Abstract
Plasmodium sporozoites are inoculated into the skin of the mammalian host as infected mosquitoes probe for blood. A proportion of the inoculum enters the bloodstream and goes to the liver, where the sporozoites invade hepatocytes and develop into the next life cycle stage, the exoerythrocytic, or liver, stage. Here, we show that a small fraction of the inoculum remains in the skin and begins to develop into exoerythrocytic forms that can persist for days. Skin exoerythrocytic forms were observed for both Plasmodium berghei and Plasmodium yoelii, two different rodent malaria parasites, suggesting that development in the skin of the mammalian host may be a common property of plasmodia. Our studies demonstrate that skin exoerythrocytic stages are susceptible to destruction in immunized mice, suggesting that their aberrant location does not protect them from the host's adaptive immune response. However, in contrast to their hepatic counterparts, they are not susceptible to primaquine. We took advantage of their resistance to primaquine to test whether they could initiate a blood-stage infection directly from the inoculation site, and our data indicate that these stages are not able to initiate malaria infection.
INTRODUCTION
Malaria remains one of the most important infectious diseases in the world, with 225 million new cases diagnosed each year and 780,000 deaths annually (31). Infection is initiated when Plasmodium sporozoites are injected into a mammalian host by an Anopheles mosquito. The sporozoites enter the bloodstream and travel to the liver, where they invade hepatocytes, differentiate, and divide asexually to produce exoerythrocytic forms (EEFs). A single successful sporozoite can produce a mature EEF containing tens of thousands of hepatic merozoites, which seed the blood and invade erythrocytes, initiating the asexual erythrocytic phase of infection (18). Blood-stage infection is accompanied by large parasite burdens and is responsible for all of the clinical symptoms of disease. In contrast, the preerythrocytic stage of infection, when parasite numbers are low, is asymptomatic and constitutes a bottleneck in the parasite's life cycle (12). Immunity to preerythrocytic stages can completely prevent clinical malaria infection and in experimental models has been shown to be an excellent target for vaccine development (reviewed in reference 6). However, attacking the liver stages of infection to prevent malaria is predicated on the fact that a blood-stage infection can only be initiated from the liver.
It was previously demonstrated that sporozoites are inoculated into the skin of the mammalian host during mosquito feeding (reviewed in reference 23). Live-imaging studies showed that after inoculation, sporozoites move within the dermis, eventually contacting a blood vessel, which they penetrate to enter the blood circulation (1, 29). Sporozoite exit from the dermis occurs in a slow trickle that lasts for hours after inoculation (32). In rodent models, approximately 20% of the inoculum goes to the draining lymph node, where parasites are ultimately destroyed (1, 32) but also initiate an adaptive immune response to infected hepatocytes (5). Despite these recent findings, there is still much to be learned about the “skin stage” of malaria infection. Here, we set out to study the fate of sporozoites that remain in the skin using the rodent malaria parasites Plasmodium berghei and Plasmodium yoelii. We found that a small proportion of the inoculum remains at this site and can begin to develop into EEFs. These skin EEFs are not sensitive to primaquine but are susceptible to destruction in immunized mice. Importantly, they do not contribute to the establishment of blood-stage infection.
MATERIALS AND METHODS
Parasites.
These studies used green fluorescent protein (GFP)-transgenic P. berghei ANKA (7) and GFP-transgenic P. yoelii 17XNL (25) parasites. Three- to 5-day-old Anopheles stephensi mosquitoes were allowed to feed on infected mice and were used for experiments on day 14 and 15 (P. yoelii) or day 18 to 20 (P. berghei) postinfective blood meal. When sporozoite isolation was necessary, the mosquitoes were rinsed in 70% ethanol and washed in Dulbecco's modified Eagle medium (DMEM), and the salivary glands were removed, homogenized, and centrifuged at 100 × g. The supernatant was taken, and the sporozoites were counted in a hemocytometer.
Animal infection.
Female Swiss Webster, C57BL/6, and BALB/c mice, aged 4 to 6 weeks, were purchased from Taconic, Jackson, or Harlan. All animal work was approved by the institutional animal care and use committee of each institution. For skin EEF experiments, animals were anesthetized with ketamine/xylazine and maintained at 37°C on a slide warmer. Ten to 15 infected mosquitoes were placed in a small feeding cage made of Plexiglas tubing with a fine mesh over one end and sealed with parafilm on the other end. This was placed mesh side down on the ventral side of the mouse's ear, and mosquitoes were allowed to probe for 10 min. At different time points after sporozoite inoculation, the mice were anesthetized, and their ears were shaved and removed for analysis. The ear leaflets were separated from one another under a dissecting microscope using fine forceps and mounted on a slide with a coverslip for observation under a Leica confocal microscope. To visualize hepatic EEFs, BALB/c mice were inoculated with 106 P. yoelii salivary gland sporozoites, and at different time points, livers from the infected mice were harvested, washed with phosphate-buffered saline (PBS), and fixed in 4% paraformaldehyde. Fifty-micrometer sections were cut using a Vibratome (Ted Pella Inc., Redding, CA), and EEFs were stained using rabbit antiserum to UIS4 to stain the parasite parasitophorous vacuole (16) and DAPI (4′,6-diamidino-2-phenylindole) to indicate the DNA.
Confocal microscopy.
A Leica inverted laser scanning confocal microscope (TCS SP2 AOBS) was used for all skin experiments. GFP-expressing parasites could be clearly seen above the fluorescent background of the ear. For each specimen, the entire ear was scanned and counted using a 20× power objective, and a 40× objective was used to take photographs of the EEFs. Each image resulted from the average projection of superimposed stacks of individual focal plane images of the entire parasite. Skin EEF sizes were measured using Leica LCS Lite software measuring the largest diameter of each EEF. For each time point, 6 to 8 randomly selected EEFs were measured. Hepatic EEF diameters were measured using an Olympus Delta Vision microscope, the pictures were deconvolved using Softworx software (Applied Precision), and measurements were taken using the measure tool in the Metamorph software. Three or four diameters for each EEF were measured and averaged.
In vitro development assays.
EEF development was quantified in the following cell lines: Hepa1-6, a mouse hepatocyte cell line (CRL-1830; ATCC, Rockville, MD); dermal fibroblasts isolated from BALB/c mice, as previously described (8); and EOMA, a mouse endothelial cell line (CRL-2586; ATCC). The cells were plated in 8-chamber Permanox Nunc Lab-Tek slides in DMEM with 10% fetal calf serum at a density of 105 cells/well and grown until they were subconfluent. On the day of the experiment, the medium was removed, and an equal number of sporozoites, obtained by aseptic dissection of infected A. stephensi salivary glands, were added to each well. The sporozoites were incubated with the cells for 3 h at 37°C in 5% CO2; then, the cultures were washed with medium to remove unattached sporozoites. Subsequently, the medium was changed daily, and the infected cells were processed for observation 24 h to 72 h later by washing them twice with phosphate-buffered saline and fixation with 4% paraformaldehyde. The cells were stained with monoclonal antibody (MAb) 2E6, specific for parasite Hsp70 (26), and nuclei were stained with DAPI (1 μg/ml in Vectashield mounting medium). Infected cells were observed and photographed using a Leica inverted laser scanning confocal microscope (see above).
Immunization experiments.
C57BL/6 mice were immunized intravenously (i.v.) with 75,000 irradiated P. berghei sporozoites, followed by two boosts of 25,000 irradiated sporozoites at 10-day intervals. Control mice were not immunized. Ten days after the last boost, most of the immunized mice and controls were challenged by allowing 15 infected mosquitoes to feed on each ear for 10 min. The salivary gland load of the infecting mosquitoes was between 15,000 and 25,000 sporozoites. A small proportion of controls and immunized mice were challenged by intravenous inoculation of 4,000 sporozoites and monitored for blood-stage infection by Giemsa-stained blood smears prepared daily from days 4 to 10 postchallenge. On days 1 through 3 postchallenge, ears were removed from control and immunized animals, and skin EEFs were counted by confocal microscopy as outlined above. For assessment of the liver parasite burden, livers were harvested and processed as outlined above.
Primaquine experiments.
C57BL/6 mice were treated with 25 mg/kg of body weight of primaquine (Sigma-Chemie) diluted in 1× PBS, and injected intraperitoneally (i.p.) 2 and 24 h after sporozoite inoculation. Control mice were injected with 1× PBS alone. Mice were inoculated either intravenously with 10,000 P. berghei ANKA sporozoites or intradermally (i.d.) by the bites of 12 to 15 mosquitoes. Intravenously inoculated mice were tested for the effect of primaquine on hepatic EEF development as follows. Livers were harvested 40 h after sporozoite inoculation, total RNA was isolated, and the liver parasite burden was quantified by reverse transcription-quantitative PCR (RT-qPCR) as outlined previously (3) using primers that recognize P. berghei-specific sequences within the 18S rRNA (14). Tenfold dilutions of a plasmid construct containing the P. berghei 18S rRNA gene were used to create a standard curve. The effect of primaquine on skin EEFs was quantified by counting the EEFs in the dermis, as outlined previously.
Infectivity of skin EEFs.
For experiments with P. berghei, C57BL/6 mice were inoculated with sporozoites intravenously or by mosquito bite and treated with 25 mg/kg of primaquine via i.p. injection 2 and 24 h after sporozoite inoculation. For P. yoelii experiments, BALB/cJ mice (Jackson) were infected by the bites of 15 infected mosquitoes, and 60 mg/kg of primaquine was administered i.p. 12 and 24 h postinfection. For both sets of experiments, the control group was not treated with primaquine. All mice were followed by daily blood smears beginning 3 days after sporozoite inoculation until day 21 postinoculation.
To test whether primaquine affected early blood stages, mice were treated as described above, and then, 24 h after the last injection, inoculated with 1,000 infected erythrocytes intravenously. Parasitemias were monitored for 5 days by Giemsa-stained blood smears.
Statistical analysis.
Mean numbers and sizes of skin EEFs in the ears and parasite liver burdens of different groups of mice were analyzed for statistically significant differences using the Mann-Whitney test or a Kruskall-Wallis test followed by Dunn's multiple-comparison test (when more than 2 groups were compared) with GraphPad Prism software, using a P value of <0.05 as the level of significance.
RESULTS
A small number of P. berghei sporozoites begin to develop at the injection site.
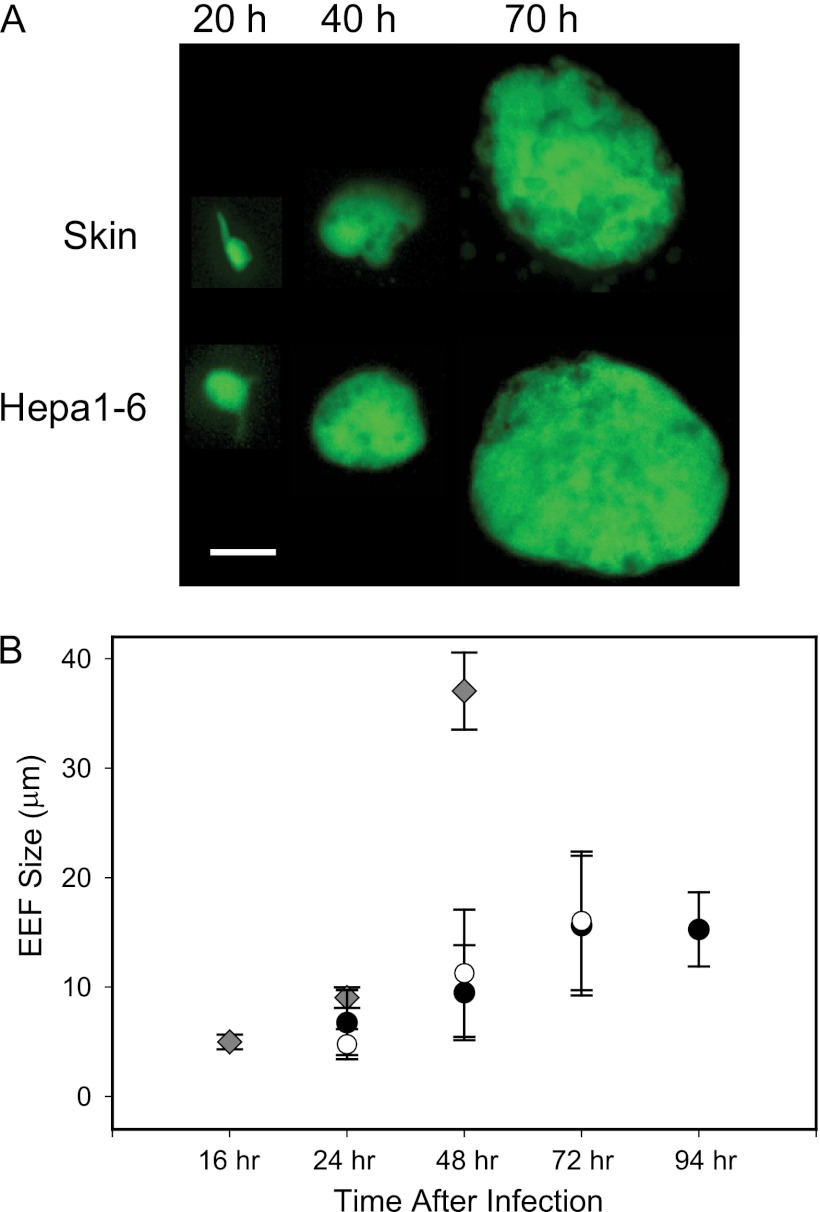
In order to investigate the fate of sporozoites that do not exit the inoculation site, mosquitoes infected with P. berghei expressing GFP (P. berghei-GFP) (7) were allowed to probe the ears of mice, which were harvested 20 h, 40 h, and 70 h later for observation by confocal microscopy. GFP-expressing parasites could be clearly seen above the fluorescent background of the ear, and it appeared that some sporozoites were developing into EEFs at the injection site. Photographs of typical EEFs are shown and compared to EEFs grown in hepatoma cells from similar time points (Fig. 1A). We also measured the diameters of skin EEFs, in vitro-cultured EEFs, and in vivo hepatic EEFs at different time points, and these data are shown in Fig. 1B. There was no statistically significant difference in size between the skin EEFs and the in vitro-cultured EEFs; however, 48-h hepatic EEFs from mice inoculated with sporozoites were significantly larger than both skin and in vitro EEFs of the same age, a result that is not surprising, given that EEF size is likely a function of how optimally adapted the parasite is to withdraw nutrients from its host cell (21).
Fig 1.
Morphology and size of P. berghei skin EEFs in Swiss Webster mice. (A) Photographs of P. berghei skin EEFs taken at the indicated time points after mosquito inoculation of sporozoites into the ears of mice. For comparison, photographs taken at the same time points of EEFs grown in Hepa1-6 cells in culture are shown. Bar, 6 μm. (B) Diameters of skin EEFs (black circles), in vitro-cultured EEFs (white circles), and hepatic EEFs (diamonds). The values are means ± standard deviations (SD) of 6 to 8 measurements for each of 3 mice or for 3 different in vitro cultures. For any given time point, no significant statistical difference was observed between the sizes of in vivo and in vitro skin EEFs. Forty-eight-hour hepatic EEFs were statistically significantly larger than skin or in vitro-cultured EEFs (P < 0.001 and P < 0.05, respectively; Kruskall-Wallis and Dunn's post hoc tests).
Numbers of P. berghei skin EEFs over time.
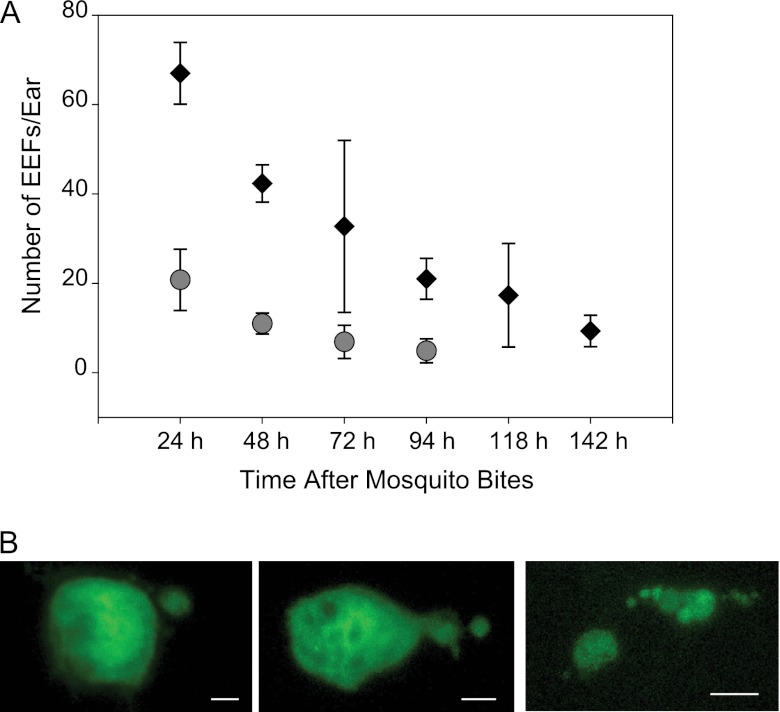
We next quantified the number of P. berghei skin EEFs in mouse ears at different time points after probing by 15 infected mosquitoes. As shown in Fig. 2A, initial numbers of skin EEFs varied between experiments, likely due to different salivary gland sporozoite loads in different batches of mosquitoes (i.e., mosquitoes used in experiment 1 had on average 87,000 sporozoites per mosquito, while those used in experiment 2 had 18,000 sporozoites per mosquito). In addition, we observed some decrease in P. berghei skin EEF numbers over time, suggesting that some of the sporozoites that remain in the skin and begin to develop are either destroyed or unable to continue growing in this environment (Fig. 2A). Previous studies have shown that the mean number of sporozoites inoculated by a single mosquito infected with rodent malaria parasites is approximately 125 (11, 15). If we use this number and extrapolate to the current study, the starting inoculum was approximately 1,800 sporozoites, although this also varies somewhat depending on the salivary gland load of the mosquito (15). Nonetheless, these numbers suggest that approximately 1 to 2% of the inoculum remains and begins to develop at the injection site. Follow-up of infected ears over several consecutive days revealed that some parasites persisted in the skin for 15 days; however, after 10 days, parasites were seen only sporadically, and the GFP signal was somewhat diffuse, suggesting the parasites were dying (data not shown). Interestingly, observation of skin EEFs between 72 and 96 h after sporozoite inoculation often revealed parasites with buds coming off the mother cell (Fig. 2B). These structures appear similar to merosomes, the “bags” of merozoites released from hepatic schizonts, although it is unclear if these structures contain mature merozoites (24).
Fig 2.
Numbers of P. berghei skin EEFs over time and morphology of late-stage skin EEFs in Swiss Webster mice. (A) Numbers of skin EEFs per ear at the indicated time points after mosquito inoculation of sporozoites in two independent experiments. Each ear was probed by 15 mosquitoes, and the values are means ± SD. In experiment 1 (diamonds), results from a minimum of 3 ears from 3 different mice are shown, and significant statistical differences were observed between the numbers of EEFs at 24 h and 142 h (P < 0.05). In experiment 2 (circles), results from a minimum of 10 ears from 5 different mice are shown, and significant statistical differences were observed between the numbers of EEFs at 24 h and at 72 and 94 h (P < 0.001). The Kruskall-Wallis and Dunn's post hoc tests were used for statistical analysis. (B) Mature P. berghei skin EEFs between 72 and 96 h after mosquito injection of sporozoites showing budding suggestive of merosome formation. Bars, 6 μm (left and center) and 12 μm (right).
Skin EEFs are also observed in P. yoelii.
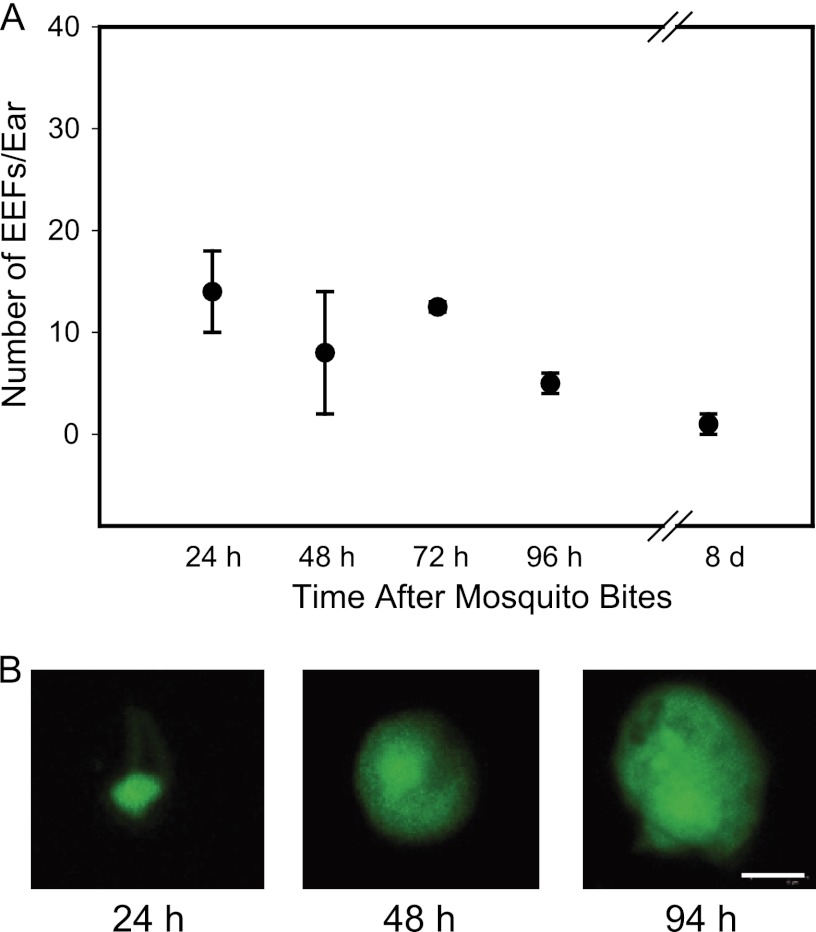
Previous studies with P. berghei sporozoites demonstrated that they were capable of invading and developing in a variety of cell types in vitro (4, 10, 20). To determine whether the skin EEFs we observed were specific to P. berghei, due to its ability to develop in cell types other than hepatocytes, we performed similar experiments with another rodent malaria parasite, P. yoelii. Fifteen mosquitoes infected with P. yoelii expressing GFP were allowed to probe on mice, and at the indicated time points, the skin EEFs in the probed skin were counted. As shown in Fig. 3, we observed what appeared to be EEFs of P. yoelii at the inoculation site; however, the numbers were significantly lower than those we observed for P. berghei, with approximately 0.5% of the inoculum remaining and developing at the site.
Fig 3.
Skin EEFs are not specific to the P. berghei-mouse experimental model. (A) Number of P. yoelii EEFs in the ear at the indicated time points after mosquito inoculation of sporozoites in Swiss Webster mice. Each ear was probed by 15 mosquitoes, and the values are means ± SD of a minimum of 3 ears from 3 different mice. (B) Photographs of P. yoelii skin EEFs at the indicated time points after infection. Bar, 6 μm.
P. berghei and P. yoelii sporozoites can develop in skin cells in vitro.
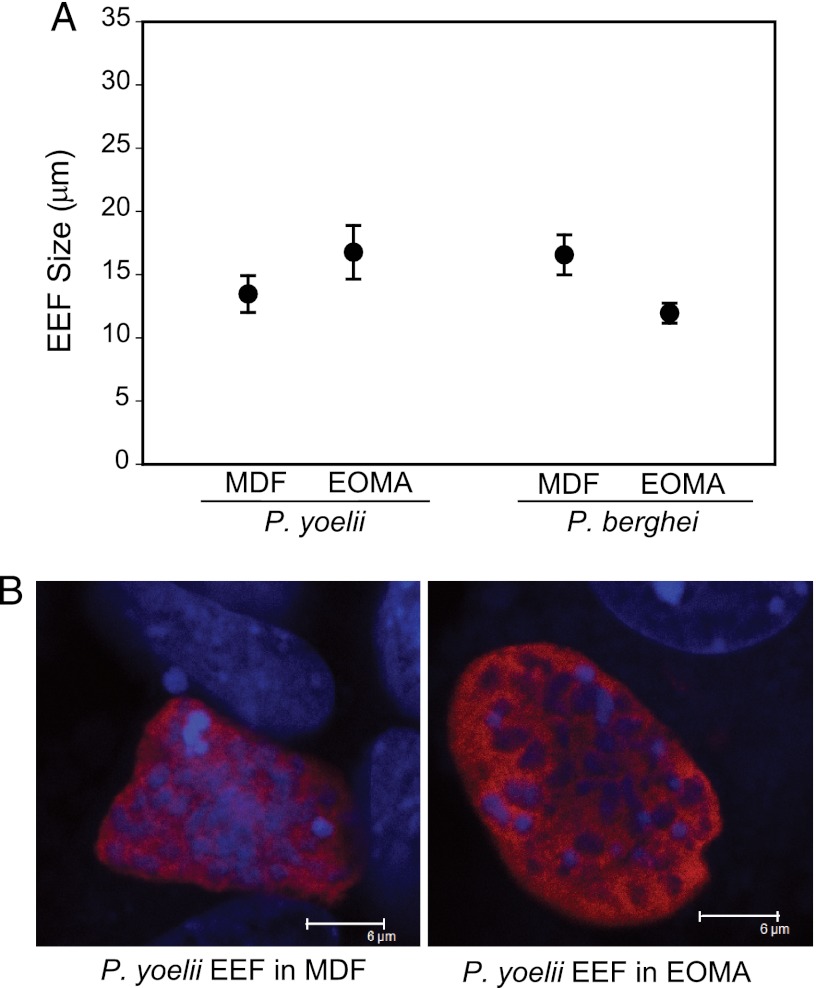
Because dermal fibroblasts and endothelial cells are among the most abundant cell types found in the skin, we tested whether P. berghei and P. yoelii sporozoites could invade and develop in dermal fibroblasts isolated from mouse skin (MDF) and in a mouse endothelial cell line (EOMA). Although the numbers of EEFs obtained were low, both P. berghei and P. yoelii parasites could develop in these cell types in vitro, and their sizes were comparable to those of EEFs grown in hepatocyte lines (Fig. 4).
Fig 4.
P. berghei and P. yoelii sporozoites can develop in skin cells in vitro. (A) Size of P. yoelii and P. berghei 48-h EEFs grown in mouse dermal fibroblasts (MDF) and a mouse endothelial cell line (EOMA) in vitro. Shown are the mean diameters ± SD of at least 8 measurements from duplicate cell cultures. (B) Representative images of 48-h P. yoelii EEFs in mouse dermal fibroblasts (left) or the endothelial cell line EOMA (right). The cells were stained for Hsp70 (red), and nuclei were stained with DAPI. Bar = 6 μm.
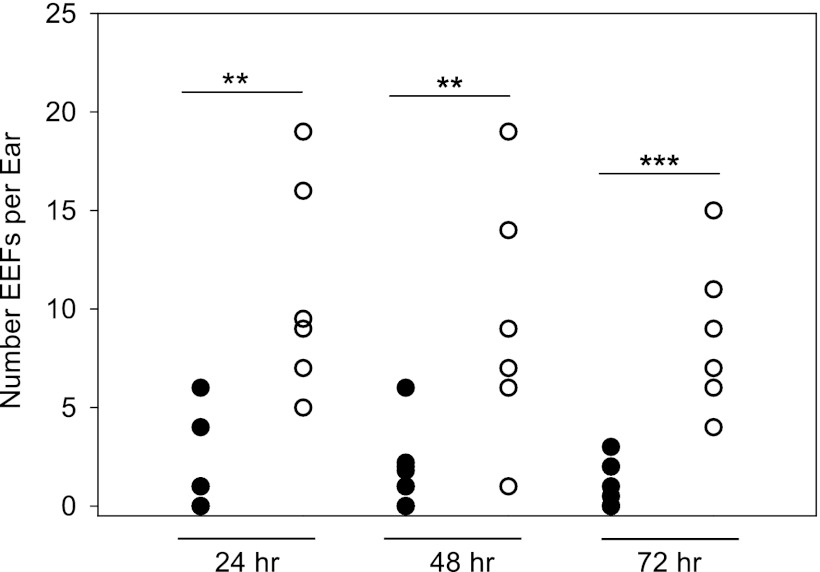
Skin EEF numbers are reduced in mice immunized with irradiated sporozoites.
The effect of vaccination on the development of skin EEFs was investigated. Immunization with irradiated sporozoites leads to protection from live sporozoite challenge due to antibodies that target sporozoites and T cells that destroy infected hepatocytes (reviewed in references 22 and 27). To test whether the skin EEFs were also destroyed, we immunized mice with 75,000 irradiated P. berghei sporozoites, followed by two boosts of 25,000 irradiated sporozoites, and challenged immunized mice with the bites of infected mosquitoes. At the indicated time points, we determined the total number of parasites developing in the skin in immunized and control mice. As shown in Fig. 5, we observed significantly fewer skin EEFs in the ears of vaccinated mice than in those of control animals. Small numbers of controls and immunized mice were challenged by intravenous inoculation of 4,000 sporozoites and monitored for blood-stage infection by Giemsa-stained blood smears. In the two experiments performed, eight immunized mice were challenged, and none of the mice became positive for blood-stage parasites, indicating that the immunization protocol was efficacious. In addition, six naive controls were challenged with sporozoites, and all of them became positive for blood-stage parasites on day 3, indicating that the sporozoites used in these experiments were infectious.
Fig 5.
Effect of immunization on the number of P. berghei skin EEFs. Mice were immunized with irradiated sporozoites (black circles) or not immunized (open circles), and the number of skin EEFs after challenge with 15 infected mosquito bites was determined at the indicated time points. Each circle represents the total number of EEFs observed in one ear. For each time point, a significant statistical difference was observed between the numbers of EEFs in the immunized and corresponding control groups. Mann-Whitney test, **, P < 0.01; ***, P < 0.001. For each time point, at least 6 ears were examined per group. The experiment was performed twice with similar results.
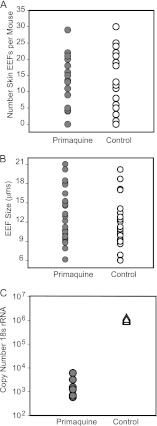
Skin EEF numbers are not affected by primaquine.
The majority of antimalarial drugs target the blood stages of the parasite, and primaquine is the only drug licensed to eliminate liver-stage parasites (reviewed in reference 2). We therefore tested the effect of primaquine on skin EEFs. P. berghei-GFP-infected mosquitoes were allowed to probe the ears of mice that were treated with two doses of primaquine 2 and 24 h after infection. The ears were harvested after 48 h, and the total number of skin EEFs was determined by confocal microscopy. Statistical analysis revealed no significant difference in the number of skin stages between primaquine-treated and control animals (Fig. 6A). Importantly, the skin EEFs observed in the primaquine-treated mice were similar in size to those found in controls (Fig. 6B). In order to confirm that liver schizonts of P. berghei were sensitive to primaquine, we inoculated P. berghei-GFP sporozoites intravenously, dosed the mice with primaquine according to the same protocol described above, and harvested livers 40 h after sporozoite inoculation to determine the liver parasite burden by RT-qPCR. A significant reduction of liver stage parasites was observed in mice that received primaquine treatment compared to their matching controls (Fig. 6C).
Fig 6.
Primaquine decreases the liver-stage parasite burden but not the number or size of skin EEFs in P. berghei. (A) Numbers of skin EEFs in the ears of primaquine-treated (gray circles) or untreated (open circles) mice. Each symbol represents the number of EEFs observed in both ears of a mouse. No statistically significant difference was observed between the treated and control animals using the Mann-Whitney test (P > 0.05). There were 3 to 5 mice per group, and the number of skin EEFs in each ear is shown. Data from 3 independent experiments are shown. (B) Diameter of skin EEFs in the ears of mice treated with primaquine (gray circles) or untreated (open circles). Shown are 25 independent measurements. No significant statistical difference was observed between the sizes of EEFs in mice treated with primaquine and those of controls using the Mann-Whitney test (P > 0.05). (C) Primaquine-treated mice (circles) and untreated controls (triangles) were inoculated with sporozoites, and the liver parasite burden was measured 40 h later by RT-qPCR using primers specific for P. berghei 18S rRNA. Shown is the copy number of parasite rRNA in each sample using a plasmid construct of the 18S rRNA gene to create a standard curve. Each symbol represents one mouse, and there were 6 mice per group. A statistically significant difference was observed between the liver parasite burdens of primaquine-treated and control mice using the Mann-Whitney test; P < 0.01. The experiment was performed twice with similar results.
Skin EEFs do not contribute to the initiation of blood-stage infection.
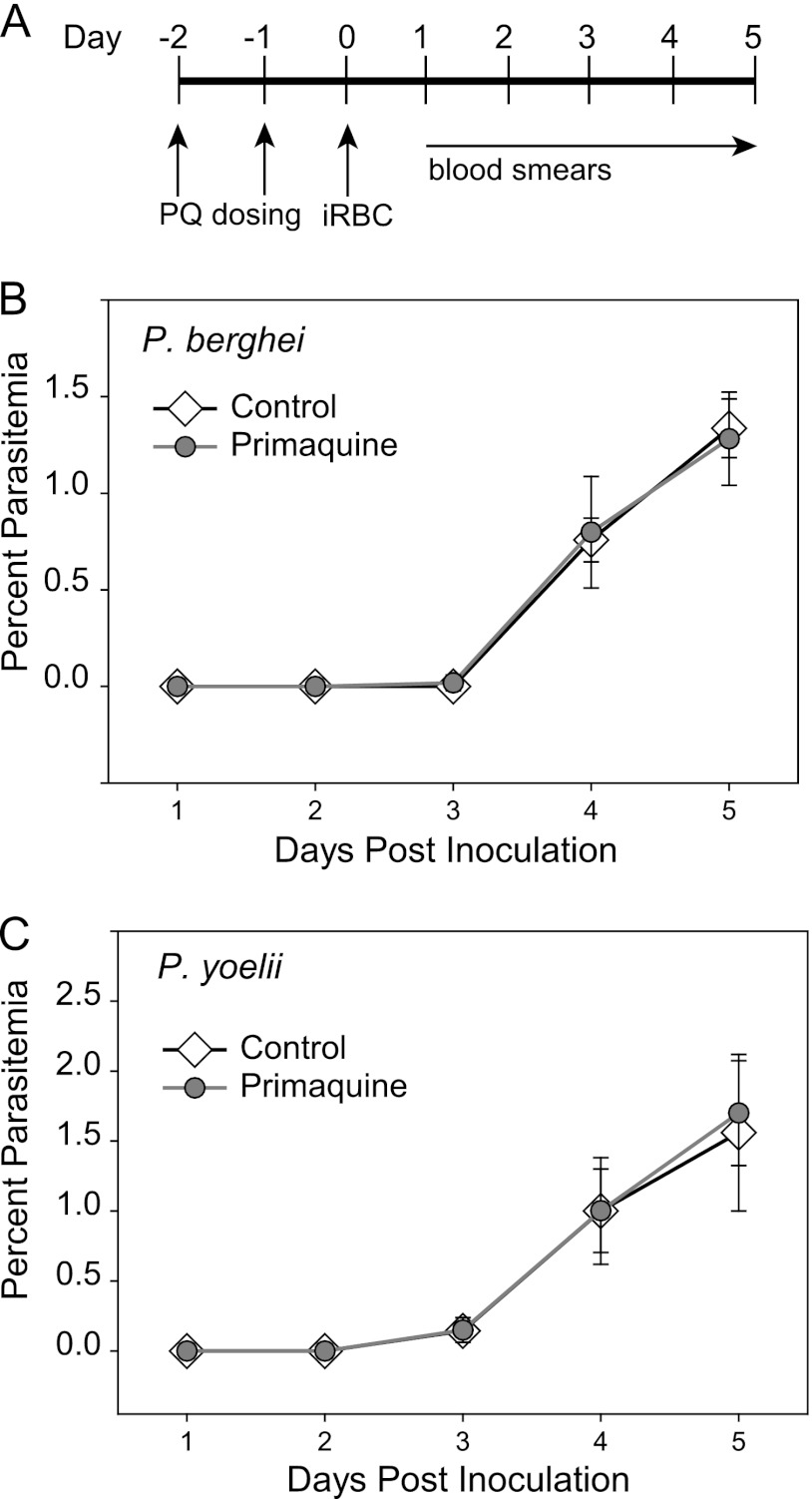
One important question that arises from these findings is whether skin EEFs generate infectious merozoites that can initiate a blood-stage infection. We performed two types of experiments to answer this question. First, we transferred mature P. berghei skin EEFs to naive mice, either by skin grafting or by inoculating homogenized ears, and then monitored the recipients for blood-stage infection. In neither case did we observe malaria infection in recipient mice (data not shown). Since it is possible that the grafting or homogenization process disturbed and ultimately killed the EEFs, we used another approach that took advantage of primaquine's selectivity for hepatic EEFs, as shown in our previous experiment (Fig. 6). We infected mice with P. berghei sporozoites by mosquito bite and treated them with primaquine. We reasoned that primaquine would kill the EEFs developing in the liver, but not in the skin, so if they are able to seed the blood, we should observe a blood-stage infection. Controls for sporozoite infectivity were mice infected by mosquito bite and not treated with primaquine; all of these mice became positive for blood-stage parasites. Controls for the efficacy of primaquine on hepatic EEFs were mice intravenously inoculated with sporozoites and treated with primaquine. In the first experiment, one mouse in each of the primaquine-treated groups, i.e., the mosquito bite group, which would have skin EEFs in addition to hepatic EEFs, and the intravenously inoculated group, became positive on day 8 after sporozoite inoculation (Table 1). This is likely because primaquine did not kill 100% of developing hepatic EEFs (Fig. 6) (19). The similarity between the mosquito bite and i.v. inoculation groups suggests that no additional blood-stage parasites arose from skin EEFs. In follow-up experiments, we inoculated fewer sporozoites and found that none of the intravenously inoculated, primaquine-treated mice became positive for blood-stage parasites. Since none of the mice infected by mosquito bite became positive for blood-stage parasites either, these data indicate that skin EEFs cannot produce merozoites that initiate blood-stage infection (Table 1). We also performed these experiments with P. yoelii sporozoites and found that, similar to those of P. berghei, P. yoelii skin EEFs were not able to initiate a blood-stage infection. To ensure that primaquine had no residual effect on the blood-stage parasites that emerged 44 to 48 h post-sporozoite inoculation, we treated mice with primaquine and inoculated them with blood-stage parasites at the time that we would expect mature liver-stage parasites to begin seeding the blood. As shown in Fig. 7, there was no effect of primaquine on blood-stage parasite growth in P. berghei or P. yoelii. Overall, these data suggest that skin EEFs do not produce infectious merozoites, or if they do, that the difference in architecture between the liver, with its abundant sinusoids, and the endothelial cells of the skin, with their tight junctions, makes it difficult for these merozoites to access the blood circulation from the skin.
Table 1.
Skin exoerythrocytic stages do not give rise to blood-stage infection
| Expt no. | Parasite speciesa | Route of inoculation | Dose | PQb | No. of mice | No. positive for blood-stage parasites | Prepatent period (days)c |
|---|---|---|---|---|---|---|---|
| 1 | P. berghei | Intravenous | 4,000 sporozoites | + | 8 | 1 | 8.0 |
| Mosquito bite | 30 bites | + | 8 | 1 | 8.0 | ||
| Mosquito bite | 30 bites | − | 3 | 3 | 3.0 | ||
| 2 | P. berghei | Intravenous | 1,000 sporozoites | + | 4 | 0 | NA |
| Mosquito bite | 10 bites | + | 8 | 0 | NA | ||
| Mosquito bite | 10 bites | − | 3 | 3 | 4.5 | ||
| 3 | P. berghei | Intravenous | 1,000 sporozoites | + | 3 | 0 | NA |
| Mosquito bite | 10 bites | + | 8 | 0 | NA | ||
| Mosquito bite | 10 bites | − | 3 | 3 | 4.6 | ||
| 4 | P. yoelii | Mosquito bite | 15 bites | + | 10 | 0 | NA |
| Mosquito bite | 15 bites | − | 3 | 3 | 3 |
C57BL/6 mice were used for P. berghei experiments, and BALB/c mice were used for P. yoelli experiments.
PQ, primaquine. +, treated; −, untreated.
NA, not applicable, i.e., none of the mice became positive for blood-stage parasites.
Fig 7.
Primaquine (PQ) has no residual effect on blood stages. (A) Time line showing time points for primaquine dosing, infected-erythrocyte (iRBC) injection, and monitoring by blood smears. (B and C) Mice were treated with primaquine and, 24 h after the last dose, inoculated with 1,000 erythrocytes infected with either P. berghei (B) or P. yoelii (C). Parasitemias were monitored by Giemsa-stained blood smears. There were 5 mice per group, and the mean parasitemia ± SD is shown for each day.
DISCUSSION
Here, we show that sporozoites of two distinct rodent malaria species, P. berghei and P. yoelii, can develop into what appear to be EEFs at the dermal inoculation site. This is the second study describing this somewhat unexpected finding (9). Canonically, EEFs of primate and rodent malaria parasites develop in the liver, a tissue that originates from the endoderm. Thus, the development of skin EEFs is somewhat reminiscent of that of EEFs of avian malaria parasites, which develop in mesodermal tissue, suggesting that this may in fact be a relic of their evolutionary history, in which they share an ancestor. It is also possible, however, that the development of a small proportion of the sporozoite inoculum in the skin is the result of a nonoptimal host-parasite combination. The natural hosts of rodent malaria parasites are African thicket rats (Thamnomys and Grammomys), likely due to the distribution of the vector, Anopheles dureni. Thus, although P. berghei and P. yoelii can infect a wide range of rodents, they may be better adapted to thicket rats, and it would be of interest to determine whether skin EEFs develop in these natural hosts.
We found that only a small percentage of the inoculum developed into skin EEFs. Calculations based upon the demonstration that on average individual mosquitoes inoculate 125 sporozoites (11, 15) indicate that approximately 1.8% of the P. berghei inoculum and 0.5% of the P. yoelii inoculum remained and developed at the inoculation site. In the case of P. yoelii, these numbers are in agreement with those published by Gueirard et al. (9); however, approximately 3 times more P. berghei skin EEFs were observed in their study. This could be due to the different mouse strains used for each study: whereas we used outbred Swiss Webster or C57BL/6 mice, Gueirard et al. used SKH1 hairless mice. Also in contrast to Gueirard et al., we did not observe skin EEFs after needle inoculation (data not shown), which may also be a result of the mouse strain used for each study. In both studies, the numbers of P. berghei skin EEFs was somewhat higher at early time points and decreased over time, whereas the numbers of P. yoelii skin EEFs were low and relatively constant over time (Fig. 3). A previous study performed with P. berghei and P. yoelii, albeit with different strains than the ones we used in our study, found that in naïve mice, P. berghei sporozoites induce a more potent inflammatory response than P. yoelii sporozoites (13), raising the possibility that the different skin EEF numbers between rodent species may be due to the host response.
We investigated the sensitivity of skin EEFs to primaquine, the only drug approved to eradicate liver stages of human malaria parasites, and found that skin EEF numbers are not decreased after primaquine treatment. Little is known about primaquine's mechanism of action; however, it is hypothesized that primaquine is a prodrug and that its toxic metabolites are responsible for killing Plasmodium liver stages (28). If this is the case, it is possible that these metabolites, which are produced in the liver, do not reach the skin in sufficient quantity to kill the parasite, thus accounting for the differential effects on liver and skin EEFs that we observed. Importantly, the different effects of primaquine on skin versus hepatic EEFs allowed us to test whether skin EEFs could directly initiate a blood-stage infection, and we found that they could not. Although Gueirard et al. emphasize the potential infectivity of these skin EEFs and indeed demonstrate that skin EEFs that finish their maturation in vitro and are then inoculated intravenously into mice can cause a blood-stage infection, our data suggest that this does not occur in vivo.
Last, we tested whether immunization with irradiated sporozoites would lead to the destruction of skin EEFs. Immunization with live attenuated sporozoites, inoculated either i.v. or i.d. (5, 30), induces sterile protective immunity in rodents, primates, and humans and is currently the gold standard to which other immunization protocols are compared (17). It is possible that extrahepatic EEFs, due to their location in an immune-privileged site or to the homing of effector T cells primarily to the liver, may resist killing by immune effector cells. Our data demonstrating that skin EEFs are also destroyed in immunized mice suggest that they are fully susceptible to the immune response generated by irradiated sporozoites, indicating that they express antigens similar to those expressed by the EEFs found in the liver.
The potential significance of these findings depends upon two as yet unanswered questions. First, it will be important to determine whether this phenomenon occurs with human malaria parasites. Second, it will be of interest to determine if these parasites play a role immunologically in natural infection, where their location may augment or possibly blunt the immune response to their sister parasites that are developing in the liver.
ACKNOWLEDGMENTS
We thank Sandra Gonzalez and Jean Nonon for expert rearing and infection of mosquitoes and Charlotte Hobbs for reading the manuscript.
This work was funded by the National Institutes of Health (R01 AI056840 to P.S.), NIH training grant 5T32AI07180 (T.V.), and PSC-CUNY Research Award 64163-00 42 (T.V.).
Footnotes
Published ahead of print 19 March 2012
REFERENCES
- 1. Amino R, et al. 2006. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat. Med. 12:220–224 [DOI] [PubMed] [Google Scholar]
- 2. Baird JK. 2005. Effectiveness of antimalarial drugs. N. Engl. J. Med. 352:1565–1577 [DOI] [PubMed] [Google Scholar]
- 3. Bruna-Romero O, et al. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 31:1499–1502 [DOI] [PubMed] [Google Scholar]
- 4. Calvo-Calle JM, Moreno A, Eling WMC, Nardin EH. 1994. In vitro development of infectious liver stages of P. yoelii and P. berghei malaria in human cell lines. Exp. Parasitol. 79:362–373 [DOI] [PubMed] [Google Scholar]
- 5. Chakravarty S, et al. 2007. CD8(+) T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat. Med. 13:1035–1041 [DOI] [PubMed] [Google Scholar]
- 6. Epstein JE, Giersing B, Mullen G, Moorthy V, Richie TL. 2007. Malaria vaccines: are we getting closer? Curr. Opin. Mol. Ther. 9:12–24 [PubMed] [Google Scholar]
- 7. Franke-Fayard B, et al. 2004. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol. Biochem. Parasitol. 137:23–33 [DOI] [PubMed] [Google Scholar]
- 8. Freshney R. 2000. Primary culture, p 149–175 In Freshney R. (ed), Culture of animal cells: a manual of basic techniques. Wiley and Sons, New York, NY [Google Scholar]
- 9. Gueirard P, et al. 2010. Development of the malaria parasite in the skin of the mammalian host. Proc. Natl. Acad. Sci. U. S. A. 107:18640–18645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hollingdale MR, Leland P, Leef JL, Schwartz AL. 1983. Entry of Plasmodium berghei sporozoites into cultured cells and their transformation into trophozoites. Am. J. Trop. Med. Hyg. 32:685–690 [DOI] [PubMed] [Google Scholar]
- 11. Jin Y, Kebaier C, Vanderberg J. 2007. Direct microscopic quantification of dynamics of Plasmodium berghei sporozoite transmission from mosquitoes to mice. Infect. Immun. 75:5532–5539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kappe SH, Vaughan AM, Boddey JA, Cowman AF. 2010. That was then but this is now: malaria research in the time of an eradication agenda. Science 328:862–866 [DOI] [PubMed] [Google Scholar]
- 13. Khan ZM, Vanderberg JP. 1991. Role of host cellular response in differential susceptibility of nonimmunized BALB/c mice to Plasmodium berghei and Plasmodium yoelii sporozoites. Infect. Immun. 59:2529–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumar KA, Oliveira GA, Edelman R, Nardin E, Nussenzweig V. 2004. Quantitative Plasmodium sporozoite neutralization assay. J. Immunol. Methods 292:157–164 [DOI] [PubMed] [Google Scholar]
- 15. Medica DL, Sinnis P. 2005. Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected Anopheline mosquitoes feeding on vertebrate hosts. Infect. Immun. 73:4363–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mueller AK, et al. 2005. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc. Natl. Acad. Sci. U. S. A. 102:3022–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Overstreet MG, Cockburn IA, Chen Y, Zavala F. 2008. Protective CD8+ T cells against Plasmodium liver stages: immunobiology of an ‘unnatural’ immune response. Immunol. Rev. 225:272–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prudêncio M, Rodriguez A, Mota MM. 2006. The silent path to thousands of merozoites: the Plasmodium liver stage. Nat. Rev. Microbiol. 4:849–856 [DOI] [PubMed] [Google Scholar]
- 19. Pukrittayakamee S, et al. 2010. A comparison of two short-course primaquine regimens for the treatment and radical cure of Plasmodium vivax malaria in Thailand. Am. J. Trop. Med. Hyg. 82:542–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sinden RE, Smith J. 1980. Culture of the liver stages (exoerythrocytic schizonts) of rodent malaria parasites from sporozoites in vitro. Trans. R. Soc. Trop. Med. Hyg. 74:134–136 [DOI] [PubMed] [Google Scholar]
- 21. Sinden RE, et al. 1990. The development and routine application of high-density exoerythrocytic-stage cultures of Plasmodium berghei. Bull. World Health Organ. 68(Suppl.):115–125 [PMC free article] [PubMed] [Google Scholar]
- 22. Sinnis P, Nardin E. 2002. Sporozoite antigens: biology and immunology of the circumsporozoite protein and thrombospondin related anonymous protein, p 70–91 In Perlmann P, Troye-Blomberg M. (ed), Malaria mmunology. S. Karger Press, Basel, Switzerland: [DOI] [PubMed] [Google Scholar]
- 23. Sinnis P, Zavala F. 2008. The skin stage of malaria infection: biology and relevance to the malaria vaccine effort. Future Microbiol. 3:275–278 [DOI] [PubMed] [Google Scholar]
- 24. Sturm A, et al. 2006. Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 313:1287–1290 [DOI] [PubMed] [Google Scholar]
- 25. Tarun AS, et al. 2006. Quantitative isolation and in vivo imaging of malaria parasite liver stages. Int. J. Parasitol. 36:1283–1293 [DOI] [PubMed] [Google Scholar]
- 26. Tsuji M, Mattei D, Nussenzweig RS, Eichinger D, Zavala F. 1994. Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol. Res. 80:16–21 [DOI] [PubMed] [Google Scholar]
- 27. Tsuji M, Zavala F. 2003. T cells as mediators of protective immunity against liver stages of Plasmodium. Trends Parasitol. 19:88–93 [DOI] [PubMed] [Google Scholar]
- 28. Vale N, Moreira R, Gomes P. 2009. Primaquine revisited six decades after its discovery. Eur. J. Med. Chem. 44:937–953 [DOI] [PubMed] [Google Scholar]
- 29. Vanderberg J, Frevert U. 2004. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int. J. Parasitol. 34:991–996 [DOI] [PubMed] [Google Scholar]
- 30. Voza T, Kebaier C, Vanderberg JP. 2010. Intradermal immunization of mice with radiation-attenuated sporozoites of Plasmodium yoelii induces effective protective immunity. Malar. J. 9:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. World Health Organization 2010. World malaria report 2010. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2010/9789241564106_eng.pdf [Google Scholar]
- 32. Yamauchi LM, Coppi A, Snounou G, Sinnis P. 2007. Plasmodium sporozoites trickle out of the injection site. Cell. Microbiol. 9:1215–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]