Abstract
We showed previously that ingested human insulin activates the insulin/IGF-1 signaling pathway in Anopheles stephensi and increases the susceptibility of these mosquitoes to Plasmodium falciparum. In other organisms, insulin can alter immune responsiveness through regulation of NF-κB transcription factors, critical elements for innate immunity that are also central to mosquito immunity. We show here that insulin signaling decreased expression of NF-κB-regulated immune genes in mosquito cells stimulated with either bacterial or malarial soluble products. Further, human insulin suppressed mosquito immunity through sustained phosphatidylinositol 3-kinase activation, since inhibition of this pathway led to decreased parasite development in the mosquito. Together, these data demonstrate that activation of the insulin/IGF-1 signaling pathway by ingested human insulin can alter NF-κB-dependent immunity, and ultimately the susceptibility, of mosquitoes to P. falciparum.
INTRODUCTION
Annually, there are over 250 million new malaria cases, the majority due to infection with the parasite Plasmodium falciparum (58). When a female mosquito ingests an infectious blood meal, insulin from vertebrate blood collects in the mosquito midgut, a critical site for Plasmodium development (reviewed in reference 57) and a tissue that is exquisitely responsive to ingested human insulin (9, 31). The amount of insulin ingested by a feeding mosquito is directly influenced by malaria infection. That is, patients with severe malaria can develop blood insulin levels that exceed that of a normal healthy adult by as much as 10- to 35-fold (13, 56). Ingested human insulin enhances malaria parasite development in exposed mosquitoes relative to mosquitoes that do not receive insulin in the infectious blood meal (50). Collectively, these observations suggest that control strategies for malaria should recognize that the burden of transmission is influenced, in part, by changes in insulin physiology in the human host that cross talk with analogous physiology in the mosquito host.
The highly conserved insulin/IGF-1 signaling (IIS) pathway consists of two main signaling branches, a mitogen-activated protein kinase (MAPK)-dependent branch and phosphatidylinositol 3-kinase (PI3K)/Akt-dependent branch, both of which are involved in the regulation of innate immune responses (26, 38). Ingested insulin can activate both the PI3K and MAPK branches of the IIS pathway in mosquitoes (9, 31, 37, 38, 50). In addition, at least two IIS proteins—Akt/PKB and ERK—have proven critical for the control of malaria infection in the mosquito host (9, 51).
Our previous work suggested that human insulin ingested in a blood meal can signal mosquito midgut epithelial cells directly (31), altering the susceptibility of mosquitoes to malaria infection (50). Specifically, when mosquitoes were provided human insulin in an infectious blood meal, we observed a significant increase in P. falciparum development relative to mosquitoes that did not receive insulin in the blood meal (50). While these data suggest that human insulin-induced signaling in the mosquito midgut alters the immune responsiveness of mosquitoes to malaria parasites, the effects of insulin signaling on mosquito immunity have not been well characterized, and no direct connections between the IIS pathway and mosquito immunity have been established.
Plasmodium parasites undergo a series of complicated developmental transformations upon ingestion by the Anopheles mosquito and, during this process, mosquito immunity can result in significant reductions in parasite numbers (reviewed in reference 48). The regulation of mosquito immunity during Plasmodium infection occurs in part through the activation of the highly conserved NF-κB transcription factors Rel1 and Rel2 that control mosquito responses to bacterial, fungal, and parasitic pathogens (7, 20, 39–41).
NF-κB binding motifs have been found in the upstream regions of numerous insect immune genes, including antimicrobial effectors such as defensin 1 (Def), cecropin 1 (Cec), and gambicin (Gam), as well as nitric oxide synthase (18, 25, 39, 45, 54, 55). Increased NF-κB-dependent transcription can reduce both bacterial load and Plasmodium development in anopheline mosquitoes (2, 18, 20, 23, 41). In mammals, a number of well-characterized cell signaling pathways, including IIS, can network with NF-κB activation to fine tune the response to infection. For example, physiological levels of human insulin can suppress both lipopolysaccharide (LPS)- and tumor necrosis factor alpha (TNF-α)-induced NF-κB activation in human cell lines (11, 28). In addition, IIS activation in human cell lines can attenuate the degradation of the negative regulators of NF-κB, resulting in increased NF-κB sequestration in the cytoplasm (10). Therefore, we hypothesized that human insulin ingested in an infectious blood meal alters mosquito immunity to malaria parasite infection through the regulation of NF-κB activity.
We show here that human insulin can inhibit the expression of the NF-κB-regulated immune gene expression both in immortalized mosquito cells in vitro and in the Anopheles stephensi midgut epithelium in vivo in response to bacterial and malarial soluble products. The presence of human insulin resulted in the sustained activation of the PI3K, but not the MAPK, branch of the mosquito IIS pathway. In addition, inhibition of PI3K activity both in immortalized mosquito cells and in the A. stephensi midgut resulted in the reversal of the immunosuppressive effects of human insulin. Taken together, our data indicate that activation of the mosquito IIS by human insulin inhibits the mosquito immune response to malaria parasites at least in part through the regulation of NF-κB activity.
MATERIALS AND METHODS
Cell culture, transfection, and luciferase reporter assays.
A. stephensi embryonic (ASE) cells (a gift from H.-M. Müller, EMBL) were maintained as previously described (51). Cec and Gam promoters were cloned into the promoterless luciferase reporter pGL3 (Promega, Madison, WI). Transfections were performed using Effectene transfection reagent (Qiagen) as previously described (43). At 24 h posttransfection, cells were challenged with 100 μg of LPS (Escherichia coli serotype O26:B6; Sigma-Aldrich, St. Louis, MO)/ml or with 150 × 106 parasite equivalents of P. falciparum soluble products (PfsPs) with or without 0.017 to 1.7 μM human insulin (Sigma-Aldrich). For assays of kinase inhibition, cells were treated with the inhibitors LY294002 (50 μM; Sigma-Aldrich), wortmannin (1 μM; EMD Chemicals, Gibbstown, NJ), PD98059 (10 μM; Sigma-Aldrich), or U0126 (10 μM; Promega) for 1 h prior to immune challenge. Luciferase activity was measured 24 h after immune challenge with the Dual-Glo system (Promega).
Immunoblotting.
Protein extracts were prepared, separated, and transferred to membranes as previously described (37). Membranes were blocked in 5% nonfat dry milk (wt/vol) in Tris-buffered saline (pH 7.0) containing 0.1% Triton-100 (TBS-T) for 1 h at room temperature. Membranes were incubated at 4°C overnight with the following: 1:10,000 mouse monoclonal anti-diphosphorylated ERK1/2 (p-ERK; Sigma-Aldrich), 1:1,000 anti-phospho-FOXO1/FOXO3a (p-FOXO; Cell Signaling, Danvers, MA), or 1:10,000 anti-GAPDH (Sigma-Aldrich) antibody in 5% nonfat dry milk in TBS-T. Membranes were washed three times, for 5 min each time, and incubated with a 1:2,000 (p-FOXO) or 1:20,000 (GAPDH) dilution of horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Biosource International, Camarillo, CA) or with 1:20,000 (p-ERK) HRP-conjugated rabbit anti-mouse IgG (Pierce, Rockford, IL) at 4°C overnight. To reveal antibody-bound proteins, membranes were incubated with SuperSignal West Dura chemiluminescent reagent for 5 min and visualized using the Kodak Image Station 4000MM Pro and Carestream molecular imaging software (Carestream Health, New Haven, CT). The levels of phospho-proteins in each treatment were first normalized to total protein levels as determined by GAPDH and then to the appropriate control group.
Mosquito rearing and experimental treatments.
A. stephensi Liston (Indian wild-type strain) were reared and maintained at 27°C and 75% humidity. All mosquito rearing and feeding protocols were approved and in accordance with regulatory guidelines and standards set by the Institutional Animal Care and Use Committee of the University of California, Davis. For experimental treatments, laboratory reared 3- to 5-day-old female mosquitoes were kept on water for 48 h and then allowed to feed for 30 min on reconstituted human blood meals provided through a Hemotek insect feeding system (Discovery Workshops, Accrington, United Kingdom). For signaling studies, mosquitoes were fed washed red blood cells (RBCs) alone or with 170 pM human insulin, 20 μM LY294002, or insulin plus LY294002. For Western blot analyses, midguts were dissected from 60 mosquitoes in each treatment group at various time points after blood feeding and processed as previously described (51).
For life span studies, ∼1-day-old A. stephensi females (n = 50) were placed into four treatment groups and fed a single blood meal containing (i) 170 pM human insulin, (ii) heat-killed bacteria (HK-bacteria), (iii) HK-bacteria plus insulin, or (iv) an equivalent volume of phosphate-buffered saline (PBS) as a control. HK-bacterial stocks were prepared by combining equivalent volumes of log-phase liquid cultures of Staphylococcus aureus and Pseudomonas aeruginosa (Carolina Biological Supply, Burlington, NC) grown overnight and autoclaving the liquid culture for ∼20 min. The HK-bacteria were pelleted, and the supernatant was removed. HK-bacteria were washed twice with sterile PBS before being added to a blood meal. After provision of this single blood meal, mosquitoes were provided 10% sucrose solution ad libitum thereafter. Experiments were repeated four times separately, and mortality was recorded daily.
PfsP preparation.
To produce P. falciparum soluble products (PfsPs) for in vitro experiments, infected RBCs (iRBCs) from day 15 parasite cultures were lysed in 15 ml of 1× RBC lysis buffer (eBioscience, San Diego, CA) for 15 min and then brought to a final volume of 100 ml in PBS. Paramagnetic, hemozoin-containing parasites were enriched using a MACS LS column (Miltenyi Biotec, Auburn, CA) in a magnetic holder as described previously (33). Column elutant, consisting primarily of parasites, was then frozen at −80°C for 10 min and thawed at 37°C 10 times and applied to new LS columns to remove the hemozoin. Pooled PfsPs preparations were determined to be endotoxin-free (E-Toxate kit; Sigma-Aldrich) and contained <1 μM hemin/heme/hemozoin (hemin assay kit; BioVision, Mountain View, CA). The number of parasite equivalents was determined with the following formula: (iRBCS/RBCs) × (total number of RBCs) minus 30% estimated loss during purification process (32, 33).
P. falciparum culture and mosquito infection.
For mosquito infection, cultures of P. falciparum strain NF54 MCB (a gift from Sanaria, Inc., Rockville, MD) were grown in 10% heat-inactivated human serum and 5% washed human RBCs (Interstate Blood Bank) in RPMI 1640 with HEPES (Invitrogen) and hypoxanthine (Sigma-Aldrich). Mosquitoes were allowed to feed on day 15 mature gametocyte cultures diluted with human RBCs and heat-inactivated human serum with or without 170 pM human insulin and with or without 20 μM LY294002. All treatments were added to the diluted P. falciparum culture immediately prior to blood feeding. Protocols involving the culture and handling of P. falciparum for mosquito feeding were approved and in accordance with regulatory guidelines and standards set by the Biological Safety Administrative Advisory Committee of the University of California, Davis. After 10 days, midguts from 50 mosquitoes with fully developed eggs (to confirm complete engorgement) from each group were dissected in PBS and stained with 0.1% mercurochrome for direct counting of P. falciparum oocysts. Means of oocysts per midgut in each treatment group were calculated from all dissected mosquitoes, including zeros for mosquitoes that contained no oocysts.
P. falciparum growth assays.
Aliquots of P. falciparum NF54 culture were synchronized 48 h prior to the assay as previously described (35) and then plated in 96-well flat-bottom plates in complete RPMI 1640 with HEPES, hypoxanthine, and 10% heat-inactivated human serum. Parasites were treated with LY294002 inhibitor at 2, 20, or 200 μM or with an equivalent volume of dimethyl sulfoxide (DMSO) diluent for 48 and 96 h in a candle jar in a 37°C incubator. The assays were terminated by replacing the culture medium with RPMI 1640 with 10% formalin in PBS. Erythrocytes were stained with 10 μg of propidium iodide (Sigma-Aldrich)/ml in PBS for 1 h at room temperature. Infected RBCs were counted with FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). The levels of parasite growth in response to treatment were normalized to DMSO controls, which were set to 100%.
RESULTS
Human insulin inhibits LPS-induced antimicrobial promoter activity in immortalized mosquito cells.
To determine whether human insulin could regulate the mosquito immune response in vitro, we utilized a luciferase-reporter assay to quantify LPS-induced NF-κB-dependent antimicrobial peptide (AMP) gene promoter activity in immortalized ASE cells. For ease of manipulation, ∼1.5 kb upstream of the transcriptional start site for each AMP gene was used to construct the reporter plasmids. Although all three promoter-reporters were inducible, the upstream sequences for Cec and Gam may not include all necessary regulatory elements for expression given the lower induction of these gene promoters compared to Def (Fig. 1).
Fig 1.
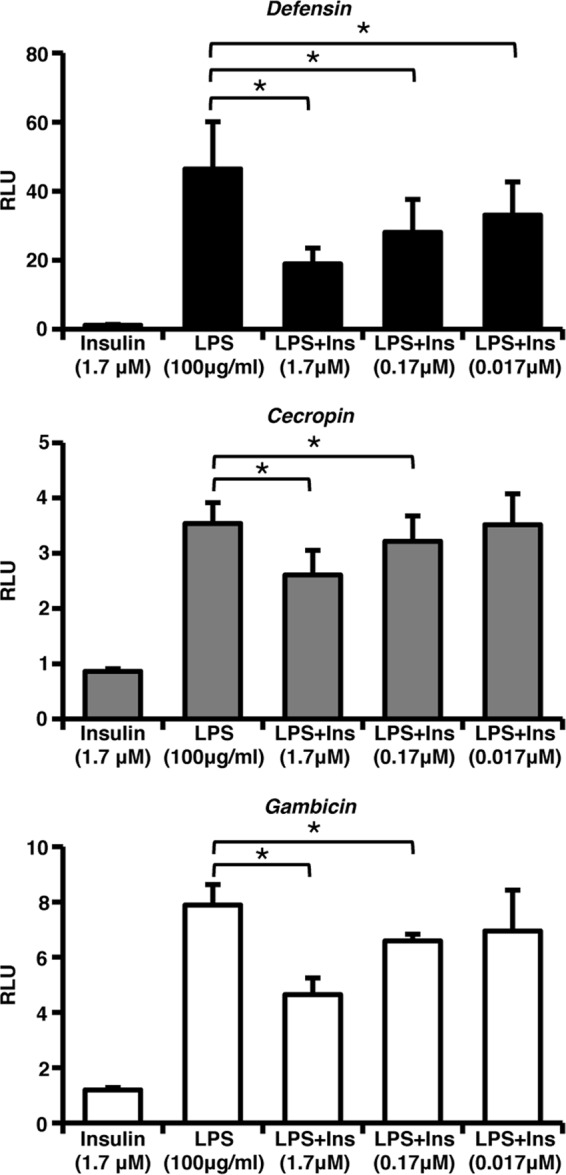
Human insulin decreases LPS-induced antimicrobial promoter activity in immortalized A. stephensi cells. ASE cells were transfected with Defensin, Cecropin, or Gambicin luciferase promoter-reporter plasmid constructs and then stimulated 24 h later with 1.7 μM human insulin, 100 μg of LPS/ml, or LPS and decreasing concentrations of human insulin. Graphs represent means ± the standard errors of the mean (SEM) of luciferase activity (relative light units [RLU]) normalized to untreated controls (n = 3). Pairwise comparisons of treatments were analyzed by using the Student t test (*, P < 0.05).
As expected, human insulin alone did not alter activity of the Def, Cec, or Gam promoters in ASE cells. However, treatment with increasing concentrations of human insulin from 0.017 to 1.7 μM significantly decreased the activity of all three promoters in response to LPS (Fig. 1), demonstrating that activation of the IIS pathway by human insulin can downregulate the innate immune response of mosquito cells. Despite the fact that human insulin is a heterologous ligand for A. stephensi cells, our results were still consistent with the attenuation of LPS-induced NF-κB activity by 0.1 μM insulin reported in mammalian macrophages (10). Although immortalized mosquito cells provide a useful model for studying cell signaling, they are not identical to the highly specialized epithelial cells present in the midgut of mosquitoes and, as such, require the use of a higher concentration of human insulin.
Inhibition of the PI3K branch of the IIS pathway reverses the negative effects of human insulin on the mosquito innate immune response.
In mammals, activation of the PI3K branch of the IIS pathway has been shown to negatively regulate NF-κB activation and the expression of inflammatory genes, while activation of the MEK/ERK branch of the IIS pathway has been shown to have many proinflammatory effects (10, 24, 28). Thus, the IIS-mediated effects of human insulin on the mosquito immune response, via regulation of NF-κB activity, may be specific to which branch of the IIS pathway is being activated.
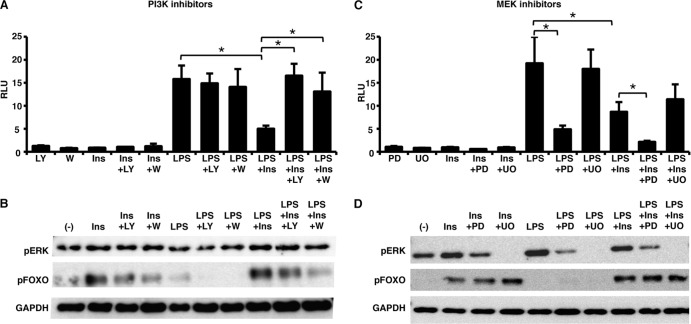
If inhibition of the mosquito immune response by human insulin observed in Fig. 1 occurs via PI3K activation, then treatment with a PI3K inhibitor should recover AMP promoter activity. To test this hypothesis, ASE cells transfected with pDef-luc were treated separately with two PI3K inhibitors, LY294002 or wortmannin, for 1 h and subsequently challenged with 1.7 μM human insulin, 100 μg of LPS/ml, or LPS plus insulin (LPS+insulin). Pretreatment of ASE cells with either inhibitor reversed the insulin-dependent inhibition of Def promoter activity (Fig. 2A). Western blot analysis of phosphorylated FOXO (p-FOXO), a transcription factor downstream of PI3K, confirmed that both LY294002 and wortmannin inhibited PI3K signaling in ASE cells. As expected, treatment of the cells with either insulin alone or LPS+insulin and either LY294002 or wortmannin reduced p-FOXO levels (Fig. 2B). From these data, we concluded that the inhibitory effects of human insulin on LPS-induced NF-κB activity are dependent on PI3K signaling.
Fig 2.
Inhibition of PI3K, but not MEK, reverses the negative effects of human insulin on LPS-induced antimicrobial promoter activity in immortalized A. stephensi cells. ASE cells were transfected with the Defensin luciferase promoter-reporter plasmid (pDef-luc) and treated with specific small molecule inhibitors of PI3K (20 μM LY294002 [LY] or 1 μM wortmannin [W]) (A) or MEK (10 μM PD98059 [PD] or 10 μM U0126 [UO]) (C) for 1 h prior to stimulation with 1.7 μM human insulin, 100 μg of LPS/ml, or LPS+insulin. Luciferase activities were determined 24 h after stimulation. The graphs represent the means ± the SEM of luciferase activity (relative light units [RLU]) normalized to untreated controls (not shown) (n = 5). Pairwise comparisons of treatments were analyzed by using the Student t test (*, P < 0.05). Representative, replicated Western blots of phosphorylated ERK (p-ERK) and FOXO (p-FOXO) from cell lysates treated as described above for 5 min are shown to confirm the inhibition of the IIS pathway by PI3K (B) or MEK (D) inhibitors. GAPDH provided an assessment of protein loading (n = 2).
In contrast to the PI3K branch, the MEK/ERK branch of the IIS pathway has been shown to be involved in the activation of NF-κB in other organisms (10, 24, 28). Therefore, we hypothesized that inhibition of MEK activity, in the presence of insulin, would lead to a greater decrease in AMP promoter activity. To test this hypothesis, ASE cells transfected with a Def promoter-reporter plasmid were treated with the MEK inhibitors PD98059 or U0126 for 1 h and stimulated as previously described. In support of our hypothesis, ASE cells pretreated with PD98059 exhibited a greater reduction in Def promoter activity compared to LPS+insulin-treated cells (Fig. 2C). In addition, cells treated with LPS plus PD98059 (LPS+PD) in the absence of insulin exhibited a significant decrease in Def promoter activity compared to cells treated with LPS alone (Fig. 2C; compare LPS versus LPS+PD).
In contrast to the results with PD98059, cells pretreated with U0126 showed no significant differences in Def promoter activity compared to either LPS or LPS+insulin (Fig. 2C). We noted that cells treated with PD98059 and stimulated with LPS or LPS+insulin had decreased levels of phosphorylated ERK (p-ERK), whereas cells treated with U0126 had undetectable levels of p-ERK (Fig. 2D). Numerous studies have documented the increased potency of U0126 to inhibit MEK-ERK-dependent signaling compared to PD98059 (49, 52). Inhibition of MEK-ERK signaling directly reduces activation of the mitogen- and stress-associated kinases (MSKs), substrates of activated ERK (6) that can act as negative regulators of Toll-like receptor signaling in humans (1). Hence, the more potent inhibition of MEK-ERK signaling by U0126 could be offset by a greater inhibition of MSK signaling and, therefore, increased NF-κB-dependent signaling relative to that observed with PD98059.
Stimulation of mosquito cells by human insulin results in sustained FOXO, but not ERK, phosphorylation.
The MEK/ERK and PI3K/Akt branches of the IIS pathway have distinct and opposing effects on NF-κB-dependent immune responses in mammalian cells (10, 24, 28). These effects are partially mediated by acute inhibitory (<6 h) PI3K/Akt signaling and stimulatory effects of MAPK signaling that are not evident until 24 to 48 h after insulin treatment (28). Based on these observations, we hypothesized that activation of the PI3K/Akt branch of the IIS pathway by insulin should occur rapidly and persist for some hours to exert the negative effects on NF-κB-dependent promoter activity observed in Fig. 1. To test this hypothesis, ASE cells were treated with 100 μg of LPS/ml, 1.7 μM human insulin, or LPS+insulin for 5 min, 30 min, 1 h, 3 h, 6 h, and 24 h, and the levels of p-ERK and p-FOXO were determined by immunoblotting. No significant changes in p-ERK or p-FOXO were observed following LPS stimulation (Fig. 3, top panel). Similar to previous results (31, 50), we observed a trend toward increased p-ERK levels at 5 min after stimulation with human insulin, followed by a rapid drop-off by 30 m, but responses at all other times were not significantly different from control levels. In contrast to p-ERK, we detected statistically significant increases in p-FOXO levels at all time points through 24 h after treatment with human insulin, both in the presence and in the absence of LPS (Fig. 3, middle and bottom panels). These data suggested that the dominant activation of PI3K signaling is necessary for insulin-induced downregulation of NF-κB-dependent promoter activity.
Fig 3.
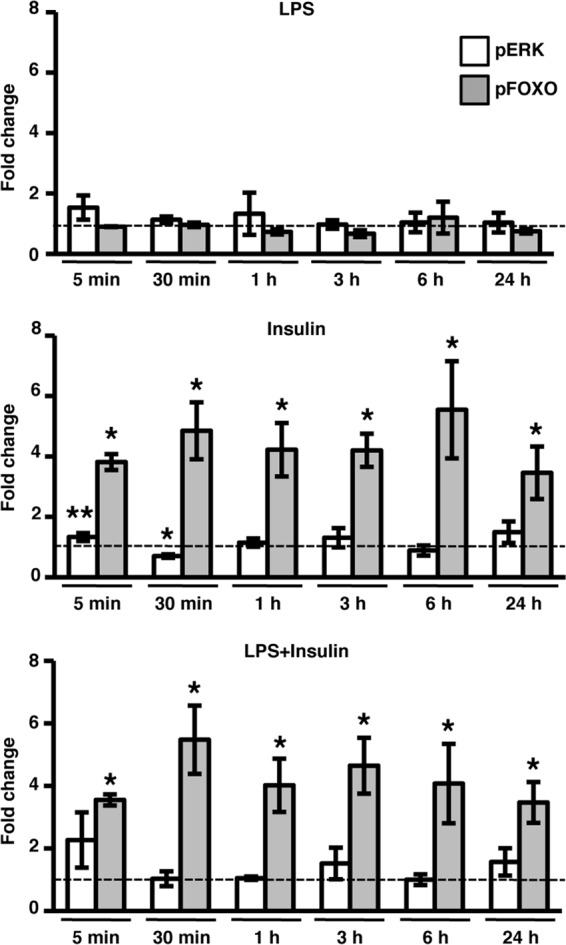
Stimulation of immortalized A. stephensi cells with human insulin results in sustained FOXO, but not ERK, phosphorylation. Cell lysates from ASE cells treated with 100 μg of LPS/ml, 1.7 μM human insulin, or LPS+insulin were collected at the indicated time points after treatment, and the levels of phosphorylated ERK (pERK) and FOXO (pFOXO) were determined by Western blotting. The graphs represent the average fold change ± the SEM of phospho-specific protein levels normalized to untreated controls (n = 4). The dashed line represents phospho-specific protein levels in untreated controls. GAPDH provided an assessment of protein loading. Pairwise comparisons of treatments versus controls at each time point were analyzed by using the Student t test (*, P < 0.05; **, P < 0.10).
Provision of human insulin increases survival of A. stephensi following feeding of heat-killed bacteria.
Mosquitoes possess a sophisticated innate immune system that protects them from a variety of bacterial, fungal, and parasitic pathogens. However, immune responses are costly because they can consume energy, induce irreversible pathologies, and can—ultimately—feed back to reduce insulin signaling. For example, hyperactivated immune responses reduce the life span of Drosophila melanogaster and Caenorhabditis elegans (reviewed in reference 14). Specifically, activation of the Toll signaling pathway in D. melanogaster led to both induction of immunity and repression of insulin signaling, nutrient storage, and growth (15). We hypothesized that immune-stimulated mosquitoes provided human insulin would have a reduced immune response resulting in decreased immunopathology and reallocation of resources, compared to immune-stimulated mosquitoes that had not received insulin. In other words, the sum of insulin-induced alterations in immune-stimulated mosquitoes should lead to increased survivorship relative to controls. To test this, mosquitoes were fed a single blood meal containing 170 pM insulin, HK-bacteria, HK-bacteria+insulin, or an equivalent volume of buffer as a control. The dose of insulin used here significantly increased P. falciparum development in A. stephensi in previous studies (50) and is within the normal fasting to nonfasting range (17 to 590 pM) in human blood (13). Daily mortality for each treatment was recorded, and dead mosquitoes were removed daily for 18 days. A proportional-hazards model was utilized to compare the survivorship of mosquitoes in the control and treatment groups. Across all four replicates, experimental treatment significantly predicted time to death (P < 0.0001). Insulin-fed mosquitoes had reduced survivorship compared to buffer-fed controls (Fig. 4A), a finding consistent with previous observations (31). As expected, mosquitoes fed HK-bacteria had significantly reduced survivorship compared to both buffer-fed and insulin-fed controls (Fig. 4A). Most importantly, the increased survivorship of mosquitoes fed HK-bacteria+insulin relative to those fed HK-bacteria alone was significant when analyzed separately with a proportional hazards model that accounted for variation among replicates (Fig. 4B, combined results). These data suggested that human insulin reverses the life span reductions normally associated with immune activation.
Fig 4.
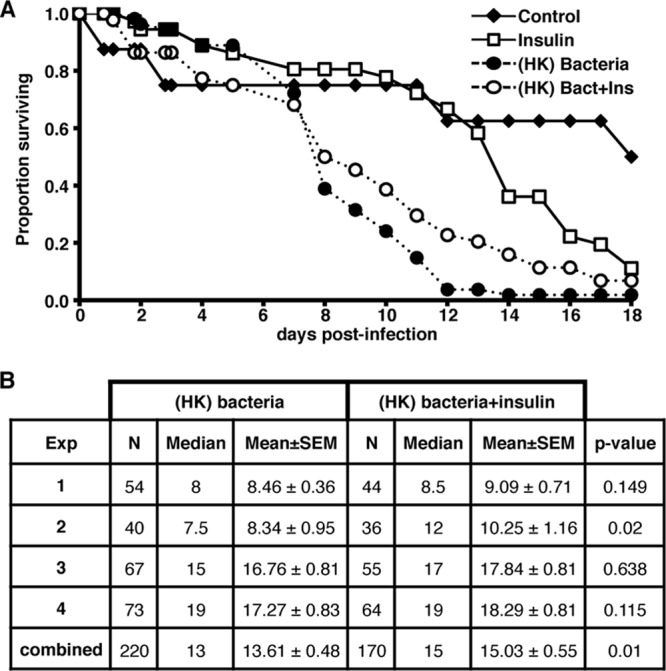
Provision of human insulin increases survival of female A. stephensi fed heat-killed bacteria (HK-bacteria). Approximately 50 3- to 5-day-old female A. stephensi were fed a single blood meal containing PBS as a control, 170 pM human insulin, HK-bacteria, or HK-bacteria+human insulin. All mosquitoes were provided 10% sugar ad libitum after blood feeding. Daily mortality for each treatment was recorded, and dead mosquitoes were removed and counted daily for 18 days. (A) A representative survivorship curve is shown. Life span experiments were replicated four times with separate cohorts of mosquitoes. (B) Summary of sample sizes, medians, means, and statistical significance comparing mosquitoes fed HK-bacteria versus HK-bacteria+insulin.
Human insulin decreases P. falciparum-induced antimicrobial promoter activity in mosquito cells.
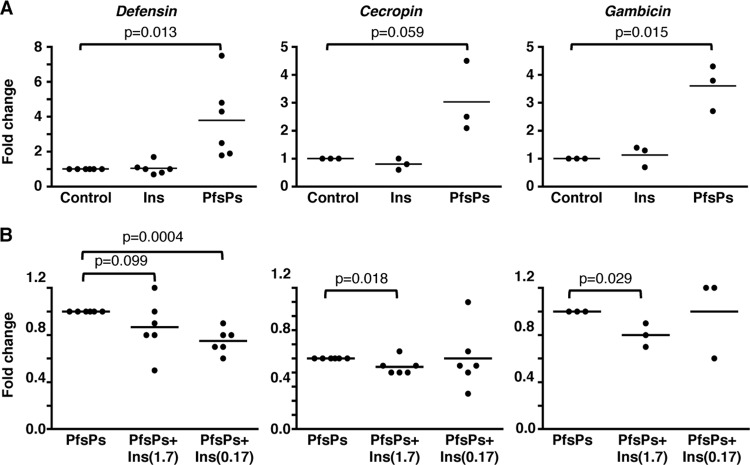
Insulin-induced PI3K activation can alter A. stephensi responsiveness to LPS and to HK-bacteria. However, the obvious question for this important disease vector is whether insulin can alter mosquito responsiveness to malarial soluble products and to parasite infection. To address the first part of this question, we developed a magnetic purification method for the enrichment and isolation of soluble P. falciparum parasite products (PfsPs) based on the work of Kim et al. (33). We utilized PfsPs to mimic the mosquito immune response in vitro to the exclusively extracellular parasites that are normally present during development in the mosquito midgut. For these studies, ASE cells were transfected with AMP promoter-reporter plasmids and 24 h later challenged with 150 × 106 parasite equivalents of PfsPs alone, with PfsPs plus 1.7 or 0.17 μM human insulin, or with an equivalent volume of buffer as a control. Under our conditions, female A. stephensi ingest 375 to 825,000 parasites (10% parasitemia) in a midgut volume of approximately 1 to 2 μl (37), which is comparable to 300,000 PfsP parasite equivalents per μl for these assays. We found that stimulation of transfected cells by PfsPs significantly increased the activity levels of all three AMP promoters compared to buffer controls (Fig. 5A). No increase in promoter activity was observed, however, with human insulin alone (Fig. 5A). In contrast, ASE cells stimulated with PfsPs plus human insulin exhibited a significant decrease in Def, Cec, and Gam promoter activity levels compared to cells stimulated with PfsPs alone (Fig. 5B). Together with our LPS and HK-bacteria results, these data indicated that human insulin can downregulate both bacterium-specific and malaria-specific NF-κB-dependent innate immune responses in A. stephensi cells.
Fig 5.
Antimicrobial promoter activity is increased by stimulation with P. falciparum soluble products, and this activity is decreased by human insulin in immortalized mosquito cells. ASE cells were transfected with Defensin, Cecropin, or Gambicin luciferase promoter-reporter plasmid constructs and stimulated 24 h later with 150 × 106 parasite equivalents of P. falciparum soluble products (PfsPs), 1.7 μM human insulin, or PfsPs plus 1.7 or 0.17 μM insulin. The luciferase activity (relative light units [RLU]) was normalized to untreated controls. The graphs show the fold change relative to untreated controls (A) or PfsPs-treated cells (B). Horizontal lines represent means from three to six independent experiments. Pairwise comparisons of treatments were analyzed by using the Student t test; the P values are indicated.
Inhibition of PI3K signaling in insulin-fed A. stephensi results in decreased P. falciparum development.
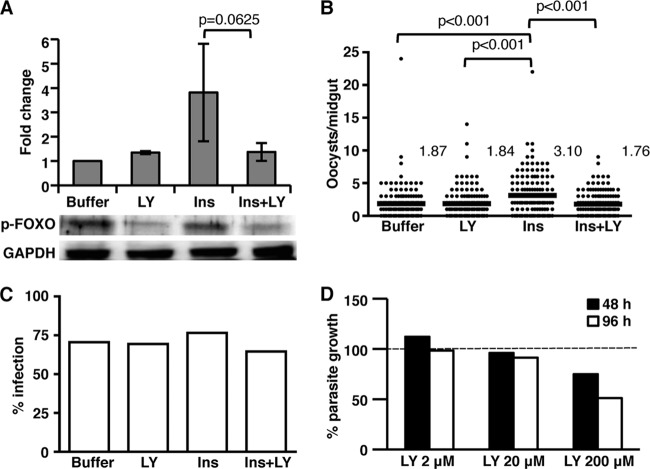
To confirm that LY294002 could inhibit PI3K activity in vivo, we examined p-FOXO protein levels in A. stephensi fed a meal containing PBS as a control, 20 μM LY294002, 170 pM human insulin, or insulin+LY294002. Similar to our in vitro results (Fig. 2B), mosquitoes fed insulin+LY294002 exhibited reduced levels of p-FOXO compared to those fed insulin alone (Fig. 6A), demonstrating that LY294002 can inhibit PI3K activity in the mosquito. Our data suggest that insulin acts through PI3K-dependent signaling to inhibit the mosquito immune response and enhance P. falciparum development (50). Thus, we hypothesized that inhibition of insulin-induced PI3K signaling in mosquitoes fed an infectious blood meal would recover the innate immune response and reduce P. falciparum development. To test this hypothesis, we fed A. stephensi an infectious blood meal containing 20 μM LY294002, 170 pM human insulin, insulin+LY294002, or an equivalent volume PBS added to the blood meal as a control. As expected, mosquitoes fed insulin had higher oocyst numbers than controls (Fig. 6B). However, A. stephensi fed insulin in the presence of the PI3K inhibitor LY294002 exhibited significantly lower oocyst development compared to those fed only insulin (Fig. 6B). Indeed, addition of the inhibitor to insulin-fed mosquitoes reduced oocyst infection levels to that of buffer and inhibitor controls. There were no significant differences in infection prevalence among control and treatment groups (Fig. 6C).
Fig 6.
Inhibition of PI3K signaling in insulin-fed A. stephensi results in decreased P. falciparum development. (A) Mosquitoes were fed a meal of saline or washed RBCs supplemented with PBS as a control (buffer), 20 μM LY294002 (LY), 170 pM human insulin (Ins), or insulin+LY294002 (ins+LY). The graphs represent the fold change of phosphorylated FOXO (p-FOXO) normalized to untreated controls (n = 4). GAPDH provided an assessment of protein loading. The data were not normally distributed; therefore, pairwise comparisons of treatments were analyzed by Wilcoxon test (P values are shown). A representative Western blot of p-FOXO and GAPDH is shown. (B) Mosquitoes were fed with P. falciparum-infected RBCs supplemented with PBS (buffer) as a control, 20 μM LY294002 (LY), 170 pM human insulin (Ins), or insulin+LY294002 (Ins+LY). The data from three independent experiments with separate cohorts of mosquitoes were analyzed for the main effects of experiment and treatment. Horizontal lines indicate the means for three combined sets of 50 mosquitoes (150 mosquitoes total) per treatment group. The data were not normally distributed and, therefore, analyzed using the Kruskal-Wallis test and Dunn's post test. Significant differences between treatment groups are indicated. (C) The prevalence of infection (mosquitoes with at least one P. falciparum oocyst) is shown as a percentage of the dissected mosquitoes (n = 3). No significant differences were noted. (D) P. falciparum cultures were incubated with 2, 20, or 200 μM LY294002. The graph represents the average relative growth compared to DMSO controls at 48 h (□) or 96 h (■) (n = 2).
Vaid et al. (53a) reported that P. falciparum possesses an ortholog of PI3K, whose activity is essential for parasite growth within RBCs. An alternative explanation for our results, therefore, is that LY294002 ingested in an infectious blood meal inhibits parasite development in the mosquito by directly altering parasite PI3K activity that may be necessary for parasite development. To test whether LY294002 could directly affect parasite growth under our conditions, we examined the effects of increasing concentrations of LY294002 on the growth of synchronized asexual-stage P. falciparum parasites in vitro. Although the highest concentration of LY294002 (200 μM) appeared to have a nonspecific cytotoxic effect, the concentration used in our feeding studies (20 μM) had no significant effect on parasite growth at either 48 or 96 h (Fig. 6D).
DISCUSSION
The provision of physiological concentrations of human insulin in a blood meal, and subsequent activation of the IIS pathway, enhance P. falciparum development in A. stephensi (50). In addition to the metabolic role of IIS activation, the IIS cascade can broadly regulate the innate immune responses of a variety of organisms (reviewed in reference 38) and notably inhibits TNF-α- and LPS-induced activation of mammalian NF-κB-dependent immune responses (11, 28). Mosquito NF-κB-dependent immune responses control malaria parasite infection via activation of the Toll and/or Imd signaling pathways (reviewed in reference 8), suggesting that NF-κB-dependent signaling could be similarly targeted by insulin action in the mosquito host. In support of this observation, we confirmed that human insulin downregulates NF-κB-dependent immune responses in A. stephensi cells to both bacterial (Fig. 1) and parasite (Fig. 5) soluble products. In the mosquito host, human insulin reversed the physiological cost of responding to heat-killed bacteria (Fig. 4), while blocking PI3K-dependent signaling reversed the effects of human insulin on P. falciparum development (Fig. 6). Hence, our data confirmed that human insulin activates the mosquito IIS to downregulate LPS- and parasite-induced NF-κB-dependent innate immune responses in the midgut epithelium, a tissue that is critical for parasite development.
Invertebrate immunity can be induced with noninfectious agents such as pathogen membrane components, microscopic beads, or HK-bacteria (reviewed in references 17 and 46). Inducing immunity in this manner negates the effects live pathogens have on host mortality. Immune induction alone can reduce survival and reproduction (14, 42, 47). Therefore, we used HK-bacteria to examine the negative effects of immune induction on mosquito life span in the absence of an ongoing, persistent infection. In agreement with previous studies in other systems (14, 42, 47), we observed that immune induction was costly: mosquitoes fed HK-bacteria alone died significantly faster than controls (Fig. 4). Further, mosquitoes that were provided with a blood meal containing HK-bacteria and insulin exhibited increased survival relative to those fed HK-bacteria alone (Fig. 4), suggesting that insulin signaling attenuates the physiologically costly mosquito immune response in vivo. However, the effects of insulin are pleiotropic and can impact a broad range of cell functions, including the storage and utilization of nutrients. We cannot exclude the possibility, therefore, that immune-independent signaling effects, such as those observed in Mycobacterium-infected D. melanogaster (16), contribute to the insulin-dependent increase in survival observed in our life span studies (Fig. 4).
To identify the IIS cascade components responsible for the immunosuppressive action of insulin, we demonstrated that mosquitoes fed an infectious blood meal containing human insulin and an inhibitor of PI3K (LY294002) had significantly fewer parasites compared to those fed insulin alone (Fig. 6B). These data confirmed that human insulin signals through PI3K to inhibit the mosquito innate immune response to malaria parasites, an effect that is clearly analogous to the inhibitory effects of insulin-induced PI3K activation on proinflammatory markers in mammalian cells (28, 29, 36). PI3Ks are a conserved family of signal transduction enzymes that are involved a wide variety of cellular processes, including inflammation (reviewed in reference 5). In particular, PI3K-dependent signaling has been shown to suppress NF-κB signaling by attenuating the degradation of the negative regulators of NF-κB in mammalian cells (10, 21). Two negative regulators of NF-κB, Cactus and Caspar (40), have been identified in mosquitoes, and the anti-inflammatory effects of human insulin may be mediated through inhibition of the degradation of these. Activated PI3K can also decrease proinflammatory MAPK and Toll-like receptor signaling pathways in mammals (22, 24). Given that mosquitoes possess orthologous MAPK signaling cascades (26) as well as Toll signaling orthologs (reviewed in reference 27), inhibition of these pathways by PI3K signaling could enhance the anti-inflammatory effect of human insulin on mosquito immunity.
Studies in mammals have suggested that distinct temporal activation patterns of the two IIS branches—MEK/ERK and PI3K/Akt—differentially regulate NF-κB signaling (10, 24, 28). We previously showed that human insulin can activate both branches of the IIS pathway (50). However, the kinetics of this response remained unclear until the present studies. We found that whereas p-ERK activation peaked at 5 min and declined rapidly by 30 m, p-FOXO levels were maintained to 24 h after insulin treatment (Fig. 3). These results suggested that PI3K/Akt signaling, and the associated downstream inhibitory effects on the mosquito immune response, are sustained after insulin stimulation. In the context of P. falciparum development, this insulin-induced signaling persistence would extend from zygote and ookinete development to the early invasion of the midgut epithelium, thereby protecting developing parasites during a broad period of critical morphogenesis.
In previous work, we showed that P. falciparum infection of A. stephensi can be completely inhibited by the overexpression of activated Akt in the midgut epithelium (9), an outcome that initially appears to be at odds with the effects of insulin-induced activation of PI3K/Akt presented here. However, Akt can interact with a variety of proteins that both regulate its activity and allow for stimulus-specific differences in its substrate utilization (reviewed in reference 19). Therefore, we would not expect the overexpression of constitutively active Akt in transgenic mosquitoes to replicate the ligand-specific effects of insulin stimulation in nontransgenic mosquitoes. Certainly, insulin-induced activation of MEK/ERK (Fig. 3) and p38 MAPK activation (26) would not contribute to the phenotype of Akt-overexpressing transgenic A. stephensi since these signaling molecules are not downstream of Akt. Finally, our observations regarding the differences between overexpression of a signaling regulator versus signaling by a ligand are not unique. The overexpression of antioxidant genes, purported to extend life span, in fact reduce the life span of D. melanogaster in the absence of oxidative stress (3). Hence, functional biology is often only revealed when ligand and signaling are connected.
By 2030, one in five adults will have type 2 diabetes in Africa (53). Our data, combined with these predictions, suggest that hyperinsulinemia in diabetics could reach levels that would be predicted to inhibit the mosquito immune response to P. falciparum, thereby increasing the ability of mosquitoes to transmit malaria. These interactions could increase the spread of malaria infection in populations where this comorbidity is increasing in prevalence and where malaria transmission is already a significant problem. Our observations suggest that a critical component of future control strategies for both of these diseases will be based on understanding the effects of hyperinsulinemia and clinical interventions on the relative risk of parasite infection and transmission by Anopheles mosquitoes.
ACKNOWLEDGMENTS
We thank Edwin E. Lewis for statistical analyses and Andrew Ross for technical assistance in completing these studies. We also thank Joseph L. DeRisi and Charles C. Kim for the kind gift of the magnetic purification stand.
Funding for these studies was provided by NIH NIAID R01 AI080799 and R01 AI073745.
Footnotes
Published ahead of print 2 April 2012
REFERENCES
- 1. Ananieva O, et al. 2008. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat. Immunol. 9:1028–1036 [DOI] [PubMed] [Google Scholar]
- 2. Barillas-Mury C, et al. 1996. Immune factor Gambif1, a new rel family member from the human malaria vector, Anopheles gambiae. EMBO J. 15:4691–4701 [PMC free article] [PubMed] [Google Scholar]
- 3. Bayne AC, Mockett RJ, Orr WC, Sohal RS. 2005. Enhanced catabolism of mitochondrial superoxide/hydrogen peroxide and aging in transgenic Drosophila. Biochem. J. 391:277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reference deleted.
- 5. Cantley LC. 2002. The phosphoinositide 3-kinase pathway. Science 296:1655–1657 [DOI] [PubMed] [Google Scholar]
- 6. Cargnello M, Roux PP. 2011. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 75:50–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Christophides GK, et al. 2002. Immunity-related genes and gene families in Anopheles gambiae. Science 298:159–165 [DOI] [PubMed] [Google Scholar]
- 8. Cirimotich CM, Dong Y, Garver LS, Sim S, Dimopoulos G. 2010. Mosquito immune defenses against Plasmodium infection. Dev. Comp. Immunol. 34:387–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corby-Harris V, et al. 2010. Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes. PLoS Pathog. 6:e1001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cuschieri J, Bulger E, Grinsell R, Garcia I, Maier RV. 2008. Insulin regulates macrophage activation through activin A. Shock 29:285–290 [DOI] [PubMed] [Google Scholar]
- 11. Dandona P, et al. 2001. Insulin inhibits intranuclear nuclear factor κB and stimulates IκB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J. Clin. Endocrinol. Metab. 86:3257–3265 [DOI] [PubMed] [Google Scholar]
- 12. Reference deleted.
- 13. Darby SM, Miller ML, Allen RO, LeBeau M. 2001. A mass spectrometric method for quantitation of intact insulin in blood samples. J. Anal. Toxicol. 25:8–14 [DOI] [PubMed] [Google Scholar]
- 14. DeVeale B, Brummel T, Seroude L. 2004. Immunity and aging: the enemy within? Aging Cell 3:195–208 [DOI] [PubMed] [Google Scholar]
- 15. DiAngelo JR, Bland ML, Bambina S, Cherry S, Birnbaum MJ. 2009. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc. Natl. Acad. Sci. U. S. A. 106:20853–20858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dionne MS, Pham LN, Shirasu-Hiza M, Schneider DS. 2006. Akt and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Curr. Biol. 16:1977–1985 [DOI] [PubMed] [Google Scholar]
- 17. Dionne MS, Schneider DS. 2008. Models of infectious diseases in the fruit fly Drosophila melanogaster. Dis. Model Mech. 1:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dong Y, et al. 2006. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Franke TF. 2008. PI3K/Akt: getting it right matters. Oncogene 27:6473–6488 [DOI] [PubMed] [Google Scholar]
- 20. Frolet C, Thoma M, Blandin S, Hoffmann JA, Levashina EA. 2006. Boosting NF-κB-dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity 25:677–685 [DOI] [PubMed] [Google Scholar]
- 21. Fukao T, Koyasu S. 2003. PI3K and negative regulation of TLR signaling. Trends Immunol. 24:358–363 [DOI] [PubMed] [Google Scholar]
- 22. Fukao T, et al. 2002. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat. Immunol. 3:875–881 [DOI] [PubMed] [Google Scholar]
- 23. Garver LS, Dong Y, Dimopoulos G. 2009. Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog. 5:e1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guha M, Mackman N. 2002. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J. Biol. Chem. 277:32124–32132 [DOI] [PubMed] [Google Scholar]
- 25. Hillyer JF, Estevez-Lao TY. 2010. Nitric oxide is an essential component of the hemocyte-mediated mosquito immune response against bacteria. Dev. Comp. Immunol. 34:141–149 [DOI] [PubMed] [Google Scholar]
- 26. Horton AA, et al. 2011. The mitogen-activated protein kinome from Anopheles gambiae: identification, phylogeny and functional characterization of the ERK, JNK and p38 MAP kinases. BMC Genomics 12:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Imler JL, Zheng L. 2004. Biology of Toll receptors: lessons from insects and mammals. J. Leukoc. Biol. 75:18–26 [DOI] [PubMed] [Google Scholar]
- 28. Iwasaki Y, et al. 2009. Insulin exhibits short-term anti-inflammatory but long-term proinflammatory effects in vitro. Mol. Cell Endocrinol. 298:25–32 [DOI] [PubMed] [Google Scholar]
- 29. Jeschke MG, Klein D, Herndon DN. 2004. Insulin treatment improves the systemic inflammatory reaction to severe trauma. Ann. Surg. 239:553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reference deleted.
- 31. Kang MA, Mott TM, Tapley EC, Lewis EE, Luckhart S. 2008. Insulin regulates aging and oxidative stress in Anopheles stephensi. J. Exp. Biol. 211:741–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karl S, Davis TM, St Pierre TG. 2010. Parameterization of high magnetic field gradient fractionation columns for applications with Plasmodium falciparum infected human erythrocytes. Malaria J. 9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim CC, Wilson EB, DeRisi JL. 2010. Improved methods for magnetic purification of malaria parasites and haemozoin. Malaria J. 9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reference deleted.
- 35. Lambros C, Vanderberg JP. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418–420 [PubMed] [Google Scholar]
- 36. Leffler M, et al. 2007. Insulin attenuates apoptosis and exerts anti-inflammatory effects in endotoxemic human macrophages. J. Surg. Res. 143:398–406 [DOI] [PubMed] [Google Scholar]
- 37. Lim J, Gowda DC, Krishnegowda G, Luckhart S. 2005. Induction of nitric oxide synthase in Anopheles stephensi by Plasmodium falciparum: mechanism of signaling and the role of parasite glycosylphosphatidylinositols. Infect. Immun. 73:2778–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luckhart S, Riehle MA. 2007. The insulin signaling cascade from nematodes to mammals: insights into innate immunity of Anopheles mosquitoes to malaria parasite infection. Dev. Comp. Immunol. 31:647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Luna C, et al. 2006. Expression of immune responsive genes in cell lines from two different anopheline species. Insect Mol. Biol. 15:721–729 [DOI] [PubMed] [Google Scholar]
- 40. Meister S, et al. 2005. Immune signaling pathways regulating bacterial and malaria parasite infection of the mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A. 102:11420–11425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meredith JM, et al. 2006. A novel association between clustered NF-κB and C/EBP binding sites is required for immune regulation of mosquito Defensin genes. Insect Mol. Biol. 15:393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moret Y, Schmid-Hempel P. 2000. Survival for immunity: the price of immune system activation for bumblebee workers. Science 290:1166–1168 [DOI] [PubMed] [Google Scholar]
- 43. Pakpour N, Cheung KW, Souvannaseng L, Concordet JP, Luckhart S. 2010. Transfection and mutagenesis of target genes in mosquito cells by locked nucleic acid-modified oligonucleotides. J. Vis. Exp. pii:e2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reference deleted.
- 45. Richman AM, Dimopoulos G, Seeley D, Kafatos FC. 1997. Plasmodium activates the innate immune response of Anopheles gambiae mosquitoes. EMBO J. 16:6114–6119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schmid-Hempel P. 2005. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 50:529–551 [DOI] [PubMed] [Google Scholar]
- 47. Schmid-Hempel P. 2005. Natural insect host-parasite systems show immune priming and specificity: puzzles to be solved. Bioessays 27:1026–1034 [DOI] [PubMed] [Google Scholar]
- 48. Sinden RE. 1999. Plasmodium differentiation in the mosquito. Parasitologia 41:139–148 [PubMed] [Google Scholar]
- 49. Son MH, Kang KW, Lee CH, Kim SG. 2001. Potentiation of cadmium-induced cytotoxicity by sulfur amino acid deprivation through activation of extracellular signal-regulated kinase1/2 (ERK1/2) in conjunction with p38 kinase or c-jun N-terminal kinase (JNK): complete inhibition of the potentiated toxicity by U0126 an ERK1/2 and p38 kinase inhibitor. Biochem. Pharmacol. 62:1379–1390 [DOI] [PubMed] [Google Scholar]
- 50. Surachetpong W, Pakpour N, Cheung KW, Luckhart S. 2011. Reactive oxygen species-dependent cell signaling regulates the mosquito immune response to Plasmodium falciparum. Antioxid. Redox Signal. 14:943–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Surachetpong W, Singh N, Cheung KW, Luckhart S. 2009. MAPK ERK signaling regulates the TGF-β1-dependent mosquito response to Plasmodium falciparum. PLoS Pathog. 5:e1000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Traore K, Sharma RB, Burek CL, Trush MA. 2007. Role of ROS and MAPK in TPA-induced ICAM-1 expression in the myeloid ML-1 cell line. J. Cell Biochem. 100:1010–1021 [DOI] [PubMed] [Google Scholar]
- 53. Unwin N, Gan D, Whiting D. 2010. The IDF Diabetes Atlas: providing evidence, raising awareness, and promoting action. Diabetes Res. Clin. Pract. 87:2–3 [DOI] [PubMed] [Google Scholar]
- 53a. Vaid A, Ranjan R, Smythe WA, Hoppe HC, Sharma P. 2010. PfPI3K, a phosphatidylinositol-3 kinase from Plasmodium falciparum, is exported to the host erythrocyte and is involved in hemoglobin trafficking. Blood 115:2500–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vizioli J, et al. 2000. Cloning and analysis of a cecropin gene from the malaria vector mosquito, Anopheles gambiae. Insect Mol. Biol. 9:75–84 [DOI] [PubMed] [Google Scholar]
- 55. Vizioli J, et al. 2001. Gambicin: a novel immune responsive antimicrobial peptide from the malaria vector Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A. 98:12630–12635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. White NJ, et al. 1983. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N. Engl. J. Med. 309:61–66 [DOI] [PubMed] [Google Scholar]
- 57. Whitten MM, Shiao SH, Levashina EA. 2006. Mosquito midguts and malaria: cell biology, compartmentalization and immunology. Parasite Immunol. 28:121–130 [DOI] [PubMed] [Google Scholar]
- 58. World Health Organization 2008. World malaria report 2008. World Health Organization, Geneva, Switzerland [Google Scholar]