Abstract
Natural killer T (NKT) cells are known to play a protective role in the immune responses of mice against a variety of infectious pathogens. However, little is known about the detailed information of NKT cells in patients with Mycobacterium tuberculosis infection. The aims of this study were to examine NKT cell levels and functions in patients with active M. tuberculosis infection, to investigate relationships between NKT cell levels and clinical parameters, and to determine the mechanism responsible for the poor response to α-galactosylceramide (α-GalCer). NKT cell levels were significantly lower in the peripheral blood of pulmonary tuberculosis and extrapulmonary tuberculosis patients, and the proliferative responses of NKT cells to α-GalCer were also lower in patients, whereas NKT cell levels and responses were comparable in latent tuberculosis infection subjects and healthy controls. Furthermore, this NKT cell deficiency was found to be correlated with serum C-reactive protein levels. In addition, the poor response to α-GalCer in M. tuberculosis-infected patients was found to be due to increased NKT cell apoptosis, reduced CD1d expression, and a defect in NKT cells. Notably, M. tuberculosis infection was associated with an elevated expression of the inhibitory programmed death-1 (PD-1) receptor on NKT cells, and blockade of PD-1 signaling enhanced the response to α-GalCer. This study shows that NKT cell levels and functions are reduced in M. tuberculosis-infected patients and these deficiencies were found to reflect the presence of active tuberculosis.
INTRODUCTION
Tuberculosis (TB) remains one of the infectious causes of illness with high morbidity and mortality in humans. In 2009, ∼9.4 million new cases of active infection were diagnosed and the disease was responsible for roughly 1.7 million deaths (48). Although 32% of the world's population has been estimated to be currently infected by Mycobacterium tuberculosis, the majority are latently infected and remain healthy (1, 14). Nevertheless, approximately 5 to 10% of individuals infected with M. tuberculosis will eventually develop active disease. In addition, the risk of developing active tuberculosis is increased by a variety of immunocompromised conditions, such as HIV infection, diabetes, renal failure, immunosuppressant treatment, and malnutrition (29). Accordingly, host immune status might play an important role in the control of progression to active disease. Furthermore, emerging evidence indicates that cellular immunity to M. tuberculosis requires the coordinated responses of the innate and adaptive immune systems (23, 37).
Human natural killer T (NKT) cells are a distinct subset of T lymphocytes and are characterized by the restricted expression of an invariant Vα24-Jα18 T cell receptor (TCR) chain paired with the Vβ11 TCR chain. The pair of TCR chains recognizes glycolipid antigens, such as α-galactosylceramide (α-GalCer), presented by the major histocompatibility complex (MHC) class I-like molecule CD1d (20). In addition, NKT cells play a bridging role between innate and adaptive immunity due to their ability to activate a variety of innate and adaptive immune cells, such as dendritic cells, monocytes, natural killer (NK) cells, T cells, and B cells, by rapidly producing large amounts of Th1 and Th2 cytokines, like gamma interferon (IFN-γ) and interleukin-4 (IL-4) (45). Furthermore, NKT cells are known to serve as regulatory and/or effector cells and are implicated in a broad range of diseases, including autoimmunity, cancer, and infectious diseases (19, 47).
NKT cells are known to play a protective role in the immune responses of mice against a variety of infectious pathogens, including bacteria, viruses, and protozoan parasites, although some of these results are controversial (18, 28). More specifically, several studies have shown that murine or human NKT cells may mediate protection against M. tuberculosis (4, 17, 37, 38, 46). For example, it was demonstrated in a recent study that α-GalCer administration, alone or in combination with classic chemotherapy, can improve the clinical outcomes of M. tuberculosis infection in mice (38). It has also been shown that α-GalCer incorporation into bacillus Calmette-Guérin vaccine enhances the host immune response by modulating T cell priming via murine NKT cell activation (46). However, much less is known about human NKT cell levels and functions in patients with M. tuberculosis infection. A numerical deficiency of NKT cells has been found in pulmonary tuberculosis (PTB) patients (33, 40, 42), but the NKT cell level has not been determined in extrapulmonary tuberculosis (EPTB) patients. Furthermore, the response of NKT cells to α-GalCer has not previously been investigated in TB patients, and the clinical relevance of NKT cell level in peripheral blood remains to be clarified.
The aims of the present study were to examine NKT cell levels and proliferative responses to α-GalCer in the peripheral blood of PTB and EPTB patients, latent tuberculosis infection (LTBI) subjects, and healthy controls (HCs), to investigate potential relationships between NKT cell levels and the clinical parameters of active TB. In addition, we sought to determine the mechanism responsible for the poor response to α-GalCer in TB patients.
MATERIALS AND METHODS
Study subjects.
The study cohort was composed of 73 patients with active TB (55 PTB patients and 18 EPTB patients), 13 LTBI subjects, and 90 age- and sex-matched HCs. The clinical and laboratory characteristics of patients and HCs are summarized in Table 1. On the basis of the guidelines of the American Thoracic Society and the U.S. Centers for Disease Control and Prevention, active TB was diagnosed and classified into PTB and EPTB according to infected sites (2). LTBI was defined using the following criteria: (i) no clinical or radiographic evidence of an active TB infection, (ii) known exposure to an individual with an active TB infection, and (iii) positivity for tuberculin skin test or IFN-γ release assay at the time of sample collection (2, 31). All HCs met the following criteria: (i) no known exposure to any individual with an active TB infection, (ii) no symptoms of TB infection, (iii) no detectable tuberculin skin test or IFN-γ release assay reactivity, and (iv) no history of an autoimmune disease, an infectious disease, a malignancy, or immunosuppressive therapy (2, 40). The study was approved by the Institutional Review Board of Chonnam National University Hospital, and written informed consent was obtained from all participants.
Table 1.
Clinical and laboratory characteristics of the TB patients and HCs
| Characteristic | HC | LTBI | EPTB | PTB |
|---|---|---|---|---|
| Total no. | 90 | 13 | 18 | 55 |
| Sex (no. male/no. female) | 46/44 | 6/7 | 9/9 | 35/20 |
| Mean ± SD age (yr) | 54.6 ± 13.0 | 47.9 ± 9.8 | 50.9 ± 18.1 | 55.2 ± 20.9 |
| No. (%) with previous TB medication | ||||
| No | NDa | ND | 17 (94.4) | 44 (80) |
| Yes | ND | ND | 1 (5.5) | 11 (20) |
| No. (%) with positive sputum AFBb smear | ND | ND | 0 | 35 (63.6) |
| No. (%) with cavity on chest radiograph | ND | ND | 0 | 19 (34.5) |
| No. (%) with the following extent of pulmonary disease: | ||||
| Stage 1 (segmental disease) | ND | ND | 0 | 4 (7.3) |
| Stage 2 (lobar disease) | ND | ND | 0 | 13 (23.6) |
| Stage 3 (bi/trilobar disease) | ND | ND | 0 | 10 (18.2) |
| Stage 4 (bilateral disease) | ND | ND | 0 | 28 (50.9) |
| No. (%) with the following sites of EPTB: | ||||
| Lymph node | ND | ND | 6 (33.3) | 0 (0) |
| Bone and joint | ND | ND | 8 (33.3) | 4 (7.3) |
| Pericardium | ND | ND | 1 (5.6) | 1 (1.8) |
| Peritoneum | ND | ND | 1 (5.6) | 2 (3.6) |
| Pharynx | ND | ND | 1 (5.6) | 0 (0) |
| Pleura | ND | ND | 1 (5.6) | 0 (0) |
| Mean ± SD hemoglobin concn (mg/dl) | 14.0 ± 1.2 | 13.8 ± 1.4 | 11.6 ± 2.3 | 12.0 ± 1.7 |
| Mean ± SD leukocyte count (cells/μl) | 5,592 ± 1,457 | 6,885 ± 1,485 | 5,758 ± 2,046 | 7,669 ± 3,014 |
| Mean ± SD lymphocyte count (cells/μl) | 2,041 ± 719 | 2,102 ± 526 | 1,422 ± 590 | 1,389 ± 666 |
| Mean ± SD platelet count (103 cells/μl) | 243 ± 58 | 228 ± 43 | 242 ± 96 | 297 ± 124 |
| Mean ± SD CRPc concn (mg/dl) | ND | ND | 2.05 ± 2.38 | 5.21 ± 5.42 |
ND, not done.
AFB, acid-fast bacilli.
CRP, C-reactive protein.
MAbs and flow cytometry.
The following monoclonal antibodies (MAbs) and reagents were used in this study: fluorescein isothiocyanate (FITC)-conjugated MAbs against CD3, CD4, and CD14; FITC-conjugated annexin V; phycoerythrin (PE)-conjugated MAbs against NKT cell TCR (clone 6B11) and CD1d; peridinin chlorophyll-a protein (PerCP)-conjugated anti-CD3 MAb; allophycocyanin (APC)-conjugated MAbs against CD3 and CD8; and 7-aminoactinomycin D (AAD) (all were from BD Biosciences, San Diego, CA) and PE-conjugated anti-human programmed death-1 (PD-1) MAb (eBiosciences, San Diego, CA). Cells were stained with combinations of appropriate MAbs at 4°C for 20 min and analyzed on a FACSCalibur flow cytometer using Cell Quest software (BD Biosciences, Mountain View, CA).
Isolation of PBMCs, monocytes, and T cells and identification of NKT cells.
Peripheral venous blood samples were collected in tubes containing heparin. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll-Paque Plus solution (Amersham Bioscience, Uppsala, Sweden), and monocytes and T cells were purified from PBMCs by magnetically activated cell sorting (MACS) as previously described (9, 36). NKT cells were identified phenotypically as CD3+ 6B11+ cells by flow cytometry (3, 9, 16, 34, 43).
NKT cell proliferation assays.
The proliferative abilities of NKT cells were assayed by flow cytometry as previously described (9). Briefly, freshly isolated PBMCs were suspended in complete medium (RPMI 1640, 2 mmol/liter l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin) supplemented with 10% fetal bovine serum (Gibco BRL, Grand Island, NY), seeded in a 24-well plate at 1 × 106 cells/well, and cultured for 7 days at 37°C in a 5% CO2 humidified incubator in the presence of 100 U/ml of IL-2 (BD PharMingen, San Jose, CA) and 100 ng/ml of α-GalCer (Alexis Biochemicals, Lausen, Switzerland) or 0.1% dimethyl sulfoxide (DMSO; used as a control). Cells were harvested and stained with PerCP-conjugated anti-CD3 and PE-conjugated anti-6B11 MAbs. Percentages of CD3+ 6B11+ NKT cells were determined by flow cytometry on day 0 and after culture on day 7. Proliferation index was defined as the ratio of NKT cell percentage within CD3+ T cells on day 7 to NKT cell percentage within CD3+ T cells on day 0, and indices are referred to as fold increases (see Fig. S1 in the supplemental material for a representative fluorescence-activated cell sorter plot highlighting the gating strategy). To quantify NKT cell death in culture, cells were stained with FITC-conjugated annexin V, PE-conjugated 6B11 MAb, 7-AAD, and APC-conjugated anti-CD3 MAb, as previously described (13, 21). Percentages of apoptotic (annexin V-positive) and necrotic (7-AAD-positive) NKT cells were measured by flow cytometry on days 0 and 7. In some experiments, anti-PD-L1 and anti-PD-L2 Abs (5 μg/ml each; eBiosciences, San Diego, CA) or purified mouse IgG1 (used as an isotype control; 10 μg/ml; eBiosciences, San Diego, CA) was added to the wells to block the interaction between PD-1 and its ligands and then cultured for 14 days.
Coculture of APCs and T cells with α-GalCer.
CD14+ monocytes were purified from PBMCs by MACS and used as a source of antigen-presenting cells (APCs). CD3+ T cells were also isolated from the remaining PBMCs by MACS. APCs (1 × 105 cells/well) and T cells (9 × 105 cells/well) were seeded in a 24-well plate and cultured for 7 days in the presence of 100 U/ml of IL-2 and 100 ng/ml of α-GalCer or 0.1% DMSO (control). Cocultures were subdivided into 4 subgroups according to the donor origins of the APCs and T cells: APCs and T cells from the same HCs, APCs from HCs and T cells from TB patients, APCs from TB patients and T cells from HCs, and APCs and T cells from the same TB patients.
Statistical analysis.
Clinical and laboratory characteristics were analyzed descriptively. Spearman's correlation coefficient was used to examine relationships between NKT cell numbers and clinical parameters. Percentages and absolute numbers of NKT cells, proliferation indices, and expression levels of CD1d and PD-1 were compared among groups using the Mann-Whitney U test. The Wilcoxon signed-rank test was used to compare changes in the proliferative responses of NKT cells after blockade of PD-1 signaling with anti-PD-L Abs. All statistical analyses were performed using SPSS (version 17.0) software (SPSS, Chicago, IL), and significance was accepted for P values of <0.05.
RESULTS
Reduced numbers of circulating NKT cells in EPTB and PTB patients.
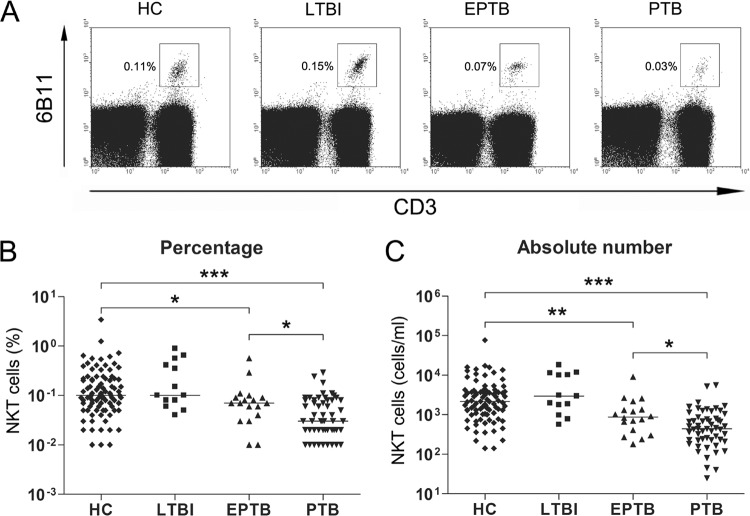
The percentages and absolute numbers of NKT cells in the peripheral blood samples of 55 PTB patients, 18 EPTB patients, 13 LTBI subjects, and 90 age- and sex-matched HCs were determined by flow cytometry. Percentages of NKT cells were significantly lower in EPTB and PTB patients than in HCs (medians, 0.07% and 0.03%, respectively, versus 0.10% [P < 0.05 and P < 0.001, respectively]) (Fig. 1A and B). Absolute NKT cell numbers were calculated by multiplying NKT cell percentages by total lymphocyte numbers per milliliter in peripheral blood. EPTB and PTB patients had significantly lower absolute NKT cell numbers than HCs (medians, 871 and 440 cells/ml, respectively, versus 2,151 cells/ml [P < 0.005 and P < 0.001, respectively]) (Fig. 1C). Reductions in the percentages and absolute numbers of NKT cells were more prominent in PTB than in EPTB patients (medians, 0.03% versus 0.07% [P < 0.05] and 440 cells/ml versus 871 cells/ml [P < 0.05], respectively). However, no significant differences between NKT cell levels in LTBI subjects and HCs were observed.
Fig 1.
Reduced circulating NKT cell numbers in the peripheral blood of EPTB and PTB patients. Freshly isolated PBMCs from 90 age- and sex-matched HCs, 13 LTBI subjects, 18 patients with EPTB, and 55 patients with PTB were stained with PerCP-conjugated anti-CD3 and PE-conjugated anti-6B11 MAbs and then analyzed by flow cytometry. Symbols represent subjects, and horizontal lines are median values. (A) Representative NKT cell percentages determined by flow cytometry; (B) percentages of NKT cells in peripheral blood lymphocytes; (C) absolute numbers of NKT cells in peripheral blood samples (per ml of blood). P values were determined by Mann-Whitney U test. *, P < 0.05; **, P < 0.005; ***, P < 0.001.
Correlation between reductions in NKT cells and CRP in TB patients.
To evaluate the clinical relevance of NKT cell levels in EPTB and PTB patients, we investigated relations between absolute NKT cell numbers in peripheral blood and clinical parameters using Spearman's correlation analysis (Table 2). The analysis showed that absolute NKT cell numbers were significantly correlated with lymphocyte counts and C-reactive protein (CRP) levels (P = 0.002 and P < 0.001, respectively). However, no significant correlations between age, body mass index, smoking, leukocyte counts, or platelet counts and absolute NKT cell numbers were found (Table 2).
Table 2.
Spearman's correlation coefficients for absolute NKT cell numbers with respect to clinical and laboratory findings in 73 TB patients
| Variable | Correlation coefficient (γ) | P value |
|---|---|---|
| Age (yr) | −0.129 | 0.279 |
| Body mass index (kg/m2) | 0.142 | 0.354 |
| Smoking (no. of pack-years) | −0.164 | 0.175 |
| Hemoglobin concn (g/dl) | 0.208 | 0.077 |
| Leukocyte count (cells/μl) | −0.185 | 0.117 |
| Lymphocyte count (cells/μl) | 0.361 | 0.002 |
| Platelet count (103 cells/μl) | −0.075 | 0.531 |
| CRP concn (mg/dl) | −0.428 | <0.001 |
Impaired proliferative response of NKT cells to α-GalCer in EPTB and PTB patients.
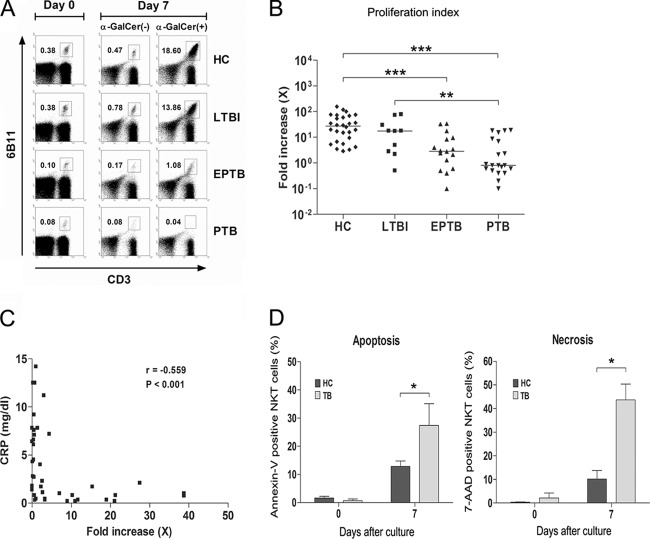
To examine the proliferative effects of α-GalCer on NKT cells, PBMCs from 24 PTB patients, 16 EPTB patients, 9 LTBI subjects, and 30 age- and sex-matched HCs were cocultured with α-GalCer for 7 days in the presence of 100 U/ml of IL-2. Percentages of NKT cells and proliferation indices were evaluated by flow cytometry, as described in Materials and Methods. The percentages of NKT cells among PBMCs from an HC subject markedly increased in response to α-GalCer, from 0.38% on day 0 to 18.6% on day 7. Similarly, NKT cells from an LTBI subject expanded from 0.38% to 13.9% over the same period. In contrast, NKT cells from a PTB patient and an EPTB patient did not proliferate or proliferated only slightly (Fig. 2A). Although the magnitudes of responses to α-GalCer were highly variable among all study subjects, overall proliferation indices were significantly lower in EPTB and in PTB patients than in HCs (medians, 2.9 versus 27.1 [P < 0.001] and 0.8 versus 27.1 [P < 0.001], respectively) (Fig. 2B). However, no significant differences between HCs and LTBI subjects were observed. In addition, we analyzed the relationship between serum CRP levels and NKT proliferation indices in 24 PTB patients and 16 EPTB patients. It was found that the proliferative response to α-GalCer was inversely correlated with CRP level (correlation coefficient [γ] = −0.559, P < 0.001) (Fig. 2C).
Fig 2.
Decreased proliferative response of NKT cells from EPTB and PTB patients to α-GalCer. (A and B) Proliferation indices of NKT cells. Freshly isolated PBMCs (1 × 106 cells/well) from 30 age- and sex-matched HCs, 9 LTBI subjects, 16 patients with EPTB, and 24 patients with PTB were cultured for 7 days in the presence of IL-2 (100 U/ml) and α-GalCer (100 ng/ml) or 0.1% DMSO as a control. The cells were then stained with PerCP-conjugated anti-CD3 and PE-conjugated anti-6B11 MAbs and analyzed by flow cytometry. Proliferation index was defined as the ratio of NKT cell percentage within CD3+ T cells on day 7 to NKT cell percentage within CD3+ T cells on day 0, and indices are expressed as fold increases. The results shown are representative of the experiments (A). Symbols represent subjects, and horizontal lines are median values (B). (C) The relation between serum CRP level and proliferation index in 24 PTB patients and 16 EPTB patients. Serum CRP levels were plotted against the NKT cell proliferation indices. (D) Increased apoptosis of NKT cells in TB patients. Freshly isolated PBMCs from 5 age- and sex-matched HCs and 5 TB patients were cultured for 7 days in the presence of IL-2 (100 U/ml) and α-GalCer (100 ng/ml). NKT cell apoptosis was analyzed by flow cytometry using FITC-conjugated annexin V, PE-conjugated 6B11 MAb, 7-AAD, and APC-conjugated anti-CD3 MAb. Percentages of apoptotic (annexin V-positive) NKT cells and necrotic (7-AAD-positive) NKT cells on days 0 and 7 are shown. Results are presented as means ± SEMs. P values were determined by Mann-Whitney U test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Increased NKT cell death in TB patients.
To determine whether the impaired proliferative response of NKT cells to α-GalCer was due to a loss of NKT cells in culture, PBMCs from 5 TB patients and 5 age- and sex-matched HCs were cultured with α-GalCer for 7 days in the presence of 100 U/ml of IL-2. NKT cell apoptosis was then evaluated by flow cytometry, as described in Materials and Methods. Apoptotic and necrotic percentages of NKT cells were minimal on day 0, and no significant differences between HCs and TB patients were found. However, after being stimulated with α-GalCer for 7 days, NKT cell apoptosis and necrosis were greater for TB patients than for HCs (means, 27.4% versus 12.8% [P < 0.05] and 43.7% versus 10.2% [P < 0.05], respectively) (Fig. 2D). These findings suggest that increased NKT cell apoptosis in culture is related to the impaired proliferative response of NKT cells observed in TB patients.
Reduced expression of CD1d in EPTB and PTB patients.
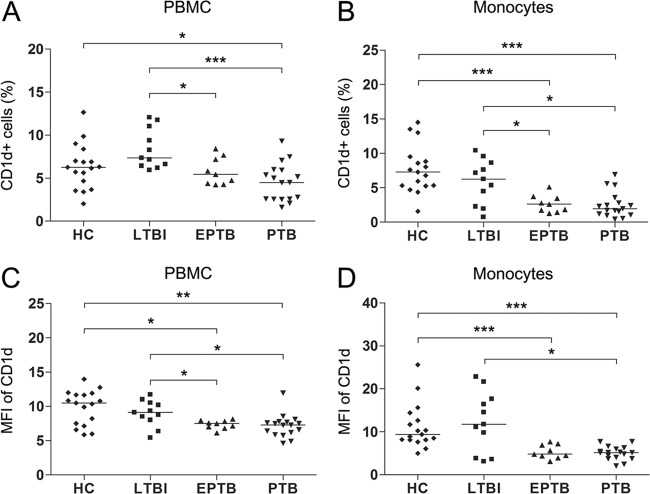
CD1d is known to be required for the presentation of glycolipid antigens to NKT cells. Thus, we hypothesized that the poor response to α-GalCer observed in EPTB and PTB patients might be due to an altered CD1d expression. To determine whether CD1d levels differed in patients and HCs, percentages of CD1d+ cells and mean fluorescence intensities (MFIs) of CD1d were evaluated by flow cytometry in the peripheral blood samples of 17 PTB patients, 9 EPTB patients, 11 LTBI subjects, and 17 age- and sex-matched HCs. The percentages of CD1d+ cells among PBMCs were found to be significantly lower in PTB patients than in HCs (medians, 4.5% versus 6.3% [P < 0.05]) (Fig. 3A). Furthermore, the MFIs of CD1d on PBMCs were also significantly lower in EPTB and in PTB patients than in HCs (medians, 7.5 versus 10.5 [P < 0.05] and 7.3 versus 10.5 [P < 0.01], respectively) (Fig. 3C). However, the percentages of CD1d+ cells and MFIs of CD1d showed no significant differences in LTBI subjects and HCs.
Fig 3.
CD1d expression levels in the peripheral blood samples of HCs, LTBI subjects, EPTB patients, and PTB patients. Freshly isolated PBMCs from 17 age- and sex-matched HCs, 11 LTBI subjects, 9 patients with EPTB, and 17 patients with PTB were stained with PE-conjugated anti-CD1d, FITC-conjugated anti-CD14, and PerCP-conjugated anti-CD3 MAbs and analyzed by flow cytometry. Symbols represent subjects, and horizontal lines are median values. (A and B) Percentages of CD1d+ cells; (C and D) MFIs of CD1d. P values were determined by Mann-Whitney U test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
It has been previously reported that NKT cell expansion is dependent on CD1d-expressing monocytes and that peripheral blood monocytes are effective APCs for NKT cells (9, 15, 41). Thus, we investigated levels of CD1d expression on peripheral blood monocytes in patients and HCs. EPTB and PTB patients were found to have significantly lower percentages of CD1d+ cells than HCs (medians, 2.6% versus 7.3% [P < 0.001] and 2.0% versus 7.3% [P < 0.001], respectively) (Fig. 3B), and the MFIs of CD1d on monocytes were found to be significantly lower in EPTB and in PTB patients than in HCs (medians, 4.8 versus 9.4 [P < 0.001] and 5.2 versus 9.4 [P < 0.001], respectively) (Fig. 3D). However, CD1d levels were similar in LTBI subjects and HCs.
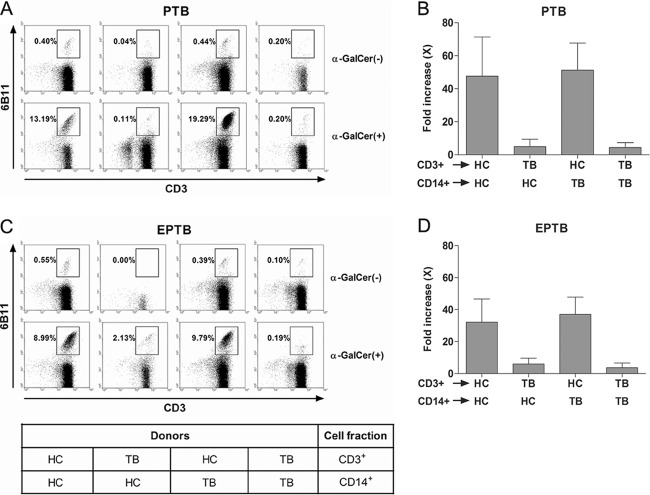
NKT cell dysfunction in EPTB and PTB patients.
To determine whether the poor response of NKT cells to α-GalCer was due to an NKT cell dysfunction, we performed cross-coculture experiments between HCs and patients (Fig. 4). As was expected, monocytes from HC subjects could expand NKT cells from an HC subject in the presence of α-GalCer (100 ng/ml) and IL-2 (100 U/ml), whereas NKT cells from PTB patients failed to proliferate in the presence of monocytes from HC subjects. Interestingly, monocytes from PTB patients expanded NKT cells from HC subjects (Fig. 4A and B). Consistently, monocytes from HC subjects did not expand NKT cells from EPTB patients, but NKT cells from HC subjects proliferated in the presence of monocytes from EPTB patients (Fig. 4C and D). Taken together, these results suggest that the poor response to α-GalCer is caused by an NKT cell dysfunction.
Fig 4.
NKT cell abnormalities in EPTB and PTB patients. (A and C) Cocultures of APCs and T cells with α-GalCer. CD14+ monocytes and CD3+ T cells were purified from PBMCs by MACS. Monocytes were used as a source of APCs. Freshly isolated monocytes (1 × 105 cells/well) were cocultured with T cells (9 × 105 cells/well) for 7 days as described in Materials and Methods. Cells were then stained with PerCP-conjugated anti-CD3 and PE-conjugated anti-6B11 MAbs and analyzed by flow cytometry. The results shown are representative of the experiments. (B and D) Results were obtained from three independent donors for each group and are presented as means ± SDs.
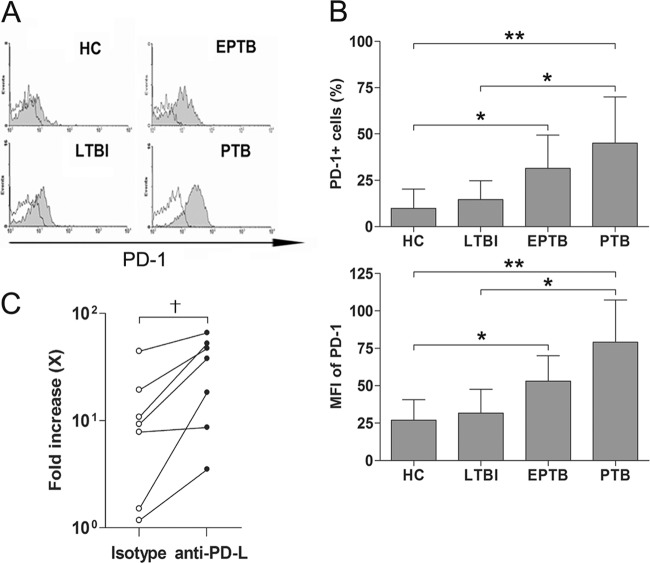
Elevated PD-1 expression in NKT cells in EPTB and PTB patients.
PD-1 and its ligands, PD-L1 and PD-L2, deliver inhibitory signals that regulate the activation of conventional T cells (25). Moreover, recent reports have shown that PD-1/PD-L interaction is involved in the induction and maintenance of NKT cell anergy (5, 35). To determine whether the NKT cell dysfunction is related to NKT cell anergy, we thus examined the expression levels of this receptor in NKT cells in the peripheral blood samples of 6 PTB patients, 6 EPTB patients, 6 LTBI subjects, and 8 age- and sex-matched HCs. (Fig. 5A and B). Interestingly, the percentages of PD-1-expressing cells in NKT cells were found to be significantly higher in EPTB and in PTB patients than in HCs (means, 31.4% versus 9.8% [P < 0.05] and 45.1% versus 9.8% [P < 0.01], respectively) (Fig. 5B). In addition, the MFIs of PD-1 in NKT cells were also significantly higher in EPTB and in PTB patients than in HCs (means, 53.1 versus 27.0 [P < 0.05] and 79.1 versus 27.0 [P < 0.01], respectively) (Fig. 5B). To examine whether PD-1 contributes to the low proliferative response of NKT cells in TB patients, PBMCs from 4 EPTB patients and 3 PTB patients were cultured with α-GalCer for 14 days in the presence of anti-PD-L1 plus anti-PD-L2 Abs or an isotype control. Although the magnitudes of responses to α-GalCer were variable among the study subjects, overall proliferation indices were found to be increased after blockade of PD-1 signaling (P < 0.05) (Fig. 5C).
Fig 5.
Elevated PD-1 expression in NKT cells in EPTB and PTB patients. (A) Representative histograms on PD-1 in the NKT cell population. Freshly isolated PBMCs were stained with PerCP-conjugated anti-CD3, APC-conjugated anti-6B11, and PE-conjugated anti-PD-1 MAbs and then analyzed by flow cytometry. Shaded regions represent anti-PD-1 MAb, and open regions represent isotype control MAb. (B) Percentages of PD-1-expressing cells and MFIs of PD-1 in NKT cells from 8 age- and sex-matched HCs, 6 LTBI subjects, 6 patients with EPTB, and 6 patients with PTB are shown. (C) Changes in the proliferative responses of NKT cells in the presence of anti-PD-L Abs. Freshly isolated PBMCs from 4 patients with EPTB and 3 patients with PTB were stimulated with α-GalCer in the presence of anti-PD-L1 plus anti-PD-L2 Abs or isotype control as described in Materials and Methods, and then proliferation indices were determined by flow cytometry. Results are presented as means ± SDs. *, P < 0.05 by Mann-Whitney U test; **, P < 0.01 by Mann-Whitney U test; †, P < 0.05 by Wilcoxon signed-rank test.
DISCUSSION
It has previously been reported that NKT cell numbers are reduced in PTB patients (33, 40, 42) and that NKT cell levels in LTBI subjects are comparable to those in HCs (40, 42). In the present study, we classified active TB into PTB and EPTB according to infected sites. Our data showed that NKT cell percentages and numbers were reduced in PTB patients and in EPTB patients but not in LTBI subjects. These findings suggest that an NKT cell deficiency reflects the presence of active TB irrespective of tuberculin skin test or IFN-γ release assay reactivity or the site infected.
In the present study, we also found that NKT cell responses to α-GalCer were markedly suppressed in PTB and EPTB patients. However, NKT cell responses were not found to be diminished in LTBI subjects or HCs. This poor responsiveness of NKT cells has previously been demonstrated in patients with autoimmune, infectious, or malignant diseases, but the precise mechanism involved has not been elucidated (9, 11, 22, 27, 32, 44). Recently, several studies have demonstrated that microorganisms can expand murine NKT cells and subsequently become unresponsive to α-GalCer-induced activation (7, 10, 26), which suggests that NKT cell function is affected by microbial infection. Our data also demonstrated that NKT cell levels and their proliferative responses to α-GalCer were inversely correlated with CRP level, which suggests that NKT cell frequency and function are suppressed by chronic microbial infection (e.g., with M. tuberculosis). A negative correlation between NKT cell frequency and CRP level was also observed in patients with chronic autoimmune arthritis (e.g., rheumatoid arthritis) (44).
Although the poor response of NKT cells is not fully understood, it might reflect the lower representation of the most responsive subset in the peripheral blood of TB patients. Human NKT cells can be classified into two or three subsets (e.g., CD4+, CD8+, and double-negative NKT cells) according to the expression of CD4 and CD8 (20, 34). NKT cell subset levels in the peripheral blood of HCs and PTB patients were different in our study cohort (see Fig. S2 in the supplemental material). The most responsive subset to α-GalCer was found to be CD4+ NKT cells (see Fig. S3 and S4 in the supplemental material). Despite the high frequency of this particular subset (i.e., CD4+ NKT cells), PTB patients showed low proliferative responses to α-GalCer. Given the overall lower frequency of NKT cells in PTB patients (>75% absolute decrease compared to HCs), the poor response of NKT cells could be due to the absence of the responding cells from the peripheral blood following recruitment at the site of infection.
The loss of NKT cells in culture due to enhanced apoptosis provides another possible explanation for the poor response of NKT cells to α-GalCer in TB patients. To examine this possibility, we measured apoptotic NKT cell numbers among PBMCs cultured with α-GalCer by flow cytometry. The data obtained clearly showed that NKT cells from TB patients were more susceptible to apoptosis during α-GalCer stimulation than NKTs from HCs. Previous studies have demonstrated that mycobacterial infections in mice and humans promote the apoptosis of activated immune cells (e.g., T cells or NK cells) (12, 30, 39). However, in our culture system, the apoptotic proportions of T cells and of NK cells were comparable for TB patients and HCs (data not shown). These findings suggest that the increased apoptosis of NKT cells in culture may reflect a poor response to α-GalCer in TB patients.
CD1d presents glycolipid antigens, such as α-GalCer, to NKT cells (24). Furthermore, previous studies have shown that blocking CD1d, using MAb or a germ line deletion, inhibits the NKT cell response to α-GalCer (24, 41, 49). Thus, we considered the possibility that numerical and functional deficiencies of NKT cells in patients might be due to alterations in CD1d expression. As we expected, CD1d expressions on PBMCs and CD14+ monocytes were found to be downregulated in TB patients. Furthermore, the downregulation of CD1d expression has also been observed in HIV (6, 8) and malignant lymphoma (22). Given that CD1d-restricted NKT cells have unique effector and regulatory functions in innate and adaptive immune responses, these findings suggest that M. tuberculosis reduces the presentation of CD1d to NKT cells and subverts host immune responses to infected cells.
The poor response to α-GalCer observed in TB patients could be caused by NKT cell dysfunction as well as by altered CD1d expression. To investigate this possibility, we established cross-coculture systems using NKT cells and monocytes from HCs and TB patients. Monocytes from patients were found to expand NKT cells from HCs as efficiently as monocytes from HCs, indicating that the monocytes of TB patients are functional. In contrast, NKT cells from patients did not proliferate even when cocultured with monocytes from HCs. Interestingly, similar findings have also been reported for autoimmune and malignant diseases (9, 22, 27). These results provide evidence that the poor response to α-GalCer is probably due to a defect in the NKT cells of TB patients.
PD-1 is well-known as a coinhibitory molecule on T cells. In conventional T cells, it is not expressed on naïve T cells but is inducibly expressed after T cell activation (25). In recent reports, PD-1 and its ligands have been implicated in the induction and maintenance of NKT cell anergy (5, 35). Our data showed an elevated PD-1 expression on NKT cells in TB patients, suggesting activation of these cells in vivo, findings that have also been observed in HIV-1 infection (32). Interestingly, blockade of the PD-1/PD-L pathway enhanced the response of NKT cells to α-GalCer. These findings suggest that PD-1 signaling at least in part contributes to the poor response of NKT cells in TB patients.
Summarizing, our findings show that NKT cells are numerically and functionally deficient in TB patients. In addition, NKT cell deficiencies were found to reflect the presence of active TB and to be correlated with CRP levels. These findings have important implications for the development of α-GalCer-based immunotherapy in M. tuberculosis patients.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the National Research Foundation of Korea funded by the Republic of Korea Government (grant nos. 2009-0070590, 2011-0011332, and 2011-0012365), the Chonnam National University Hospital Research Institute of Clinical Medicine (CRI11074-22 and CRI12057-21), and the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A100004).
We have no financial conflicts of interest.
We are grateful to Ee-Seul Park (Chonnam National University Hospital, Gwangju, Republic of Korea) for technical assistance.
Footnotes
Published ahead of print 12 March 2012
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Ahmad S. 2010. New approaches in the diagnosis and treatment of latent tuberculosis infection. Respir. Res. 11:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Thoracic Society 2000. Diagnostic standards and classification of tuberculosis in adults and children. Am. J. Respir. Crit. Care Med. 161:1376–1395 [DOI] [PubMed] [Google Scholar]
- 3. Boyson JE, et al. 2002. CD1d and invariant NKT cells at the human maternal-fetal interface. Proc. Natl. Acad. Sci. U. S. A. 99:13741–13746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chackerian A, Alt J, Perera V, Behar SM. 2002. Activation of NKT cells protects mice from tuberculosis. Infect. Immun. 70:6302–6309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang WS, et al. 2008. Cutting edge: programmed death-1/programmed death ligand 1 interaction regulates the induction and maintenance of invariant NKT cell anergy. J. Immunol. 181:6707–6710 [DOI] [PubMed] [Google Scholar]
- 6. Chen N, et al. 2006. HIV-1 down-regulates the expression of CD1d via Nef. Eur. J. Immunol. 36:278–286 [DOI] [PubMed] [Google Scholar]
- 7. Chiba A, Dascher CC, Besra GS, Brenner MB. 2008. Rapid NKT cell responses are self-terminating during the course of microbial infection. J. Immunol. 181:2292–2302 [DOI] [PubMed] [Google Scholar]
- 8. Cho S, et al. 2005. Impaired cell surface expression of human CD1d by the formation of an HIV-1 Nef/CD1d complex. Virology 337:242–252 [DOI] [PubMed] [Google Scholar]
- 9. Cho YN, et al. 2011. Numerical and functional deficiencies of natural killer T cells in systemic lupus erythematosus: their deficiency related to disease activity. Rheumatology 50:1054–1063 [DOI] [PubMed] [Google Scholar]
- 10. Choi HJ, et al. 2008. Bacterial infection alters the kinetics and function of iNKT cell responses. J. Leukoc. Biol. 84:1462–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crough T, et al. 2004. Modulation of human Valpha24(+)Vbeta11(+) NKT cells by age, malignancy and conventional anticancer therapies. Br. J. Cancer 91:1880–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dalton DK, Haynes L, Chu CQ, Swain SL, Wittmer S. 2000. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J. Exp. Med. 192:117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dong HP, et al. 2009. Evaluation of cell surface expression of phosphatidylserine in ovarian carcinoma effusions using the annexin-V/7-AAD assay: clinical relevance and comparison with other apoptosis parameters. Am. J. Clin. Pathol. 132:756–762 [DOI] [PubMed] [Google Scholar]
- 14. Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:677–686 [DOI] [PubMed] [Google Scholar]
- 15. Exley M, Garcia J, Balk SP, Porcelli S. 1997. Requirements for CD1d recognition by human invariant Valpha24+ CD4− CD8− T cells. J. Exp. Med. 186:109–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Exley MA, et al. 2008. Selective activation, expansion, and monitoring of human iNKT cells with a monoclonal antibody specific for the TCR alpha-chain CDR3 loop. Eur. J. Immunol. 38:1756–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gansert JL, et al. 2003. Human NKT cells express granulysin and exhibit antimycobacterial activity. J. Immunol. 170:3154–3161 [DOI] [PubMed] [Google Scholar]
- 18. Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. 2000. NKT cells: facts, functions and fallacies. Immunol. Today 21:573–583 [DOI] [PubMed] [Google Scholar]
- 19. Godfrey DI, Kronenberg M. 2004. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 114:1379–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. 2004. NKT cells: what's in a name? Nat. Rev. Immunol. 4:231–237 [DOI] [PubMed] [Google Scholar]
- 21. Hasper HJ, et al. 2000. A new four-color flow cytometric assay to detect apoptosis in lymphocyte subsets of cultured peripheral blood cells. Cytometry 40:167–171 [PubMed] [Google Scholar]
- 22. Imataki O, et al. 2008. Insufficient ex vivo expansion of Valpha24(+) natural killer T cells in malignant lymphoma patients related to the suppressed expression of CD1d molecules on CD14(+) cells. Cytotherapy 10:497–506 [DOI] [PubMed] [Google Scholar]
- 23. Kaufmann SH. 2006. Tuberculosis: back on the immunologists' agenda. Immunity 24:351–357 [DOI] [PubMed] [Google Scholar]
- 24. Kawano T, et al. 1997. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 278:1626–1629 [DOI] [PubMed] [Google Scholar]
- 25. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. 2008. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26:677–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim S, et al. 2008. Impact of bacteria on the phenotype, functions, and therapeutic activities of invariant NKT cells in mice. J. Clin. Invest. 118:2301–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kojo S, Adachi Y, Keino H, Taniguchi M, Sumida T. 2001. Dysfunction of T cell receptor AV24AJ18+, BV11+ double-negative regulatory natural killer T cells in autoimmune diseases. Arthritis Rheum. 44:1127–1138 [DOI] [PubMed] [Google Scholar]
- 28. Kronenberg M, Gapin L. 2002. The unconventional lifestyle of NKT cells. Nat. Rev. Immunol. 2:557–568 [DOI] [PubMed] [Google Scholar]
- 29. Landry J, Menzies D. 2008. Preventive chemotherapy. Where has it got us? Where to go next? Int. J. Tuberc. Lung Dis. 12:1352–1364 [PubMed] [Google Scholar]
- 30. Li X, McKinstry KK, Swain SL, Dalton DK. 2007. IFN-gamma acts directly on activated CD4+ T cells during mycobacterial infection to promote apoptosis by inducing components of the intracellular apoptosis machinery and by inducing extracellular proapoptotic signals. J. Immunol. 179:939–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mazurek M, et al. 2010. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recommend. Rep. 59(RR-5):1–25 [PubMed] [Google Scholar]
- 32. Moll M, et al. 2009. Severe functional impairment and elevated PD-1 expression in CD1d-restricted NKT cells retained during chronic HIV-1 infection. Eur. J. Immunol. 39:902–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Montoya CJ, et al. 2008. Invariant NKT cells from HIV-1 or Mycobacterium tuberculosis-infected patients express an activated phenotype. Clin. Immunol. 127:1–6 [DOI] [PubMed] [Google Scholar]
- 34. Montoya CJ, et al. 2007. Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11. Immunology 122:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parekh VV, et al. 2009. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J. Immunol. 182:2816–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park YW, et al. 2009. Impaired differentiation and cytotoxicity of natural killer cells in systemic lupus erythematosus. Arthritis Rheum. 60:1753–1763 [DOI] [PubMed] [Google Scholar]
- 37. Sada-Ovalle I, Chiba A, Gonzales A, Brenner MB, Behar SM. 2008. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-gamma, and kill intracellular bacteria. PLoS Pathog. 4:e1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sada-Ovalle I, Skold M, Tian T, Besra GS, Behar SM. 2010. Alpha-galactosylceramide as a therapeutic agent for pulmonary Mycobacterium tuberculosis infection. Am. J. Respir. Crit. Care Med. 182:841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schierloh P, et al. 2005. Increased susceptibility to apoptosis of CD56dimCD16+ NK cells induces the enrichment of IFN-gamma-producing CD56bright cells in tuberculous pleurisy. J. Immunol. 175:6852–6860 [DOI] [PubMed] [Google Scholar]
- 40. Snyder-Cappione JE, et al. 2007. Individuals with pulmonary tuberculosis have lower levels of circulating CD1d-restricted NKT cells. J. Infect. Dis. 195:1361–1364 [DOI] [PubMed] [Google Scholar]
- 41. Spada FM, Koezuka Y, Porcelli SA. 1998. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J. Exp. Med. 188:1529–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sutherland JS, et al. 2009. High granulocyte/lymphocyte ratio and paucity of NKT cells defines TB disease in a TB-endemic setting. Tuberculosis (Edinb.) 89:398–404 [DOI] [PubMed] [Google Scholar]
- 43. Thomas SY, et al. 2003. CD1d-restricted NKT cells express a chemokine receptor profile indicative of Th1-type inflammatory homing cells. J. Immunol. 171:2571–2580 [DOI] [PubMed] [Google Scholar]
- 44. Tudhope SJ, et al. 2010. Profound invariant natural killer T-cell deficiency in inflammatory arthritis. Ann. Rheum. Dis. 69:1873–1879 [DOI] [PubMed] [Google Scholar]
- 45. Van Kaer L. 2005. Alpha-galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat. Rev. Immunol. 5:31–42 [DOI] [PubMed] [Google Scholar]
- 46. Venkataswamy MM, et al. 2009. Incorporation of NKT cell-activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis bacillus Calmette-Guerin. J. Immunol. 183:1644–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wermeling F, Lind SM, Jordo ED, Cardell SL, Karlsson MC. 2010. Invariant NKT cells limit activation of autoreactive CD1d-positive B cells. J. Exp. Med. 207:943–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. World Health Organization 2010. Global tuberculosis control: WHO report 2010. World Health Organization, Geneva, Switzerland [Google Scholar]
- 49. Yang JQ, et al. 2007. Examining the role of CD1d and natural killer T cells in the development of nephritis in a genetically susceptible lupus model. Arthritis Rheum. 56:1219–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.