (19) found that GR1+ MDSCs from a tumor-bearing mouse can differentiate into macrophages and dendritic cells when adoptively transferred into a healthy mouse. Others found that treatment of tumor-bearing mice with all-trans-retinoic acid results in differentiation of MDSCs to macrophages and dendritic cells in vivo and in vitro (1, 19, 21). However, Cuenca et al. (9) were unable to differentiate MDSCs in vivo in septic mice using GM-CSF, although they could do so ex vivo (10). These investigators postulated that MDSCs retain the potential to differentiate but are trapped in an immature phenotype within a chronic inflammatory environment, such as in cancer or sepsis (9). Since immature myeloid cells with a similar GR1+ CD11b+ phenotype arising during normal myelopoiesis, are not immunosuppressive (13) and can quickly differentiate ex vivo (13, 20), it is likely that the immunosuppressive Gr1+ CD11b+ MDSCs are reprogrammed in the bone marrow during septic inflammation by a process that arrests differentiation. Our results support this in that early MDSCs are more responsive to differentiation than late MDSCs, and the MDSC subpopulation enriched for immature myeloid cell antigen CD31 is almost totally unresponsive to GM-CSF.
A recent study with important implications for understanding the origin and nature of the differentiation arrest of MDSCs during sepsis was reported by Rodriguez et al. (36). These investigators found that sepsis or bacterial lipopolysaccharide directly affects the most proximal hematopoietic stem cell through a Toll-like receptor 4 (TLR4)-dependent process, which increases replication of the TLR-expressing stem cells but arrests their differentiation into mature myeloid-derived phagocytic neutrophils and monocytes. This arrest of progenitor cells proximal to MDSCs might explain the formation of immature CD31+ MDSCs.
Transfer of MDSCs provides a good opportunity to study their immunomodulatory abilities in early versus late sepsis. Transfer of GR1+ CD11b+ cells derived from early (day 3) septic mice exaggerated the early sepsis proinflammatory response in mice, as manifested by increases in circulating proinflammatory cytokines and mortality. Unexpectedly, these mice had lower counts of intraperitoneal bacteria; this might reflect activation of the more mature myeloid cells within the Gr1+ CD11b+ MDSC population that retain their potential to further generate a protective innate immune reaction. In contrast, GR1+ CD11b+ MDSCs from late (day 12) septic mice generated opposite effects by increasing circulating levels of anti-inflammatory cytokines and reducing mortality. By 8 days after MDSC transfer (i.e., in late sepsis), there was no further impact on either the cell phenotype assessed by inflammatory cytokine production (data not shown) or survival (Fig. 2). These diminished effects of GR1+ CD11b+ cell transfer on the late sepsis response might be explained by the endogenous GR1+ CD11b+ cell population of septic mice, which has already expanded enough to overshadow any effect from the transferred cells. In support of this, an immature CD31+ subset of GR1+ CD11b+ cells in late (day 12) septic mice represented ∼79% of the total GR1+ CD11b+ cell population. Taken together, the data identify differences between the host inflammatory reaction and immune competence.
Two recent studies reported effects of GR1+ CD11b+ MDSCs on chronic inflammatory processes. Xia et al. (42) found that adoptive transfer of bone marrow GR1+ CD11b+ cells from normal mice into obese mice decreases inflammation, whereas their depletion by anti-Gr1 antibody increases inflammation and reduces insulin sensitivity. That study further showed that adoptive transfer of MDSCs skews macrophages toward the alternatively activated M2 and anti-inflammatory phenotype that expresses arginase and produces IL-10 and/or TGF-β. Sander et al. (37) used a murine model of polymicrobial sepsis to show that depletion of Gr1+ CD11b+ MDSCs 24 h before and after CLP increases septic mortality; however, the Gr1 antibody used in this study was not specific for MDSCs and would also deplete mature Gr1+ immunocompetent cells, such as neutrophils. That study—which discovered that the liver acute-phase response and activation of gp130, the signaling receptor shared by IL-6, are required to generate MDSCs—also found that adoptive transfer of day 5 postseptic Gr1+ CD11b+ MDSCs into gp130-deficient mice 24 h after CLP improves survival. These authors concluded that the Gr1+ CD11b+ MDSC response depends on IL-6 interaction with gp130 as a crucially important protective process during murine sepsis. The gp130-dificient mice did not accumulate Gr1+ MDSCs due to a lack of STAT3 signaling, which is required for myeloid cell proliferation and Gr1+ cell expansion. Our results suggest that during sepsis, MDSCs are heterogeneous in a time- and inflammation-dependent context and can either promote or suppress the inflammatory response; during late sepsis their immunosuppressive phenotype is dominant and sustained.
Consistent with our findings of differences in the inflammatory phenotypes between early and late septic GR1+ CD11b+ cells were the findings showing that both cell populations had different inflammatory activities. The immunosuppressive and modulatory activities of Gr1+ CD11b+ MDSCs have been defined by their upregulated production of inflammatory mediators, such as arginase, NO, and ROS, as well as pro- and anti-inflammatory cytokines (8, 9, 13). Arginase and NO are important components of the innate immune effector cell functions against infection (9, 13). The upregulation of arginase activity we detected in late septic Gr1+ CD11b+ cells may lead to depletion of l-arginine from the T cell environment and thus inhibit T cell-mediated responses by either increasing their apoptosis or inhibiting their proliferation (6, 8, 18). Our experiments showed that Gr1+ CD11b+ cells from early septic mice produced more NO than cells from late septic mice, which was consistent with the exaggerated proinflammatory reaction and the reduced bacterial growth we observed during early sepsis.
In addition, transfer of Gr1+ CD11b+ cells from early septic mice increased levels of circulating proinflammatory TNF-α and IL-6, which were associated with increased mortality and reduced numbers of peritoneal bacteria during early the sepsis response. In contrast, cells from late septic mice increased levels of anti-inflammatory IL-10 and TGF-β, which was associated with delayed mortality and enhanced bacterial growth. Thus, our results are consistent with those of previous studies showing that Gr1+ CD11b+ MDSCs from tumor-bearing animals and patients produce increased amounts of the immunosuppressive IL-10 and TGF-β (12, 39, 43). While it is not clear from our experiments if these cytokines were directly produced by the transferred cells or by the endogenous innate myeloid cells, our results demonstrate that Gr1+ CD11b+ cells of late sepsis possess potent immunosuppressive and anti-inflammatory activities consistent with the dramatic increase in the numbers of the more immature CD31+ cell subpopulation. In contrast, Gr1+ CD11b+ cells that accumulate in early sepsis have proinflammatory activities, which might have contributed to the exaggeration of the proinflammatory response seen in the recipient mice. Also, our results showed that pro- and anti-inflammatory cytokines were markedly decreased and increased, respectively, to almost similar levels in all CLP mice (whether they received saline or Gr1+ CD11b+ cells) once they entered the late septic phase. Such a shift in the cytokine profile shows a typical shift from the early to late sepsis response, and it also suggests that the dramatic expansion of the endogenous MDSCs at this late phase (see Fig. 1) is responsible for the shift in the cytokine profile.
Our results showed that Gr1+ CD11b+ cells from early septic mice were NO-dependent proinflammatory, whereas those from late septic mice had arginase-dependent anti-inflammatory phenotypes. Also, transfer of Gr1+ CD11b+ cells from late septic mice was associated with significantly elevated levels of the immunosuppressive IL-10 and TGF-β cytokines. Although TGF-β is not strictly a Th2 cytokine, it promotes Th2 responses by inhibiting Th1 macrophage activation (26) and together with IL-10 can steer the response toward the anti-inflammatory phenotype (26, 27). Since late sepsis is a Th2-dominated response, our studies suggest that the undifferentiating late septic CD31+ subset of Gr1+ CD11b+ MDSCs may be an anti-inflammatory myeloid phenotype that mediates the immunosuppressive and anti-inflammatory responses of late sepsis.
In summary, we have shown sequential changes in Gr1+ CD11b+ cells during the sepsis response. The kinetics of the expansion and the immunomodulatory activities of these cells are governed by the ongoing inflammatory process. Early septic Gr1+ CD11b+ cells are proinflammatory, since they produce increased amounts of proinflammatory mediators and exaggerate the inflammatory process of early sepsis. In contrast, late septic Gr1+ CD11b+ cells produce anti-inflammatory and immunosuppressive mediators, and they are more immature, suggesting an arrest at an early stage of myeloid progenitor cell growth and differentiation. Recently, a number of strategies have been developed to use immunosuppressive myeloid cells for cancer immunotherapy. Manipulating the numbers and activities of Gr1+ CD11b+ cells by eliminating them or inhibiting their expansion or by neutralizing their immunosuppressive products provides promise for resolving inflammatory pathologies. Because of their dramatic expansion and immunosuppressive activity in late sepsis, targeting Gr1+ CD11b+ cells may represent an attractive therapeutic approach for the untreatable immune suppression in late/chronic sepsis. Together, an increasing number of studies support a new concept that sepsis directly arrests bone marrow progenitor cells and that novel interventions can be designed to reverse this.
Footnotes
Published ahead of print 26 March 2012
REFERENCES
- 1. Almand B, et al. 2001. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol. 166:678–689 [DOI] [PubMed] [Google Scholar]
- 2. Belikoff B, Hatfield S, Sitkovsky M, Remick DG. 2011. Adenosine negative feedback on A2A adenosine receptors mediates hyporesponsiveness in chronically septic mice. Shock 35:382–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Belikoff BG, et al. 2011. A2B adenosine receptor blockade enhances macrophage-mediated bacterial phagocytosis and improves polymicrobial sepsis survival in mice. J. Immunol. 186:2444–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bone RC, et al. 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644–1655 [DOI] [PubMed] [Google Scholar]
- 5. Bronte V. 2009. Myeloid-derived suppressor cells in inflammation: uncovering cell subsets with enhanced immunosuppressive functions. Eur. J. Immunol. 39:2670–2672 [DOI] [PubMed] [Google Scholar]
- 6. Bronte V, et al. 2003. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J. Immunol. 170:270–278 [DOI] [PubMed] [Google Scholar]
- 7. Brudecki L, et al. 5 December 2011. Hematopoietic stem-progenitor cells restore immunoreactivity and improve survival in late sepsis. Infect. Immun. doi:10.1128/IAI. 05480-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Condamine T, Gabrilovich DI. 2011. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 32:19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cuenca AG, et al. 2011. A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol. Med. 17:281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Delano MJ, et al. 2007. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med. 204:1463–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ellaban E, Bolgos G, Remick D. 2004. Selective macrophage suppression during sepsis. Cell Immunol. 231:103–111 [DOI] [PubMed] [Google Scholar]
- 12. Eruslanov E, Daurkin I, Ortiz J, Vieweg J, Kusmartsev S. 2010. Pivotal advance: tumor-mediated induction of myeloid-derived suppressor cells and M2-polarized macrophages by altering intracellular PGE catabolism in myeloid cells. J. Leukoc. Biol. 88:839–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gabrilovich DI, Nagaraj S. 2009. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9:162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. 2001. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J. Immunol. 166:5398–5406 [DOI] [PubMed] [Google Scholar]
- 15. Hotchkiss RS, Karl IE. 2003. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348:138–150 [DOI] [PubMed] [Google Scholar]
- 16. Huang X, et al. 2009. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc. Natl. Acad. Sci. U. S. A. 106:6303–6308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inoue S, et al. 2010. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J. Immunol. 184:1401–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kung JT, Brooks SB, Jakway JP, Leonard LL, Talmage DW. 1977. Suppression of in vitro cytotoxic response by macrophages due to induced arginase. J. Exp. Med. 146:665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kusmartsev S, et al. 2003. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 63:4441–4449 [PubMed] [Google Scholar]
- 20. Kusmartsev S, Gabrilovich DI. 2003. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J. Leukoc. Biol. 74:186–196 [DOI] [PubMed] [Google Scholar]
- 21. Kusmartsev S, et al. 2008. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin. Cancer Res. 14:8270–8278 [DOI] [PubMed] [Google Scholar]
- 22. Lederer JA, Rodrick ML, Mannick JA. 1999. The effects of injury on the adaptive immune response. Shock 11:153–159 [DOI] [PubMed] [Google Scholar]
- 23. Ling V, et al. 1997. Structural identification of the hematopoietic progenitor antigen ER-MP12 as the vascular endothelial adhesion molecule PECAM-1 (CD31). Eur. J. Immunol. 27:509–514 [DOI] [PubMed] [Google Scholar]
- 24. Mazuski JE, et al. 2002. The Surgical Infection Society guidelines on antimicrobial therapy for intra-abdominal infections: an executive summary. Surg. Infect. (Larchmt.) 3:161–173 [DOI] [PubMed] [Google Scholar]
- 25. Merlino JI, Yowler CJ, Malangoni MA. 2004. Nosocomial infections adversely affect the outcomes of patients with serious intraabdominal infections. Surg. Infect. (Larchmt.) 5:21–27 [DOI] [PubMed] [Google Scholar]
- 26. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. 2000. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164:6166–617310843666 [Google Scholar]
- 27. Munder M, Eichmann K, Modolell M. 1998. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J. Immunol. 160:5347–5354 [PubMed] [Google Scholar]
- 28. Oberholzer A, Oberholzer C, Moldawer LL. 2001. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock 16:83–96 [DOI] [PubMed] [Google Scholar]
- 29. Ostrand-Rosenberg S, Sinha P. 2009. Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol. 182:4499–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Osuchowski MF, Welch K, Siddiqui J, Remick DG. 2006. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J. Immunol. 177:1967–1974 [DOI] [PubMed] [Google Scholar]
- 31. Osuchowski MF, Welch K, Yang H, Siddiqui J, Remick DG. 2007. Chronic sepsis mortality characterized by an individualized inflammatory response. J. Immunol. 179:623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peranzoni E, et al. 2010. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr. Opin. Immunol. 22:238–244 [DOI] [PubMed] [Google Scholar]
- 33. Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. 2002. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock 17:463–467 [DOI] [PubMed] [Google Scholar]
- 34. Remick DG, et al. 2001. Combination immunotherapy with soluble tumor necrosis factor receptors plus interleukin 1 receptor antagonist decreases sepsis mortality. Crit. Care Med. 29:473–481 [DOI] [PubMed] [Google Scholar]
- 35. Rittirsch D, Flierl MA, Ward PA. 2008. Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 8:776–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodriguez S, et al. 2009. Dysfunctional expansion of hematopoietic stem cells and block of myeloid differentiation in lethal sepsis. Blood 114:4064–4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sander LE, et al. 2010. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J. Exp. Med. 207:1453–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shubin NJ, Monaghan SF, Ayala A. 2011. Anti-inflammatory mechanisms of sepsis. Contrib. Microbiol. 17:108–124 [DOI] [PubMed] [Google Scholar]
- 39. Vuk-Pavlovic S, et al. 2010. Immunosuppressive CD14+HLA-DRlow/− monocytes in prostate cancer. Prostate 70:443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A. 2005. Leukocyte apoptosis and its significance in sepsis and shock. J. Leukoc. Biol. 78:325–337 [DOI] [PubMed] [Google Scholar]
- 41. Wysocka M, et al. 2001. IL-12 suppression during experimental endotoxin tolerance: dendritic cell loss and macrophage hyporesponsiveness. J. Immunol. 166:7504–7513 [DOI] [PubMed] [Google Scholar]
- 42. Xia S, et al. 2011. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J. Biol. Chem. 286:23591–23599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang L, et al. 2008. Abrogation of TGF-beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13:23–35 [DOI] [PMC free article] [PubMed] [Google Scholar]