Abstract
Knowledge of the immunological correlates of Staphylococcus aureus and Streptococcus pneumoniae colonization is required for the search for future protein vaccines. We evaluated natural antibody levels against pneumococcal and staphylococcal proteins in relation to previous bacterial colonization with both pathogens. In a randomized controlled trial, nasopharyngeal samples were obtained from children at 1.5, 6, 12, 18, and 24 months and cultured for S. aureus and S. pneumoniae. Approximately 50% of the children were PCV7 vaccinated. Serum IgG against 18 pneumococcal and 40 staphylococcal proteins was semiquantified by Luminex technology from 111 12 month olds and 158 24 month olds. Previous culture-proven S. aureus colonization was associated with higher IgG levels against 6/40 staphylococcal proteins (ClfB, ClfA, Efb, CHIPS, LukD, and LukF [P ≤ 0.001]) compared to noncarriers. Previous pneumococcal colonization was associated with increased IgG levels against 12/18 pneumococcal proteins compared to noncarriers (P ≤ 0.003). Increasing age was associated with higher levels of antibodies to most pneumococcal proteins and lower levels of antibodies to over half the staphylococcal proteins, reflecting natural colonization dynamics. Anti-S. pneumoniae and anti-S. aureus protein antibodies at the age of 12 months were not negatively correlated with subsequent colonization with the homologous species in the following year and did not differ between PCV7-vaccinated and nonvaccinated children. Colonization with S. aureus and S. pneumoniae induces serum IgG against many proteins, predominantly proteins with immune-modulating functions, irrespective of PCV7 vaccination. None of them appeared to be protective against new acquisition with both pathogens, possibly due to the polymorphic nature of those proteins in the circulating bacterial population.
INTRODUCTION
Streptococcus pneumoniae and Staphylococcus aureus are both important causes of bacterial infections in children in the first years of life (14, 21). Nasopharyngeal colonization is a prerequisite for the development of diseases. Preventive strategies may aim for protection against acquisition and colonization or subsequent infection. Current pneumococcal conjugate vaccines are based on the capsule as antigen and therefore restricted to the present epidemiologically predominant capsular serotypes in children (11). Although the 7-valent pneumococcal conjugate vaccine (PCV7) has been shown to effectively eradicate the vaccine serotype pneumococci from the nasopharynx, the vacant niche is immediately filled by nonvaccine pneumococci, which may also cause disease (7, 35). For this reason, there has been growing interest in vaccines against common and conserved pneumococcal proteins, since they may target all pneumococcal strains irrespective of capsule (32). In mice, immunization with pneumococcal proteins, such as the pneumococcal histidine triad (Pht) proteins, as well as PspA, PdbD, PmpA, CbpA (PspC), and PsaA, has been proven effective against pneumococcal colonization or invasive disease (3, 11, 25, 26, 32).
Comparable strategies are needed to prevent S. aureus colonization and infection, where multidrug (methicillin) resistance has become a serious problem (19). Several S. aureus proteins, e.g., clumping factor A (ClfA), clumping factor B (ClfB), and IsdB, have been shown to protect against colonization and invasive disease in mice (13, 24, 39).
To predict which proteins might be of special interest to prevent disease in humans, we investigated the dynamics, immunogenicity, and (cross)protectiveness of virulence proteins of both species in relation to nasopharyngeal colonization. In a randomized controlled trial (RCT) setting, we determined IgG levels against 18 pneumococcal and 40 S. aureus virulence factors, which included 21 newly tested S. aureus proteins.
These antibody levels were all examined in relation to nasopharyngeal colonization and PCV7 vaccination by Luminex multiplex technology.
MATERIALS AND METHODS
Design, sample collection, and processing.
Between July 2005 and February 2006, before nationwide implementation of PCV7, 1,003 infants were enrolled in a randomized controlled trial, investigating the effects of reduced-dose PCV7 schedules on pneumococcal colonization during the first 2 years of life (NCT00189020) (35). In brief, after obtaining written informed consent from both parents or a guardian, healthy participants were randomly assigned to receive PCV7 (i) PCV7 at 2 and 4 months of age (2-dose group); (ii) PCV7 at 2, 4, and 11 months (2- + 1-dose group); or (3) no PCV7 (control group). Part of this cohort was asked to participate in the immunogenicity arm of the study on a voluntary basis (for details, see reference 31). The present study was performed in the 2- + 1-dose (n = 116) and control (n = 91) groups (baseline characteristics are shown in Table 1).
Table 1.
Baseline characteristics of participants receiving 2 + 1 doses (PCV7+) or no PCV7 vaccinations (controls)
| Parameter | No. (%) |
||
|---|---|---|---|
| All children | PCV7+ | Controls | |
| Total | 207 | 116 (56) | 91 (44) |
| Male gender | 107 (52) | 60 (56) | 47 (44) |
| Serum samples (n = 269) | |||
| 12 mo olds | 111 | 82 (74) | 29 (26) |
| 24 mo olds | 158 | 78 (49) | 80 (51) |
| Paired | 62 | 44 (71) | 18 (30) |
| Nasopharynx cultures (n = 1,030)a | |||
| Children culture positive for S. aureus | |||
| 6 wk | 96 (46) | 53 (46) | 43 (47) |
| 6 mo | 25 (12) | 15 (13) | 10 (11) |
| 12 mob | 22 (11) | 15 (13) | 7 (8) |
| 18 mo | 16 (8) | 9 (8) | 7 (8) |
| 24 mob | 17 (8) | 11 (10) | 6 (7) |
| Children culture positive for S. pneumoniae | |||
| 6 wk | 30 (15) | 20 (17) | 10 (11) |
| 6 mo | 99 (48) | 56 (48) | 43 (47) |
| 12 mob | 125 (66) | 70 (60) | 55 (60) |
| 18 mo | 132 (64) | 66 (57) | 66 (73) |
| 24 mob | 123 (59) | 66 (57) | 57 (63) |
Missing 1 and 2 cultures at 12 and 18 months, respectively.
Culture times at which blood samples were obtained.
During house visits, 269 serum samples, 111 samples from 12 month olds, and 158 samples from 24 month olds, including 62 consecutive serum samples, were collected. Sera were separated within 24 h and stored at −20°C until they were assayed. For each child, swabs were obtained by approaching the nasopharynx transnasally at 6 weeks and 6, 12, 18, and 24 months of age with a flexible, dry cotton-wool swab. Transport, isolation, and identification of pneumococci and S. aureus in these 1,013 samples were done using standard methods (35, 36). A national ethics committee from The Netherlands approved the trial. The study was performed in accordance with the European Statements for Good Clinical Practice.
Detection of anti-protein antibodies.
Antibodies to pneumococcal and S. aureus proteins were measured in serum by a multiplex fluorescent-bead-based immunoassay (xMap; Luminex). This is a validated technique for both protein panels (34, 37), where validation was repeated upon expansion of the panels, as well. We confirmed for IgG directed against TSST-1 that there is a correlation between median fluorescence intensity (MFI) values and the in vitro neutralizing capacity of antibodies (37). The antigens used in this study were selected based on (i) immunological importance, as indicated by the current scientific literature; (ii) their potential role in vaccine development; and (iii) availability (2, 6, 9, 10, 23, 29, 33, 34, 37). Isolation, purification, coupling of the antigens and determination of IgG antibodies by Luminex were performed as described previously (34, 37). In each experiment, control beads (not protein coupled) were included to determine nonspecific antibody binding. In case of nonspecific binding, these MFI values were subtracted from the antigen-specific values. We used human pooled sers (HPS) from 36 healthy donors as a standard tool to test for interassay variation. Tests were accomplished as independent duplicates. The MFI values represent antibody levels in a semiquantitative way. Since MFIs are not normally distributed, we averaged and log transformed them.
All MFI values above zero were defined as measurable antibody titers. Abbreviations of all tested S. aureus and S. pneumoniae virulence factors are listed in Table S1 in the supplemental material. IgG levels against 18 pneumococcal antigens were determined: toxins PLY and PdbD, which is a double mutant of PLY; pneumococcal cell surface proteins CbpA (PspC), PspA, PpmA, SlrA, PsaA, Hyl, NanA, BvH-3, Pht-D (Sp1003; BVH-11-2; histidine triad protein), and Pilus A; enzymes ENO and IgA-1; and proteins Sp0189 (hypothetical protein), Sp0376 (response regulator; intracellular location), Sp1633 (response regulator; intracellular location), and Sp1651 (thiol peroxidase; intracellular location) (15, 18, 23). The data on the double mutant, PdbD are not shown, since children are not exposed to this protein in nature. In addition, IgG levels against 40 S. aureus antigens were determined: the microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) ClfA, ClfB, SasG, IsdA, IsdH, FnbpA, FnbpB, SdrD, and SdrE; staphylococcal enterotoxins (SE) SEA to SEE, SEG to SEJ, SEM to SEO, SEQ, and SER; and staphylococcal superantigen-like 1 (SSL-1), SSL-3, -5, -9, and -11 and TSST-1; exfoliative toxins ETA and ETB; leukocidins LukD, LukE, LukF, and LukS; alpha-toxin; HlgB; and immunomodulatory proteins SCIN, chemotaxis inhibitory protein (CHIPS), and extracellular fibrinogen binding protein (Efb) (39).
Outcome measures.
The immunogenicity of proteins was analyzed by studying the correlation between antibody levels at 12 months of age and colonization in the previous year (measured as culture positive at the age of 6 weeks and/or 6 months) and the correlation between antibody levels at the age of 24 months and colonization in the previous year (measured as culture positive at 12 and/or 18 months of age) and in the whole previous period of 2 years.
To reflect general protein antibody responses to each organism, we performed a combined measurement. First, for each age group, titers were ranked for each protein (from low to high MFI), followed by a combined ranking for all pneumococcal or staphylococcal protein responses per child. These values were correlated with previous colonization status.
The potential protectiveness of anti-protein antibodies was studied by correlating antibody levels at 12 months of age with colonization with the homologous species in the following year (as measured at the age of 18 and/or 24 months).
Statistical analysis.
Mann-Whitney U tests were used to study immunogenicity, overall “species” antibody responses, potential protectiveness, and PCV7 vaccine effects. Wilcoxon signed rank tests were used to study the dynamics of antibodies in time for children for whom we had paired samples available. Antibody levels were determined as continuous variables. Kruskall-Wallis tests were used to test the impact of colonization frequency.
For all analysis, a Bonferroni correction was applied to adjust for multiple comparisons. In this study, we tested 40 S. aureus and 18 pneumococcal proteins; therefore, the null hypothesis was rejected for comparisons regarding anti-staphylococcal-protein and anti-pneumococcal-protein antibodies with P values of ≤0.001 and ≤0.003, respectively. The statistical analyses were performed using the Statistical Package of Social Sciences version 17.0 for Windows (SPSS Inc., Chicago, IL).
RESULTS
IgG levels against 18 pneumococcal and 40 staphylococcal virulence proteins were determined in 269 serum samples collected from 111 12-month-old and 158 24-month-old children (Table 1). In the serum samples of all 12-month-old children, the IgG levels (MFI units) against the S. aureus proteins SSL-3, SSL-9, and Hlgb remained nonconcordant when tested in duplicate and were consequently excluded from analyses. We excluded data on 2, 6, and 11 samples of all S. aureus proteins (12 months), pneumococcal proteins (12 months), and pneumococcal proteins (24 months), respectively, because of nonconcordant results. Therefore, statistical analyses are based on 105/111 (12 months) and 147/158 (24 months) samples of pneumococcal proteins and 109/111 (12 months) and 158/158 (24 months) samples of S. aureus proteins. Consequently, of the 62 paired samples, 56 were available for anti-pneumococcal protein and 60 for anti-S. aureus protein analyses.
Natural humoral responses against S. pneumoniae and S. aureus proteins in infancy.
To study the natural immunogenicity of S. pneumoniae and S. aureus proteins, we determined in the paired serum samples the number of children with detectable natural antibodies against these proteins at the ages of 12 and 24 months irrespective of colonization status. In general, all anti-pneumococcal-protein antibody titers increased between the ages of 12 and 24 months (P ≤ 0.003), except for Sp1651. In contrast, for over half of the S. aureus proteins (23/40), we observed a decrease in antibody titers between 12 and 24 months of age (P < 0.001). The other 17 anti-staphylococcal-protein antibodies remained at a more or less stable level (Fig. 1). Nevertheless, at 12 months of age, we already observed in more than three-quarters of infants measurable antibodies against most pneumococcal proteins, whereas at 24 months of age, almost all children had detectable antibodies against those proteins. The only exception was observed for IgG against Pilus A antigen, which was found in only 10% of 12-month-old children, although this increased rapidly to 87% in 24 month olds. With respect to the antibody response against S. aureus proteins, we observed antibodies directed against all but one staphylococcal protein in almost all 12-month-old children. However, at 24 months of age, detectable anti-protein IgG dropped, depending on the protein measured. For more detailed information regarding the dynamics of the natural humoral responses in infancy, see Tables S2 and S3 in the supplemental material.
Fig 1.
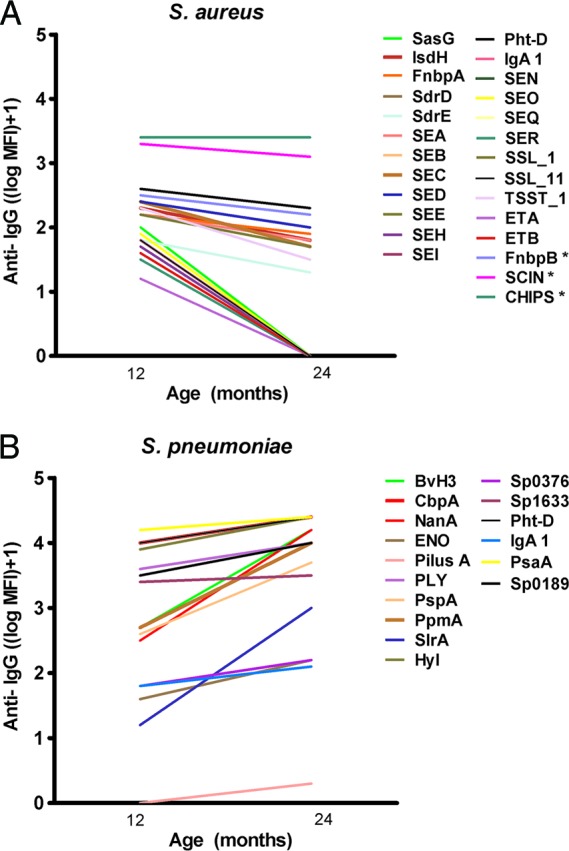
Dynamics of humoral responses against both pathogens in time. Proteins in which significant changes were observed are depicted. (A) We observed a decrease in anti-IgG levels against half the S. aureus proteins between the ages of 12 and 24 months (P ≤ 0.001; *, P ≤ 0.004). (B) We observed an increase in anti-IgG levels against almost all S. pneumoniae virulence factors between the ages of 12 and 24 months (P ≤ 0.003).
Immunogenicity of S. pneumoniae and S. aureus virulence proteins in relation to proven previous colonization in infancy.
We investigated the immunogenicities of all S. pneumoniae and S. aureus proteins in relation to recent challenge with the homologous species, defined as culture-proven carriage at the preceding two sample times.
In children with proven challenge at the age of 6 weeks and/or 6 months or at 12 and/or 18 months of age, the levels of IgG against the proteins PLY, PdbD, CbpA (PspC), PspA, PpmA, PsaA, NanA, Pht-E (BVH-3), ENO, Pht-D (Sp1003), and Sp0189 were significantly higher than those in children without preceding positive cultures at the ages of 12 and 24 months, respectively (Table 2). In addition, SlrA IgG antibodies were higher in 12 month olds with recent pneumococcal challenge than in culture-negative children. Calculations of immunogenicity at 24 months in relation to proven previous colonization in the preceding 2 years showed similar data (Table 2).
Table 2.
Immunogenic S. pneumoniae and S. aureus proteins determined by increase of antibody titers in previous culture-positive carriers compared to noncarriers
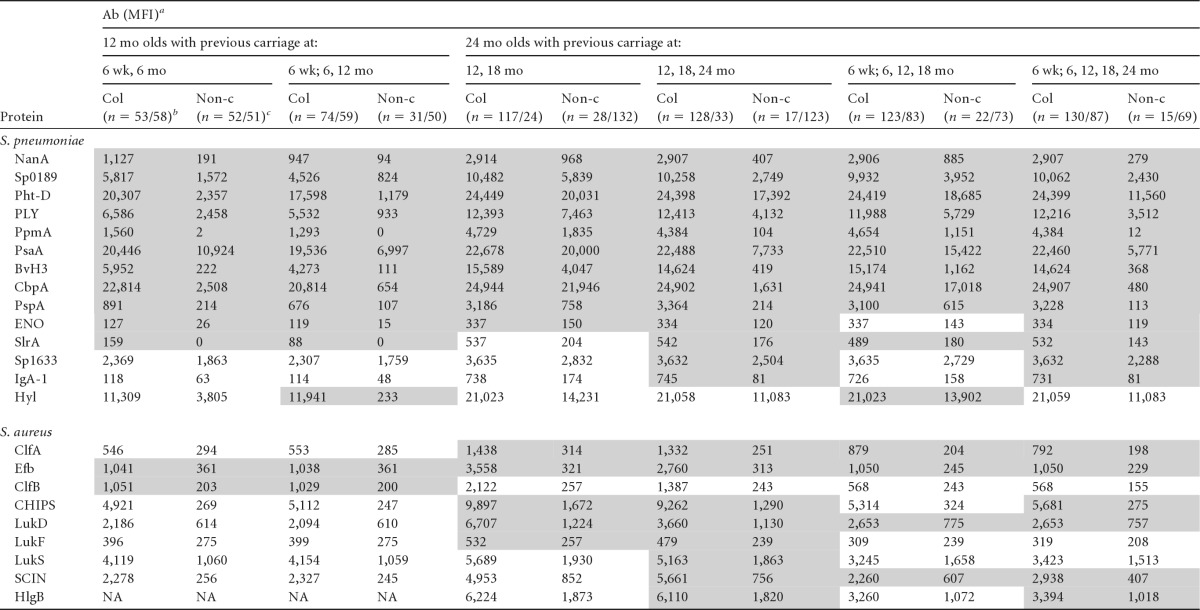
Median antibody (Ab) levels (MFI units). The shaded cells represent the immunogenic proteins (S. aureus, P ≤ 0.001, and S. pneumoniae, P ≤ 0.003). NA, not applicable. For S. pneumoniae, 12 mo olds, n = 105; 24 mo olds, n = 145 (2 out of 147 children for whom serology data were available were excluded because of missing culture data [Table 1]). For S. aureus, 12 mo olds, n = 109; 24 mo olds, n = 156 (2 out of 158 children for whom serology data were available were excluded because of missing culture data [Table 1]).
Col, culture positive (n, number of children culture positive at ≥1 of the time points for S. pneumoniae/S. aureus).
Non-c, culture negative (n, number of children culture negative at all time points for S. pneumoniae/S. aureus).
We performed identical calculations for immunogenicity of S. aureus proteins. At 12 months of age, we found significantly higher antibody titers against S. aureus proteins ClfB and Efb in children colonized at 6 weeks and/or 6 months of age compared to noncolonized children. At 24 months of age, we observed higher antibody titers against ClfA and Efb (38) and also higher antibody titers against CHIPS, LukF, and LukD in children with culture-proven colonization at 12 and/or 18 months of age (P ≤ 0.001) (Table 2).
Repeating the calculations, including “current” colonization (immunogenicity at 12 months based on culture results at 6 weeks, 6 months, and 12 months of age and immunogenicity at 24 months based on culture results at 12, 18, and 24 months), did not change the results (Table 2).
Repeating the 24-month calculations, taking into account culture results from all sample times in the previous 2 years, showed results similar to those taking into account culture results from the preceding year (Table 2).
Additionally, we assessed the impact of the number of positive cultures on antibody levels at 12 months and 24 months. Fifteen, 17, 35, 42, 29, and 7 children were positive for S. pneumoniae zero, one, two, three, four, and five times, respectively, in the preceding 2 years (5 sample times). S. aureus was cultured zero, one, two, three, four, and five times from 69, 57, 18, 9, 2, and 1 children 24 months of age, respectively (Table 3). We found a significant positive association between the number of cultures (0, 1, 2, and ≥3) positive for S. pneumoniae and S. aureus in the first 2 years of life, and antibody titers against pneumococcal proteins PspA, PpmA, SlrA, Sp0189, Pht-D (Sp1003), BvH3, ENO, Sp1633, NanA, PLY, PsaA, and CbpA (P ≤ 0.003) and S. aureus proteins ClfA, Efb, LukD, SCIN, CHIPS, HlgB, and FnbpB (P ≤ 0.001) (Table 3) measured at 24 months of age. Similar trends were observed at 12 months of age (Table 3).
Table 3.
Anti-protein antibody levels of children in relation to S. pneumoniae and S. aureus colonization frequency
| Age groupa | n | No. of times culture positiveb | Ab (MFI)c |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
S. pneumoniae | ||||||||||||||
| PspA | PpmA | SlrA | Sp0189 | Pht-D | Pht-E | ENO | Sp1633 | NanA | PLY | PsaA | CbpA | |||
| 12 mo | 31 | 0 | 107 | 0 | 0 | 824 | 1,179 | 111 | 15 | 1,759 | 94 | 933 | 6,997 | 654 |
| 35 | 1 | 493 | 603 | 0 | 3,175 | 10,694 | 3,346 | 75 | 1,949 | 577 | 4,173 | 16,558 | 17,897 | |
| 28 | 2 | 1,049 | 1,560 | 221 | 5,662 | 20,041 | 5,330 | 127 | 2,350 | 1,127 | 6,698 | 22,171 | 22,814 | |
| 11 | 3 | 593 | 2,850 | 462 | 6,858 | 23,126 | 8,176 | 146 | 3,321 | 1,195 | 7,856 | 20,579 | 24,668 | |
| 24 mo | 15 | 0 | 113 | 12 | 143 | 2,430 | 11,560 | 368 | 119 | 2,287 | 279 | 3,512 | 5,771 | 480 |
| 17 | 1 | 851 | 827 | 372 | 6,177 | 22,743 | 7,341 | 195 | 2,941 | 2,516 | 11,364 | 20,115 | 23,665 | |
| 35 | 2 | 3,322 | 3,779 | 504 | 8,815 | 23,835 | 9,966 | 270 | 3,218 | 2,042 | 12,039 | 22,467 | 24,886 | |
| 42 | 3 | 5,294 | 7,326 | 450 | 11,328 | 24,460 | 16,940 | 370 | 3,851 | 3,055 | 12,711 | 22,762 | 24,953 | |
| 29 | 4 | 5,726 | 8,084 | 787 | 12,137 | 24,796 | 16,269 | 410 | 4,293 | 3,204 | 12,432 | 22,830 | 25,193 | |
| 7 | 5 | 637 | 4,896 | 49 | 11,333 | 24,692 | 14,442 | 276 | 3,019 | 2,807 | 7,081 | 21,401 | 24,647 | |
|
S. aureus |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ClfA | ClfB | Efb | LukD | SCIN | CHIPS | HlgB | FnbpB | |||||||
| 12 mo | 50 | 0 | 285 | 200 | 361 | 231 | ||||||||
| 41 | 1 | 407 | 689 | 998 | 273 | |||||||||
| 14 | 2 | 2,980 | 2,030 | 1,240 | 749 | |||||||||
| 4 | 3 | 3,524 | 1,966 | 897 | 407 | |||||||||
| 24 mo | 69 | 0 | 198 | 229 | 757 | 407 | 275 | 1,018 | 135 | |||||
| 57 | 1 | 495 | 610 | 1,590 | 1,966 | 3,117 | 2,771 | 207 | ||||||
| 18 | 2 | 884 | 1,344 | 3,482 | 4,037 | 8,877 | 5,154 | 596 | ||||||
| 9 | 3 | 2,322 | 6,294 | 8,721 | 6,101 | 11,627 | 6,338 | 964 | ||||||
| 2 | 4 | 730 | 3,507 | 1,677 | 3,033 | 2,072 | 1,536 | 4,365 | ||||||
| 1 | 5 | 5,406 | 10,165 | 7,369 | 13,472 | 13,254 | 11,865 | 916 | ||||||
For S. pneumoniae, 12 mo olds, n = 105; 24 mo olds, n = 145 (2 out of 147 children for whom serology data were available were excluded because of missing culture data [Table 1]). For S. aureus, 12 mo olds, n = 109; 24 mo olds, n = 156 (2 out of 158 children for whom serology data were available were excluded because of missing culture data [Table 1]).
Total number of times culture positive at 6 weeks and 6 and 12 months (12 mo) or at 6 weeks and 6, 12, 18, and 24 months (24 mo).
Median antibody (Ab) levels (MFI units) against S. pneumoniae and S. aureus proteins. For the 12-month group, Kruskall-Wallis test in children 0 to ≥2 times culture positive, P < 0.003 for S. pneumoniae and P ≤ 0.001 for S. aureus. For the 24-month group, Kruskall-Wallis test in children 0 to ≥3 times culture positive, P ≤ 0.003 for S. pneumoniae and P ≤ 0.001 for S. aureus.
Finally, we performed a combined measurement of responses to all proteins to reflect the overall antibody responses to the individual organisms. At both 12 months and 24 months of age, the combined antibody response against S. pneumoniae and S. aureus was significantly higher in children with previous culture-proven carriage than in noncolonized children (all P values ≤ 0.002).
Protectiveness of S. pneumoniae and S. aureus antibodies.
We assessed the potential protectiveness of anti-protein antibodies by relating antibody levels at 12 months to subsequent colonization with the homologous species in the following year. Furthermore, we also explored whether these antibodies are protective against the heterologous species. We observed no negative correlation between antibodies to any of the S. pneumoniae and S. aureus proteins at the age of 12 months and pneumococcal and/or S. aureus colonization in the following year (data not shown).
Development of antibodies in relation to PCV7 vaccination.
To examine the effect of PCV7 on development of natural antibodies to pneumococcal and S. aureus antigens, we compared anti-IgG levels against pneumococcal and staphylococcal proteins between children previously immunized with PCV7 and nonimmunized children. In the 12-month-old group, serum antibodies were measured for 76 vaccinees and 29 controls, and in the 24 month olds for 70 vaccinees and 77 controls. We observed no significant differences in anti-protein antibodies between vaccinees and controls for both pneumococcal and S. aureus antigens at both ages. Nevertheless, we did observe a small trend toward slightly lower pneumococcal antibody levels in the vaccinated group for NanA (MFI, 2,982 versus 2,177 [P = 0.025]), Pilus A (MFI, 34 versus 19 [P = 0.034]), PspA (MFI, 3,686 versus 1,060 [P = 0.015]), and PsaA (MFI, 22,510 versus 20,281 [P = 0.054]) at 24 months of age comparing the unvaccinated and the vaccinated group, respectively.
DISCUSSION
In a search for potential future protein vaccine candidates, we studied the immunogenicities of S. aureus and S. pneumoniae proteins and the potential protective levels of these antibodies against colonization in PCV-vaccinated and nonvaccinated infants. The study was performed in a randomized control setting before nationwide PCV7 introduction in The Netherlands. We examined 18 pneumococcal and 40 S. aureus proteins in total, including 21 S. aureus proteins that were tested for immunogenicity for the first time. Our experiments demonstrated the presence of natural antibodies to all tested staphylococcal and pneumococcal proteins at both 12 and 24 months, with increasing anti-pneumococcal antibodies and declining anti-staphylococcal antibodies with age. These findings are in line with the literature (18, 38) and correspond to the natural dynamics of colonization, where nasopharyngeal colonization with pneumococcus increases and staphylococcal colonization decreases with age. Therefore, these data support the hypothesis that anti-protein antibodies are a result of natural exposure to the pathogens studied.
It is noteworthy that anti-Pilus A IgG was detectable in only 10% of the 12-month-old children but in over 80% of 24 month olds. This might be caused by lower immunogenicity of the protein, or it might be because the pilus is expressed in only a minority of strains. A previous study showed that the pilus frequency might be reduced by the conjugate pneumococcal vaccine (28), which was supported by our data showing a trend toward a lower antibody response against Pilus A in the PCV7-vaccinated group at 24 months of age.
Natural immunogenicity of many of the proteins was supported by the strong correlation between culture-proven colonization and consecutive development of antigen-specific antibodies to most pneumococcal virulence factors and several S. aureus proteins. We observed significantly higher IgG antibody levels against the S. aureus proteins Efb and CHIPS (34, 38) and against ClfA, ClfB, LukD, and LukF in children with culture-proven colonization in the preceding year(s), which underlines the immunogenicity of the organism and specifically of these proteins. Interestingly, ClfB appears to be a major determinant of persistent nasal S. aureus colonization in humans (37, 40). ClfA is known to reduce opsonophagocytosis of S. aureus by neutrophils (8). Secreted cytolytic toxins (LukD and LukF) interfere with neutrophil function by causing membrane damage (8). CHIPS blocks the neutrophil chemoattractant receptors FPR-1 and C5aR (8), while the Efb reduces neutrophil recruitment, as well as chemoattractant C5A (16). As a result, all of these proteins seem to have an immune-suppressive function, which potentially makes them ideal targets for vaccine development.
Known bacterial factors that contribute to pneumococcal colonization are, among others, CbpA, PspA, BvH-3, Hyl, and ENO (15, 23). CbpA binds to human secretory component on a polymeric Ig receptor during the first stage of translocation across the epithelium. PspA is known to prevent binding of C3 onto the pneumococcal surface, and BvH-3 is an inhibitor of the complement system as well. In our study, these proteins appeared immunogenic as well, underlining their need to be expressed at the time of colonization. The natural immunogenicity of all the proteins mentioned is further supported by the significant positive association between the frequency of culture-proven colonization and consecutive antibody titers. For SlrA and PpmA, we even observed unmeasurable antibody titers in 12-month-old children if their cultures were negative at all prior colonization time points. Children who were (repetitively) culture positive in the first year had higher titers against both proteins, which confirms previously published results (1).
Previously, we demonstrated in the same study population an increase in serotype-specific anti-capsular IgG antibodies in previous carriers compared to controls for the serotypes 19F, 23F, and 6B, as well, underlining the immunogenicity of most capsule polysaccharides (30). Since for each carrier event the host is exposed to a single or a few capsule polysaccharides but to many surface-associated proteins at the same time, we also calculated a combined overall anti-protein antibody titer for the two species separately, which confirmed strong carriage-related general anti-protein immunity.
Our experiments show no protective effect of antibodies against any of the proteins on colonization in the following year. Possibly, this is caused by downregulation of protein expression by the bacterium under immune pressure or by the limited accessibility of opsonizing antibodies to proteins shielded by the capsule. Moreover, systemic antibodies might not reach the bacteria in the nasopharyngeal niche properly. The most plausible cause might be the polymorphic nature of S. pneumoniae and, to a lesser extent, S. aureus proteins, resulting in low cross-protectiveness of protein-specific antibodies among strains, which might be a serious problem for their use for future vaccine development (12, 22). Therefore, it might not be surprising that results from murine models strongly suggest CD4+ Th17-mediated immunity rather than antibody-mediated immunity to be the primary (natural) mechanism of protection against pneumococcal colonization, although this still needs to be confirmed in humans (5, 20). Our immunogenicity data do not suggest cross-protection of S. aureus and S. pneumoniae anti-protein IgG against colonization with the heterologous bacterium, either (17). Consequently, the previously observed negative association between S. aureus and S. pneumoniae colonization cannot be explained by anti-protein IgG levels (4, 27).
To the best of our knowledge, the effect of PCV7 on early natural humoral responses to S. pneumoniae and S. aureus proteins is not yet known. No differences in responses to S. pneumoniae proteins between controls and vaccinees were observed, although we did notice a small trend toward lower pneumococcal antibody levels in the vaccinated group against NanA, Pilus A, PspA, and PsaA. This might be partly the consequence of the subtle temporary decrease in overall pneumococcal colonization that we observed at 18 months of age in the vaccinated group compared to the controls (in this subset of children, 57% versus 73%) or of the observed serotype replacement, both described in more detail previously (35, 36). The latter should imply that nonvaccine serotypes might express fewer (homologous) epitopes on their surfaces. The study was performed well before herd effects, after PCV7 introduction in the Dutch National Immunization Program. As a result, in the coming years, the impact of decreased vaccine serotype colonization and serotype replacement on natural anti-pneumococcal protein IgGs may need to be reevaluated (35). In addition, we observed no significant differences in IgG antibody responses against S. aureus between controls and vaccinees, which might be explained by similar colonization rates observed in the two groups.
Some limitations of our study need to be recognized. First, the results are derived from a post hoc analysis. It would have been ideal if we had sampled the children more frequently. Due to the 6-month sampling intervals in our study, we missed several colonization episodes in most individuals during their first 2 years of life, which skewed our results toward smaller effects. This is underlined by the presence of anti-protein antibodies to most proteins in children who remained culture negative for pneumococci at all sample times.
The strengths of our study include the randomized controlled and longitudinal design, which made it possible to estimate the effect of PCV7 vaccination on natural antibody responses against virulence proteins in time in a controlled setting. In addition, this is the first study simultaneously analyzing IgG responses against S. aureus and S. pneumoniae in this large number of samples. Moreover, we corrected for multiple testing, probably resulting in lower numbers of false-positive associations. Finally, this multiplex fluorescent-bead-based immunoassay allowed us to analyze the humoral responses of 58 proteins simultaneously, which is highly advantageous when working with small volumes of pediatric serum samples.
In conclusion, natural anti-protein antibodies to pneumococci and S. aureus showed an inverse correlation with age, which is in line with colonization dynamics. Many pneumococcal and staphylococcal proteins appear to be immunogenic in children under 24 months of age. In contrast, none of the anti-protein antibodies appeared to be either protective or cross-protective against pneumococcal and S. aureus colonization in the second year of life. The consequences of these findings for protectiveness of the proteins when used as actual vaccine candidates should be further investigated.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the members of the Linnaeus Institute, Research Center in Hoofddorp, for their dedication and work, which made this project possible; the participating children and their families for their time and commitment to the studies; and Suzan Rooijakkers, Jan-Ingmar Flock, Silva Holtfreter, Kok van Kessel, and John Fraser for kindly supplying the S. aureus proteins and toxins.
Footnotes
Published ahead of print 9 April 2012
Supplemental material for this article may be found at http://iai.asm.org.
REFERENCES
- 1. Adrian PV, et al. 2004. Development of antibodies against pneumococcal proteins alpha-enolase, immunoglobulin A1 protease, streptococcal lipoprotein rotamase A, and putative proteinase maturation protein A in relation to pneumococcal carriage and otitis media. Vaccine 22:2737–2742 [DOI] [PubMed] [Google Scholar]
- 2. Balaban N, Rasooly A. 2000. Staphylococcal enterotoxins. Int. J. Food Microbiol. 61:1–10 [DOI] [PubMed] [Google Scholar]
- 3. Bogaert D, et al. 2006. Development of antibodies against the putative proteinase maturation protein A in relation to pneumococcal carriage and otitis media. FEMS Immunol. Med. Microbiol. 46:166–168 [DOI] [PubMed] [Google Scholar]
- 4. Bogaert D, et al. 2004. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 363:1871–1872 [DOI] [PubMed] [Google Scholar]
- 5. Bogaert D, Weinberger D, Thompson C, Lipsitch M, Malley R. 2009. Impaired innate and adaptive immunity to Streptococcus pneumoniae and its effect on colonization in an infant mouse model. Infect. Immun. 77:1613–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Haas CJ, et al. 2004. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J. Exp. Med. 199:687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flasche S, et al. 2011. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med. 8:e1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foster TJ. 2009. Colonization and infection of the human host by staphylococci: adhesion, survival and immune evasion. Vet. Dermatol. 20:456–470 [DOI] [PubMed] [Google Scholar]
- 9. Foster TJ, Hook M. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484–488 [DOI] [PubMed] [Google Scholar]
- 10. Foster TJ, McDevitt D. 1994. Surface-associated proteins of Staphylococcus aureus: their possible roles in virulence. FEMS Microbiol. Lett. 118:199–205 [DOI] [PubMed] [Google Scholar]
- 11. Godfroid F, Hermand P, Verlant V, Denoel P, Poolman JT. 2011. Preclinical evaluation of the Pht proteins as potential cross-protective pneumococcal vaccine antigens. Infect. Immun. 79:238–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hakenbeck R, Madhour A, Denapaite D, Bruckner R. 2009. Versatility of choline metabolism and choline-binding proteins in Streptococcus pneumoniae and commensal streptococci. FEMS Microbiol. Rev. 33:572–586 [DOI] [PubMed] [Google Scholar]
- 13. Harro C, et al. 2010. Safety and immunogenicity of a novel Staphylococcus aureus vaccine: results from the first study of the vaccine dose range in humans. Clin. Vaccine Immunol. 17:1868–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jansen AG, et al. 2009. Invasive pneumococcal disease in the Netherlands: syndromes, outcome and potential vaccine benefits. Vaccine 27:2394–2401 [DOI] [PubMed] [Google Scholar]
- 15. Kadioglu A, Weiser JN, Paton JC, Andrew PW. 2008. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 6:288–301 [DOI] [PubMed] [Google Scholar]
- 16. Ko YP, Liang X, Smith CW, Degen JL, Hook M. 2011. Binding of Efb from Staphylococcus aureus to fibrinogen blocks neutrophil adherence. J. Biol. Chem. 286:9865–9874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lebon A, et al. 2011. The inverse correlation between Staphylococcus aureus and Streptococcus pneumoniae colonization in infants is not explained by differences in serum antibody levels in the Generation R Study. Clin. Vaccine Immunol. 18:180–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lebon A, et al. 2011. Natural antibodies against several pneumococcal virulence proteins in children in the pre-pneumococcal vaccine-era: The Generation R Study. Infect. Immun. 79:1680–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lowy FD. 2003. Antimicrobial resistance: the example of Staphylococcus aureus. J. Clin. Invest. 111:1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu YJ, et al. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 4:e1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madhi SA, Schoub B, Simmank K, Blackburn N, Klugman KP. 2000. Increased burden of respiratory viral associated severe lower respiratory tract infections in children infected with human immunodeficiency virus type-1. J. Pediatr. 137:78–84 [DOI] [PubMed] [Google Scholar]
- 22. McCarthy AJ, Lindsay JA. 2010. Genetic variation in Staphylococcus aureus surface and immune evasion genes is lineage associated: implications for vaccine design and host-pathogen interactions. BMC Microbiol. 10:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mitchell AM, Mitchell TJ. 2010. Streptococcus pneumoniae: virulence factors and variation. Clin. Microbiol. Infect. 16:411–418 [DOI] [PubMed] [Google Scholar]
- 24. Murphy E, et al. 2011. Challenges for the evaluation of Staphylococcus aureus protein based vaccines: monitoring antigenic diversity. Hum. Vaccin. 7(Suppl.):51–59 [DOI] [PubMed] [Google Scholar]
- 25. Ogunniyi AD, Grabowicz M, Briles DE, Cook J, Paton JC. 2007. Development of a vaccine against invasive pneumococcal disease based on combinations of virulence proteins of Streptococcus pneumoniae. Infect. Immun. 75:350–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olafsdottir TA, Lingnau K, Nagy E, Jonsdottir I. 2012. Novel protein-based pneumococcal vaccines administered with the Th1-promoting adjuvant IC31 induce protective immunity against pneumococcal disease in neonatal mice. Infect. Immun. 80:461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Regev-Yochay G, et al. 2004. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in children. JAMA 292:716–720 [DOI] [PubMed] [Google Scholar]
- 28. Regev-Yochay G, et al. 2010. Re-emergence of the type 1 pilus among Streptococcus pneumoniae isolates in Massachusetts, U. S. A. Vaccine 28:4842–4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roche FM, Meehan M, Foster TJ. 2003. The Staphylococcus aureus surface protein SasG and its homologues promote bacterial adherence to human desquamated nasal epithelial cells. Microbiology 149:2759–2767 [DOI] [PubMed] [Google Scholar]
- 30. Rodenburg GD, et al. 2011. Lower immunoglobulin G antibody responses to pneumococcal conjugate vaccination at the age of 2 years after previous nasopharyngeal carriage of Streptococcus pneumoniae. J. Pediatr. 159:965–970 [DOI] [PubMed] [Google Scholar]
- 31. Rodenburg GD, et al. 2010. Comparability of antibody response to a booster dose of 7-valent pneumococcal conjugate vaccine in infants primed with either 2 or 3 doses. Vaccine 28:1391–1396 [DOI] [PubMed] [Google Scholar]
- 32. Rodgers GL, Klugman KP. 2011. The future of pneumococcal disease prevention. Vaccine 29(Suppl. 3):C43–C48 [DOI] [PubMed] [Google Scholar]
- 33. Shannon O, Uekotter A, Flock JI. 2006. The neutralizing effects of hyperimmune antibodies against extracellular fibrinogen-binding protein, Efb, from Staphylococcus aureus. Scand. J. Immunol. 63:184–190 [DOI] [PubMed] [Google Scholar]
- 34. Shoma S, et al. 2011. Development of a multiplexed bead-based immunoassay for the simultaneous detection of antibodies to 17 pneumococcal proteins. Eur. J. Clin. Microbiol. Infect. Dis. 30:521–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Gils EJ, et al. 2009. Effect of reduced-dose schedules with 7-valentpneumococcal conjugate vaccine on nasopharyngeal pneumococcal carriage in children: a randomized controlled trial. JAMA 302:159–167 [DOI] [PubMed] [Google Scholar]
- 36. van Gils EJM, et al. 2011. Effect of seven-valent pneumococcal conjugate vaccine on Staphylococcus aureus colonisation in a randomised controlled trial. PLoS One 6:e20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verkaik NJ, et al. 2009. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J. Infect. Dis. 199:625–632 [DOI] [PubMed] [Google Scholar]
- 38. Verkaik NJ, et al. 2010. Induction of antibodies by Staphylococcus aureus nasal colonization in young children. Clin. Microbiol. Infect. 16:1312–1317 [DOI] [PubMed] [Google Scholar]
- 39. Verkaik NJ, van Wamel WJ, Avan B. 2011. Immunotherapeutic approaches against Staphylococcus aureus. Immunotherapy 3:1063–1073 [DOI] [PubMed] [Google Scholar]
- 40. Wertheim HF, et al. 2008. Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med. 5:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


