Abstract
An immunomodulatory role of arthropod saliva has been well documented, but evidence for an effect on Plasmodium sp. infectiousness remains controversial. Mosquito saliva may orient the immune response toward a Th2 profile, thereby priming a Th2 response against subsequent antigens, including Plasmodium. Orientation toward a Th1 versus a Th2 profile promotes IgG and IgE proliferation, respectively, where the former is crucial for the development of an efficient antiparasite immune response. Here we assessed the direct effect of mosquito bites on the density of Plasmodium falciparum asexual parasites and the prevalence of gametocytes in chronic, asymptomatic infections in a longitudinal cohort study of seasonal transmission. We additionally correlated these parasitological measures with IgE and IgG antiparasite and anti-salivary gland extract titers. The mosquito biting density was positively correlated with the asexual parasite density but not asexual parasite prevalence and was negatively correlated with gametocyte prevalence. Individual anti-salivary gland IgE titers were also negatively correlated with gametocyte carriage and were strongly positively correlated with antiparasite IgE titers, consistent with the hypothesis that mosquito bites predispose individuals to develop an IgE antiparasite response. We provide evidence that mosquito bites have an impact on asymptomatic infections and differentially so for the production of asexual and sexual parasites. An increased research focus on the immunological impact of mosquito bites during asymptomatic infections is warranted, to establish whether strategies targeting the immune response to saliva can reduce the duration of infection and the onward transmission of the parasite.
INTRODUCTION
Parasitic microorganisms, such as Plasmodium spp., use a variety of mechanisms to subvert host immune defenses (30). The manipulation of the host to reduce an effective immune response is one such method by which parasitic microorganisms can successfully exploit their hosts (1, 29). For arthropod-borne organisms, an immunomodulatory role of arthropod saliva has been reported for arboviruses (23, 45) and protozoa, including Leishmania (3, 13), Trypanosoma (31), and Plasmodium (14). While prior exposure to arthropod saliva can exacerbate infection, immunity against saliva antigens has also been shown to protect against a severe outcome of disease for both Leishmania (22) and Plasmodium (16) infections. Interestingly, immunity to saliva does not have an impact on sporozoite infectivity (25).
It is recognized that the type of immune balance driven by the parasite operates at a very early stage after parasite delivery. The response of sentinel cells, such as dendritic cells, thus determines the evolution of the immune response and can lead to protection, tolerance, or immunopathology (2). Saliva contains pharmacologically active proteins and peptides (42), which provoke a localized allergic reaction in the skin, and the injection of saliva into the skin during a mosquito bite induces the production of IgE and IgG antibodies (8, 9) as well as dermal hypersensitivity reactions (21, 41). This suggests that the saliva can orient the immune response toward a Th2 profile. Dendritic cells that are oriented toward a Th2 phenotype by an antigen are more susceptible to orienting the immune response toward a Th2 profile when confronted by a second antigen (12). Thus, saliva could orient the response mounted against the arthropod-borne pathogen. The orientation of the immune response toward a Th1 profile is crucial for immunity to intracellular pathogens (34), whereas orientation toward a Th2 profile drives immunity to extracellular pathogens and antigens, resulting in class switching, giving rise to IgE-producing B cells (55).
The acquisition of immunity to the human lethal malaria parasite Plasmodium falciparum develops very slowly and is not sterilizing. Even in zones where the transmission intensity is high, the development of immunity results only in premunition, leading to a reduction in the number of clinical episodes and the progressive control of parasite density. Cytophilic immunoglobulins (IgG1 and IgG3), which are capable of eliminating the parasites by opsonization, play an important role in this premunition (51). Although individuals living in regions where malaria is endemic have elevated total and P. falciparum parasite-specific IgE levels, the role of this class of immunoglobulins is unclear. Elevated levels are observed for severe acute clinical episodes, suggesting a pathogenic role of IgE (37), whereas high levels in asymptomatic infections are seemingly protective against subsequent clinical episodes (4).
Studies of immunomodulation have focused on the direct interaction between the host and the pathogen during the infectious process and the immediate consequences thereafter (e.g., see references 24, 25, and 49). Surprisingly, however, no attention has been paid to the longer-term consequences of immunomodulation that impact an existing infection. In areas with highly seasonal transmission where malaria is endemic, individuals can carry P. falciparum parasites without symptoms for the duration of the nontransmission season. The production of gametocytes, specialized sexual parasite stages, is required for transmission from humans to the mosquito. Gametocyte production is associated with nonspecific immune responses occurring during febrile episodes of symptomatic infections. Specific immune responses have also been suggested to induce gametocyte production. Gametocytes are induced following the addition of lymphocytes from naturally infected Gambian children but not after the addition of the same components from European controls (47). Furthermore, it was suggested previously that parasites increase their conversion rate to gametocytes in individuals with acquired immunity (18).
Here we examine the immunomodulatory impact of mosquito saliva in a setting where malaria is endemic and address the hypothesis that saliva impacts existing malaria infections over the long term through the orientation of the Th1/Th2 response, as revealed by specific IgG3 and IgE. Specifically, we carried out a family-based longitudinal cohort study in a region of Senegal where P. falciparum malaria is endemic, to determine whether mosquito biting intensity and individual immunoglobulin profiles are associated with quantitative parasite phenotypes in chronic, long-term, asymptomatic infections.
MATERIALS AND METHODS
Study sites, subjects, and ethical clearance.
A family-based longitudinal cohort study was performed in 2005 in Gouye Kouly (14°43′N, 16°36′W), Senegal. Family structures were constructed by using a questionnaire, interviewing each individual or key representatives of the household to obtain both demographic information, such as birth date, age, and sex, and genetic relationships between children, their parents, and sometimes their grandparents or nonrelatives in the same household and other households. The population was composed of 482 individuals, 387 (80.3%) of whom were enrolled in the study. The majority of individuals were of the Serere ethnic group. Transmission is highly seasonal at this site, with an entomological inoculation rate measured to be approximately 2 infectious bites per person per year. P. falciparum prevalence rates in humans varied from 8% to 15% in the dry and wet seasons. The project protocol and objectives were carefully explained to the assembled village population, and informed consent was individually obtained from all subjects by a signature on a voluntary consent form written in both French and Wolof. The request for volunteers to perform mosquito landing catches, as a specific task within the protocol, was made and discussed during the village meeting and with each individual prior to obtaining written consent. Such volunteers were not placed under chemoprophylaxis given the long length of the study and the estimated high frequency of glucose-6-phosphate dehydrogenase deficiency in the region. The installation of a health clinic at the study site enabled the treatment of all clinical cases of malaria with appropriate antimalarial treatment according to the recommendation of the Malaria Division, Ministry of Public Health. The protocol was approved by the Ethical Committee of the Ministère de la Santé du Senegal.
Blood sample collection for P. falciparum parasite analyses.
An intensive sampling schedule was implemented: prior to the rains in June and then every week for 8 weeks following the onset of the rains (first week of July) and after the end of the transmission season in November. At each time point, a thick blood smear was taken from all individuals. In the June survey and every 2 weeks in July, approximately 300 to 500 μl of blood was collected by finger prick from each individual into an EDTA Microvette system (Sarstedt), 200 μl of which was mixed with 1 ml TRIzol (Invitrogen), kept on dry ice, and then frozen at −80°C for RNA extraction. The remainder of the sample was used for DNA extraction and serological analyses. Following DNA extraction and PCR amplification, all samples that were found to be positive for P. falciparum were then analyzed for the presence of gametocytes by reverse transcription (RT)-PCR. The cohort was randomly divided into two groups (by household), such that half of the cohort provided such a blood sample every week of the 8-week continuous survey. A final survey of the entire population was carried out after the rainy season in November, at which time another 300- to 500-μl blood sample was taken for immunoglobulin analysis. In all cases, parasite positivity was established as follows. Thick and thin blood smears were prepared and stained with 3% Giemsa stain. Blood smears were examined under an oil immersion objective at a ×1,000 magnification by trained laboratory technicians, and 200 thick-smear fields were examined before being declared negative for asexual-stage or gametocyte-stage parasites. The total numbers of leukocytes and parasites were counted, and a parasite/leukocyte ratio established. The number of parasites per microliter was then estimated on the basis of a mean number of 8,000 leukocytes per microliter of blood.
PCR and RT-PCR for P. falciparum gametocyte detection.
DNA was extracted from all samples by using a standard phenol-chloroform extraction method and amplified by using a small-subunit-rRNA (ssrRNA) gene nested PCR method described previously by Snounou et al. (48). RNA extraction was performed with the TRIzol (Invitrogen)-conserved samples of those samples found positive, according to protocols recommended by the manufacturer. The extracted RNA was directly analyzed or stored at −80°C. RT-PCR was carried out as described previously (26). Briefly, the “Plasmodium falciparum meiotic recombination protein DMC1-like protein” gene (GenBank accession number AF356553) was selected because it is expressed exclusively in gametocytes (27) and contains introns. Primers spanning an exon-exon junction were thus selected, amplifying a 101-bp segment, in the middle of which was a probe designed by using Primer3 software (43). Primer sequences used were forward primer GAM8_F (5′-ATATCGGCAGCGAAAATGTGT-3′), reverse primer GAM8_R (5′-GACAATTCCCCTCTTCCACTGA-3′), and probe GAM_PRO (5′–6-carboxyfluorescein [Fam]–TGCCCTTCTCGTAGTTGATTCGATTATT–black hole quencher 1 [BHQ1]–3′). cDNA was synthesized, and the reaction was primed with GAM8_R. Eight microliters of extracted RNA was mixed with buffer, deoxynucleoside triphosphates (dNTPs) (final concentration, 1 mM), RNase-free water, avian myoblastosis virus (AMV) reverse transcriptase (20 U; Promega), and an RNase inhibitor (20 U; Promega). Amplification cycle conditions were as follows: 10 min at 65°C, 60 min at 42°C, and 5 min at 95°C. The quantification of cDNA was carried out by using a fluorescent probe assay. Two microliters of synthesized cDNA was mixed with 2× Mastermix (ABGene), GAM8_R (final concentration, 400 nM), GAM8_F (final concentration, 400 nM), GAM8_PRO (final concentration, 300 nM), and sterile water. The reaction was analyzed with a Rotor Gene real-time PCR machine (Corbett Research). Each sample was analyzed in triplicate. A dilution series containing 1,000, 100, 10, 1, and 10−1 gametocytes/μl was used as controls.
Entomological surveys.
Indoor and outdoor mosquito landing catches on volunteers were performed in 5 locations within the village for 2 nights (from 7 p.m. to 7 a.m.) every week during the rainy season and 2 nights monthly for the rest of the year. The five sites were selected to provide good coverage of the village. Species identification was performed on each individual mosquito on the following day. Anopheline mosquitoes were identified by use of a key reported previously by Diagne et al. (15). A PCR technique described previously by Paskewitz and Collins (35) was used to differentiate the members of the Anopheles gambiae species complex. Culicine mosquitoes were identified by use of a key described previously by Edwards (19).
Preparation of salivary gland extract for enzyme-linked immunosorbent assay (ELISA).
Salivary glands of Anopheles gambiae (Yaounde strain) were dissected under sterile conditions, placed in 1× phosphate-buffered saline (PBS), and sonicated 5 times for 4 min. The solution was then centrifuged at 8,000 × g for 15 min at 4°C. The protein concentration was determined by Nanodrop and diluted in 1× PBS to a concentration of 5 μg/ml.
Parasite (P. falciparum) preparation for ELISA.
P. falciparum (strain 89F5, Palo Alto) in vitro intraerythrocytic cultures that were schizont rich were mixed with water (1 packed cell volume of red blood cells in 4 volumes of water) and an equal volume of lysis buffer (10 mM Tris [pH 8], 0.4 M NaCl, EDTA 10 mM, 2% Triton X-100) and incubated at 4°C for 15 min. The solution was then centrifuged at 4°C for 10 min at 8,000 × g, and the supernatant was reserved for analysis.
IgE ELISA.
Salivary gland (or parasite) extract was diluted in 0.1 M NaHCO3 (pH 9.6), and 50 μl was added per well and incubated at 37°C for 1 h. Plates were then washed three times in wash buffer (1× PBS–0.05% Tween 20). One hundred microliters of blocking buffer (1×PBS–1% bovine serum albumin) was added per well, incubated at 37°C for 1 h, and then washed three times. Serial dilutions of approximately 5% of preseason plasma samples enabled the optimal dilution for measurements of IgE titers. Each plasma sample was diluted 1/5 in 100 μl blocking buffer, and 50 μl per well was added to 2 duplicate wells. Plates were incubated overnight with gentle agitation at room temperature. The next day, plates were washed five times, and 50 μl goat IgG anti-human IgE immunoglobulin (precoupled with alkaline phosphatase) diluted 1/400 in blocking buffer (Sigma-Aldrich, Saint Quentin Fallavier, France) was added to each well and incubated for 2 h at 37°C. The plates were then washed five times, and 50 μl PNPP (4-nitrophenylphosphate disodium salt hexahydrate) (Sigma-Aldrich, Saint Quentin Fallavier, France), the substrate of alkaline phosphatase, dissolved in 0.1 M Tris-HCl (pH 8.8) at 1 mg/ml was added per well. Plates were placed in the dark for 3 h, the reaction was terminated by the addition of 25 μl 1 N NaOH to the mixture, and results were read at 410 nm.
IgG ELISA.
Salivary gland (or parasite) extract was diluted in 0.1 M NaHCO3 (pH 9.6), and 50 μl was added per well and incubated at 37°C for 1 h. Plates were then washed three times in wash buffer (1×PBS–0.05% Tween 20). One hundred microliters of blocking buffer (1× PBS–1% bovine serum albumin) was added per well, incubated at 37°C for 1 h, and then washed three times. Again, serial dilutions of approximately 5% of preseason plasma samples enabled the optimal dilution for measurements of IgG titers. Each plasma sample was diluted 1/50 (for IgG3 [mouse monoclonal antibody ZG4] and IgG4 [mouse monoclonal antibody RJ4]) or 1/100 (total IgG [mouse monoclonal antibody R10Z8E9]) in 100 μl blocking buffer, and 50 μl per well was added to 2 duplicate wells. All monoclonal antibodies were obtained from Skybio Ltd. (Wyboston, Bedfordshire, United Kingdom). Plates were incubated overnight at 4°C. The next day, plates were washed five times, and 50 μl mouse anti-human IgG (diluted 5/1,000 in blocking buffer for IgG3 and IgG4 and diluted 2/1,000 for IgG total) was added and incubated for 2 h with gentle agitation at room temperature. Plates were then washed five times, and 50 μl rabbit anti-mouse antibody coupled with horseradish peroxidase (HRP) (Dako Ltd., Trappes, France) at a dilution of 1/2,000 in blocking buffer was added to each well. Plates were then washed five times, and 50 μl o-phenylenediamine (Sigma-Aldrich, Saint Quentin Fallavier, France), the substrate of HRP, dissolved in citrate buffer (5.1 ml 0.1 M citric acid, 14.9 ml 0.1 M Na3 citrate [pH 5.1]) at 1 mg/ml with 2 μl/ml H2O2 (Sigma-Aldrich, Saint Quentin Fallavier, France), was added to each well. Plates were then placed in the dark for 7 to 10 min, the reaction was stopped with 25 μl 1 N HCl, and results were read at 490 nm.
Statistical analyses.
Statistical analyses and model fitting were conducted by using the statistical package Genstat 7.1 (50). Mean biting rates (averaged over the 2 days of each weekly or monthly survey) were not normally distributed (P < 0.001 by Shapiro-Wilk test) and thus were Box-Cox transformed. Immunoglobulin titers were similarly not normally distributed (P < 0.001 by Shapiro-Wilk test) and thus were also Box-Cox transformed. When the parasite density was used as an explanatory variable, it was ln+1 transformed. For the analysis of the effect of the mosquito biting rate (anopheline mosquitoes only or all mosquitoes) on the individual parasite density, a generalized linear mixed model (GLMM) with a Poisson error structure (log linear regression) was fitted with the individual person as a factor in the random model, to account for multiple measures at different time points for the same individual. Additional fitted explanatory variables were gender and age as continuous variables. For the analysis of the effect of the mosquito biting rate (anopheline mosquitoes only or all mosquitoes) on parasite prevalence rates and the presence or absence of gametocytes in individual blood smears, binomial error structures were implemented (thus, a logistic regression). The ln + 1-transformed parasite density was fitted as an additional explanatory factor in the gametocyte prevalence analysis.
Analyses of the effect of immunoglobulin titers on parasite density, parasite prevalence rates, and the proportion of parasite infections with gametocytes were similarly performed by fitting GLMM with a Poisson or binomial error structure. The immunoglobulin titer measured in June, prior to the transmission season, was used. In addition, because the duration of gametocyte carriage for a single infection in settings where the disease is endemic can last up to 30 days (6, 17), a more conservative analysis of gametocyte positivity was performed; i.e., we analyzed the effect of the immunoglobulin titer on whether an individual ever carried gametocytes by fitting a generalized linear model (GLM) and weighting for the number of samples for each person. F statistics in the GLM and Wald statistics, which approximate to a χ2 distribution, in the GLMM were established.
The following data exclusion criteria were implemented prior to analyses: (i) data for any individual during the week of and 3 weeks following a clinical malaria episode and (ii) data from the 2 weeks following an absence from the village. These exclusion criteria enabled the analysis of only confirmed asymptomatic infections.
RESULTS
Parasite and gametocyte prevalence rates and asexual parasite density.
During the 9 weeks of the survey, a total of 185 samples were found to be positive for P. falciparum by thick smear alone (n = 178) or smear and PCR (n = 185). The mean parasite density was 4.5 parasites/μl (standard deviation [SD], 1.4 parasites/μl; n = 178), increasing from a mean of 3.5 parasites/μl (SD, 0.2 parasites/μl) before the transmission season to a mean of 5.0 parasites/μl (SD, 0.5 parasites/μl) during the transmission season. One hundred forty-five people were positive at least once (range, 1 to 4; median, 1). There were only 8 smears positive for gametocytes, zero of which occurred in the pretransmission season (June). Same-day RNA samples for RT-PCR detection of gametocytes were available for 121 of the 185 parasite-positive samples from 82 of the 145 individuals. Seventy-nine of these samples from 49 individuals were gametocyte positive; thus, 42 samples from 33 individuals were gametocyte negative. For those parasite-positive samples for which a same-day RNA sample was not available, RNA samples from the week before and the week after were analyzed. Two of these 119 samples were found to be gametocyte positive, both from the same individual. In addition, 42 randomly selected RNA samples that were parasite negative were tested for gametocytes; none were found to be positive. A total of 88% of parasite-positive infections prior to the rains had gametocytes (none were smear positive) and comprised 24 of the 81 total gametocyte-positive infections. Of the remaining gametocyte-positive samples, 30 were from apparently new infections, 12 were from infections from previous weeks that were at that time gametocyte negative, and 15 were from infections from previous weeks that were at that time gametocyte positive.
Mosquito biting rates and effect on parasitological measures.
The mosquito biting rate for all species ranged from 14 bites per person per night during the dry season survey to over 100 bites per person per night during the rains. Anopheles biting rates ranged from zero during the dry season to 3 bites/person/night during the rains. The most prevalent mosquitoes were Aedes aegypti and Aedes furcifer, accounting for 71% of all the mosquitoes captured, and Culex tritaeniorhyncus and Culex quinquefasciatus, accounting for 22%. Other species captured were Aedes metallicus, Aedes unilineatus, Aedes vittatus, Aedes neavei, Aedes decens, Aedes vexans, Aedes argenteopunctatus, Aedes sudanensis, Culex lutzia tigripes, Culex perfuscus, Culex antennatus, and Culex univitatus. The only anopheline species observed was Anopheles arabiensis, accounting for 3% of all the mosquitoes captured. Despite some local variation, the coefficient of variation (CV) of the biting density, whether for culicine or anopheline mosquitoes, was <1, suggesting a low variance in mosquito biting rates across the five sampling stations of the study site.
The number of anopheline bites per person was found to be positively associated with a significant increase in parasite density for the same week (P = 0.001), explaining 9% of the overall variation (Table 1). Age was negatively associated with parasite density (P = 0.002). Although similar associations were found when using the anopheline biting density from the week of or 2 weeks before an episode, they were less significant. Age was marginally negatively associated with parasite prevalence rates (P = 0.012); in contrast, there was no effect of mosquito biting rates. The proportion of parasite-positive infections that were also gametocyte positive was found to be negatively associated with anopheles biting rates for that same week (P < 0.001), explaining 10% of the observed variation in gametocyte rates. Parasite density was also negatively correlated with the presence of gametocytes (P < 0.001); there was no effect of age (P = 0.50). As before, the association was less significant when using the anopheline biting density from the week of or 2 weeks before an episode. When using the mosquito biting density of all species, the same patterns were found but were consistently less significant. The biting rates for all species were highly positively correlated with anopheline biting rates (r = 0.85).
Table 1.
Effect of anopheline mosquito biting density on P. falciparum phenotypesa
| Phenotype and parameter | Wald value | P value | Parameter estimate (SE) | Adjusted R2 |
|---|---|---|---|---|
| Parasite density | ||||
| Anopheles density, same wk | 10.36 | 0.001 | 0.076 (0.023) | 0.086 |
| Anopheles density, wk before | 9.49 | 0.002 | 0.066 (0.002) | 0.082 |
| Anopheles density, 2 wk before | 3.89 | 0.049 | 0.032 (0.016) | 0.055 |
| Age | 9.62 | 0.002 | −0.005 (0.002) | |
| Parasite prevalence rate | ||||
| Anopheles density, same wk | 1.77 | 0.183 | ||
| Anopheles density, wk before | 0.47 | 0.495 | ||
| Anopheles density, 2 wk before | 0.98 | 0.322 | ||
| Age | 6.26 | 0.012 | −0.011 (0.004) | 0.004 |
| Proportion of infections harboring gametocytes | ||||
| Anopheles density, same wk | 14.1 | 0.001 | −1.08 (0.29) | 0.10 |
| Anopheles density, wk before | 12.5 | <0.001 | −0.886 (0.250) | 0.12 |
| Anopheles density, 2 wk before | 8.26 | 0.004 | −0.525 (0.183) | 0.09 |
| ln(parasite density + 1) | 12.57 | <0.001 | −0.962 (0.271) |
Shown are Wald statistics, P values, parameter estimates (with standard errors) from the fitted model, and adjusted R2 values. The statistics for the parameters age and ln(parasite density + 1) are those in the best-fit model.
Impact of immunoglobulin titers on parasitological measures.
We then tested the effect of immunoglobulin titers before the transmission season on parasite phenotypes, both including and excluding the impact of anopheline biting described above. Preseason IgE anti-salivary gland extract (anti-SG) titers were strongly negatively associated with the proportion of parasite-positive infections also harboring gametocytes (P = 0.004) (Fig. 1), and this association was increased in coanalyses with the number of anopheline bites (P = 0.002) (Table 2). Similarly, these titers were negatively associated with individuals ever carrying gametocytes when they were parasite positive (P = 0.003). No other tested immunoglobulin titers against salivary glands or parasites had any significant association with any parasite phenotype. The effect of an immunoglobulin class may be influenced by the titers of other Igs. Notably, the effect of IgE titers can be strongly muted by IgG4 titers. Ratios of anti-SG IgG4 to IgE were not found to be associated with any parasite phenotype.
Fig 1.
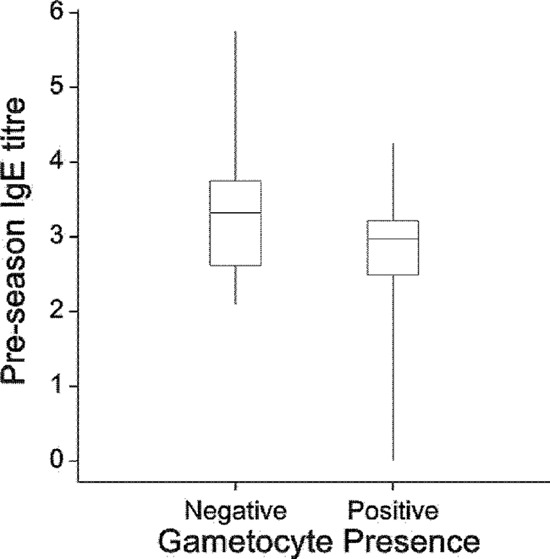
Box plot of pre-anopheline mosquito season IgE anti-salivary gland extract titers (Box-Cox transformed) in individuals who have had or had never carried gametocytes during a P. falciparum infection. The box spans the interquartile range of the values so that the middle 50% of the data lie within the box, with a line indicating the median. Whiskers extend beyond the ends of the box as far as the minimum and maximum values.
Table 2.
Effect of IgE anti-salivary gland extract titer (pre-anopheline mosquito season) on the proportion of P. falciparum infections harboring gametocytesa
| Parameter | Wald value | P value | Parameter estimate (SE) | Adjusted R2 |
|---|---|---|---|---|
| Excluding anopheline mosquito biting rate | ||||
| IgE anti-SG antibody | 8.24 | 0.004 | −2.77 (0.97) | 0.082 |
| ln(parasite density + 1) | 34.9 | <0.001 | −2.17 (0.37) | |
| Including anopheline mosquito biting rate | ||||
| IgE anti-SG antibody | 9.38 | 0.002 | −0.788 (0.257) | 0.131 |
| ln(parasite density + 1) | 13.75 | <0.001 | −0.502 (0.135) | |
| Anopheles density, same wk | 11.69 | <0.001 | −0.580 (0.171) |
Shown are Wald statistics, P values, parameter estimates (with standard errors) from the fitted model, and adjusted R2 values The statistics for ln(parasite density + 1) are those in the best-fit model.
Immunoglobulin profiles and correlation among anti-salivary gland extract and antiparasite immunoglobulin titers.
IgE antiparasite titers were highly positively correlated with IgE anti-SG titers (r = 0.72) (Fig. 2). There were no other strong correlations (r > 0.5) among immunoglobulins. IgE and IgG4 anti-SG titers decreased with age (P = 0.002 and P < 0.001, respectively), whereas IgG3 antiparasite titers increased with age (P < 0.001); IgE antiparasite showed a trend toward a decrease with age, but this was not significant (P = 0.15). There were several striking seasonal changes in immunoglobulin titers. Notably, IgE and IgG4 anti-SG titers increased, as did antiparasite IgG3 titers. Paradoxically, however, antiparasite IgE levels diminished. The differences in antiparasite IgE titers (November versus June) strongly correlated with the IgG3 antiparasite titer in June (r = 0.77); i.e., those individuals who showed the lowest decrease in antiparasite IgE titers had the highest June IgG3 antiparasite titers.
Fig 2.
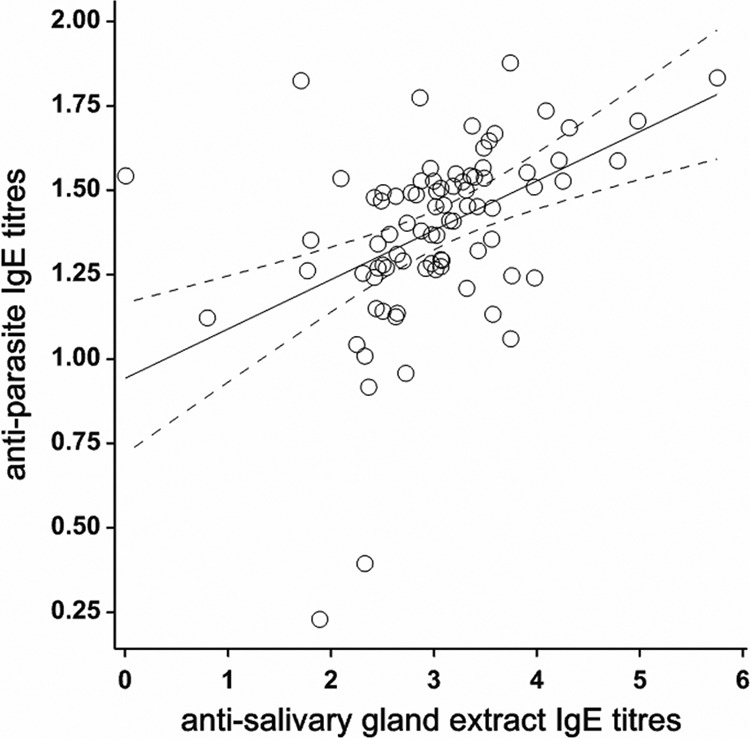
Correlation between antiparasite and anti-salivary gland extract titers (Box-Cox transformed). Shown is the linear correlation (solid line) and the 95% confidence intervals estimated from a log linear regression (dashed lines).
DISCUSSION
In this study, we observed that the mosquito biting density was strongly positively associated with parasite density but with no impact on parasite prevalence rates. This seasonal trend was noted previously for a very different setting in Liberia (33). Although new infections could lead to such an increased parasite density, the absence of any change in parasite prevalence rates and the association with mosquito biting rates for the same week, rather than the week before (given the development period of at least 1 week in the liver) argue against this. Furthermore, this confirms previously reported observations of an experimental mouse model which found that mosquito bites accelerated the malaria parasite asexual replication rate even during primary infections (5), although this was not confirmed (46).
We found a strong negative impact of mosquito bites on the production of gametocytes but that it was notably due to the high percentage of dry season (when anopheline mosquitoes were absent and other mosquito spp. were found at much reduced abundances) infections that carried gametocytes. The time since infection (40) and time since treatment (28) were previously highlighted as increasing gametocyte carriage in symptomatic infections. The relevance of this for chronic, asymptomatic infections is unclear. We are unable to differentiate the age of infection and the much reduced level of mosquito biting during the dry season in this study. However, as discussed below, the strong negative correlation between anti-SG titers and the occurrence of gametocytes does suggest some influence of mosquito bites on gametocyte production.
The individual anti-SG IgE titer was also found to be strongly positively correlated with the IgE antiparasite titer. This is consistent with the hypothesis that mosquito bites predispose individuals to develop an IgE antiparasite response, potentially by the orientation of the immune response to a Th2 profile (53). Such an orientation of the immune response would be expected to lead to a reduced Th1-type environment, resulting in a lower acquisition of asexual parasite-targeting effectors and, thus, a more fertile ground for asexual parasite survival. This is consistent with evidence suggesting that individuals with higher levels of acquired immunity induce a higher level of gametocyte conversion in infecting parasites (7). Although IgG3 antiparasite titers did not impact gametocyte prevalence or parasite density, they increased with age, which itself had a significant negative impact on parasite density. Interestingly, IgG3 antiparasite titers were negatively correlated with the seasonal decrease in IgE antiparasite titers. Such a seasonal decrease might be indicative of an exhaustion of circulating IgE, potentially being bound to effector cells. If this is the case, there is clearly competitive interference of antiparasite IgE by antiparasite IgG3, with potential consequences for the parasite.
There is conflicting evidence concerning a role for mosquito saliva in facilitating the initiation of an infection by Plasmodium sporozoites (25, 49). Here we provide evidence that mosquito saliva has a demonstrable impact on the parasite during the chronic asymptomatic stage of infection. We previously proposed that with seasonal mosquito activity in regions where malaria is endemic, such chronic-infection parasites may respond to the effects of anopheline bites by producing gametocytes in order to transmit rapidly after the expansion of the anopheline population (36). The general effect of all mosquito species bites on parasite phenotypes and the predominance of culicine mosquitoes observed here suggest that parasites may respond generally to increased mosquito bites. Parasites need to produce gametocytes to transmit to mosquitoes, and they are generated from the circulating asexual parasite population. Consequently, parasites are faced with a tradeoff between, on the one hand, producing a sufficient number of asexual parasites to maintain an effective population size to withstand immunological destruction and, on the other, generating a sufficient number of gametocytes to be able to transmit. Chronic-infection parasites persist at very low densities, often being detectable only by PCR. The parasite must therefore generate gametocytes (at a density of at least 1 gametocyte/μl) from a very-low-density asexual population. Accelerated parasite replication following anopheline mosquito bites would provide parasites with a sufficient biomass to generate gametocytes at densities high enough to ensure transmission, a phenomenon observed previously for mouse model studies (5). An alternative explanation for such an accelerated rate of parasite replication is that it is a parasite response enabling it to outcompete coinfecting clones either for resources (i.e., direct competition) or for “enemy-free” space (i.e., avoiding the immunological consequences induced by another clone—apparent competition [32]). An investment in asexual stages would thus be at a cost to gametocyte production, hence the observed negative impact of parasite density on gametocyte prevalence.
The role of IgE in the immunoallergic response has been well documented, but its role in the outcome of malaria infection remains controversial and poorly understood. The levels of P. falciparum-specific IgE are elevated during a malaria episode, and it was proposed previously that it plays a pathogenic role in severe episodes (20, 38, 39), whereas for asymptomatic infections, IgE levels were associated with protection (4). As described above, an important role for the Th1/Th2 balance in the outcome of infection was suggested previously by several studies (20). A recent genomewide linkage study identified several loci that were linked to asymptomatic parasite densities (44), and all these loci were previously linked to asthma/atopic disease or related phenotypes (e.g., see references 52 and 54). The acquisition of premunition following successive infections may therefore include the development of immunotolerance as well as immunoprotection.
Strategies that reduce the development of effective immune responses will not only enable an increased duration of the concurrent infection but also potentially enable the reinfection of the same host by the same strain. The extent to which parasites actively manipulate the Th1/Th2 balance, rather than simply profit from the allergenic nature of mosquito saliva, is not clear. Parasites are known to alter the expressions of several salivary gland proteins during their development within the salivary gland (10), but their immunogenicity has not been characterized and would in any case be relevant only for the initial invasion of the host. It seems more likely that the parasite is profiting from the allergenic nature of mosquito bites to then induce a Th2 response against itself. While repeated infections will eventually lead to the development of an effective immune response, it will be substantially delayed. A single infection by a clone of P. falciparum can last up to 2 years, a duration which may be facilitated by such immunomodulation in addition to mechanisms such as antigenic variation (11). Sterilizing immunity, if ever achieved, takes a lifetime of regular exposure to infection, and a single strain can infect the same individual twice. It is thus possible that immunomodulation can enable repeated infections of the same host and, hence, is a key mechanism for maintaining a permissive host population.
In conclusion, this work contributes to the ongoing debate concerning the targeting of mosquito saliva components as a strategy for malaria control (16, 25). In contrast to the focus on the initial stages of infection in naïve hosts, our work suggests that there may be longer-term effects of mosquito saliva that promote parasite persistence in chronic infections. Thus, while the prevention of infections is optimal, mechanisms aimed at reducing the duration of infection will contribute to reducing the prevalence and onward transmission of the parasite.
ACKNOWLEDGMENTS
We are grateful to the villagers of Gouye Kouly for their participation and continued collaboration and to the field workers for their active contribution to this project. We thank Genevieve Milon for constructive criticism of the manuscript. We also thank the Center for the Production and Infection of Anopheles, Institut Pasteur, for providing Anopheles mosquitoes for salivary gland dissection and gametocytes for the positive-control dilution series.
This work was supported by the Strategic Anopheles Horizontal Research Programme, Institut Pasteur.
Footnotes
Published ahead of print 26 March 2012
REFERENCES
- 1. Alcami A, Koszinowski UH. 2000. Viral mechanisms of immune evasion. Trends Microbiol. 8:410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Banchereau J, et al. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811 [DOI] [PubMed] [Google Scholar]
- 3. Belkaid Y, et al. 1998. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 188:1941–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bereczky S, et al. 2004. Elevated anti-malarial IgE in asymptomatic individuals is associated with reduced risk for subsequent clinical malaria. Int. J. Parasitol. 34:935–942 [DOI] [PubMed] [Google Scholar]
- 5. Billingsley PF, Snook LS, Johnston VJ. 2005. Malaria parasite growth is stimulated by mosquito probing. Biol. Lett. 1:185–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bousema JT, et al. 2004. Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malar. J. 3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bousema T, Drakeley C. 2011. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin. Microbiol. Rev. 24:377–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brummer-Korvenkontio H, Lappalainen P, Reunala T, Palosuo T. 1994. Detection of mosquito saliva-specific IgE and IgG4 antibodies by immunoblotting. J. Allergy Clin. Immunol. 93:551–555 [DOI] [PubMed] [Google Scholar]
- 9. Chen YL, Simons FE, Peng Z. 1998. A mouse model of mosquito allergy for study of antigen-specific IgE and IgG subclass responses, lymphocyte proliferation, and IL-4 and IFN-gamma production. Int. Arch. Allergy Immunol. 116:269–277 [DOI] [PubMed] [Google Scholar]
- 10. Choumet V, et al. 2007. The salivary glands and saliva of Anopheles gambiae as an essential step in the Plasmodium life cycle: a global proteomic study. Proteomics 7:3384–3394 [DOI] [PubMed] [Google Scholar]
- 11. Deitsch KW, Moxon ER, Wellems TE. 1997. Shared themes of antigenic variation and virulence in bacterial, protozoal, and fungal infections. Microbiol. Mol. Biol. Rev. 61:281–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Jong EC, et al. 2002. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J. Immunol. 168:1704–1709 [DOI] [PubMed] [Google Scholar]
- 13. de Moura TR, et al. 2007. Enhanced Leishmania braziliensis infection following pre-exposure to sandfly saliva. PLoS Negl. Trop. Dis. 1:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Depinay N, Hacini F, Beghdadi W, Peronet R, Mécheri S. 2006. Mast cell-dependent down-regulation of antigen-specific immune responses by mosquito bites. J. Immunol. 176:4141–4146 [DOI] [PubMed] [Google Scholar]
- 15. Diagne N, et al. 1994. Les anophèles du Sénégal: liste commentée et illustrée. Bull. Soc. Pathol. Exot. 87:267–277 [PubMed] [Google Scholar]
- 16. Donovan MJ, et al. 2007. Uninfected mosquito bites confer protection against infection with malaria parasites. Infect. Immun. 75:2523–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drakeley CJ, Flobbe K, Greenwood BM, Targett GA. 2000. Plasmodium falciparum gametocytes in Gambian adults. Ann. Trop. Med. Parasitol. 94:399–401 [DOI] [PubMed] [Google Scholar]
- 18. Drakeley C, Sutherland C, Bousema JT, Sauerwein RW, Targett GA. 2006. The epidemiology of Plasmodium falciparum gametocytes: weapons of mass dispersion. Trends Parasitol. 22:424–430 [DOI] [PubMed] [Google Scholar]
- 19. Edwards FW. 1941. Mosquitoes of the Ethiopian region. III. Culicine adults and pupae. British Museum of Natural History, London, United Kingdom [Google Scholar]
- 20. Elghazali G, Perlmann H, Rutta AS, Perlmann P, Troye-Blomberg M. 1997. Elevated plasma levels of IgE in Plasmodium falciparum-primed individuals reflect an increased ratio of IL-4 to interferon-gamma (IFN-gamma)-producing cells. Clin. Exp. Immunol. 109:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. French FE, West AS. 1971. Skin reaction specificity of guinea pig immediate hypersensitivity to bites of four mosquito species. J. Parasitol. 57:396–400 [PubMed] [Google Scholar]
- 22. Gomes R, et al. 2008. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc. Natl. Acad. Sci. U. S. A. 105:7845–7850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hajnická V, et al. 2005. Manipulation of host cytokine network by ticks: a potential gateway for pathogen transmission. Parasitology 130:333–342 [DOI] [PubMed] [Google Scholar]
- 24. Hiscott J, Nguyen TL, Arguello M, Nakhaei P, Paz S. 2006. Manipulation of the nuclear factor-kappaB pathway and the innate immune response by viruses. Oncogene 25:6844–6867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kebaier C, Voza T, Vanderberg J. 2010. Neither mosquito saliva nor immunity to saliva has a detectable effect on the infectivity of Plasmodium sporozoites injected into mice. Infect. Immun. 78:545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lawaly YR, et al. 2010. Heritability of the human infectious reservoir of malaria parasites. PLoS One 5:e11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Le Roch KG, et al. 2003. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science 301:1503–1508 [DOI] [PubMed] [Google Scholar]
- 28. Loucoubar C, et al. 2011. Impact of changing drug treatment and malaria endemicity on the heritability of malaria phenotypes in a longitudinal family-based cohort study. PLoS One 6:e26364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maizels RM, Pearce EJ, Artis D, Yazdanbakhsh M, Wynn TA. 2009. Regulation of pathogenesis and immunity in helminth infections. J. Exp. Med. 206:2059–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matthews KR. 2011. Controlling and coordinating development in vector-transmitted parasites. Science 331:1149–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mesquita RD, et al. 2008. Trypanosoma cruzi infection is enhanced by vector saliva through immunosuppressant mechanisms mediated by lysophosphatidylcholine. Infect. Immun. 76:5543–5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mideo N. 2009. Parasite adaptations to within-host competition. Trends Parasitol. 25:261–268 [DOI] [PubMed] [Google Scholar]
- 33. Miller MJ. 1958. Observations on the natural history of malaria in the semi-resistant West African. Trans. R. Soc. Trop. Med. Hyg. 52:152–168 [DOI] [PubMed] [Google Scholar]
- 34. Mosmann TR, Coffman RL. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145–173 [DOI] [PubMed] [Google Scholar]
- 35. Paskewitz SM, Collins FH. 1990. Use of polymerase chain reaction to identify mosquitoes species of the Anopheles gambiae complex. Med. Vet. Entomol. 4:367–373 [DOI] [PubMed] [Google Scholar]
- 36. Paul REL, Diallo M, Brey PT. 2004. Mosquitoes and transmission of malaria parasites—not just vectors. Malar. J. 3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perlmann H, et al. 1994. IgE elevation and IgE anti-malarial antibodies in Plasmodium falciparum malaria: association of high IgE levels with cerebral malaria. Clin. Exp. Immunol. 97:284–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Perlmann P, et al. 1997. Immunoglobulin E, a pathogenic factor in Plasmodium falciparum malaria. Infect. Immun. 65:116–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perlmann P, Perlmann H, ElGhazali G, Blomberg MT. 1999. IgE and tumor necrosis factor in malaria infection. Immunol. Lett. 65:29–33 [DOI] [PubMed] [Google Scholar]
- 40. Price R, et al. 1999. Risk factors for gametocyte carriage in uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 60:1019–1023 [DOI] [PubMed] [Google Scholar]
- 41. Reunala T, Brummer-Korvenkontio H, Rasanen L, Francois G, Palosuo T. 1994. Passive transfer of cutaneous mosquito-bite hypersensitivity by IgE anti-saliva antibodies. J. Allergy Clin. Immunol. 94:902–906 [DOI] [PubMed] [Google Scholar]
- 42. Ribeiro JM. 1987. Role of saliva in blood-feeding by arthropods. Annu. Rev. Entomol. 32:463–478 [DOI] [PubMed] [Google Scholar]
- 43. Rozen S, Skaletsky HJ. 2000. Primer3 on the WWW for general users and for biologist programmers, p 365–386 In Krawetz S, Misener S. (ed), Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, NJ: [DOI] [PubMed] [Google Scholar]
- 44. Sakuntabhai A, et al. 2008. Genetic determination and linkage mapping of Plasmodium falciparum malaria related traits in Senegal. PLoS One 3:e2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schneider BS, Higgs S. 2008. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans. R. Soc. Trop. Med. Hyg. 102:400–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shutler D, Reece SE, Mullie A, Billingsley PF, Read AF. 2005. Rodent malaria parasites Plasmodium chabaudi and P. vinckei do not increase their rates of gametocytogenesis in response to mosquito probing. Proc. Biol. Sci. 272:2397–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smalley ME, Brown J. 1981. Plasmodium falciparum gametocytogenesis stimulated by lymphocytes and serum from infected Gambian children. Trans. R. Soc. Trop. Med. Hyg. 75:316–317 [DOI] [PubMed] [Google Scholar]
- 48. Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. 1993. Identification of the four human malaria parasite species in field samples by polymerase chain reaction and detection of high prevalence of mixed infections. Mol. Biochem. Parasitol. 58:283–292 [DOI] [PubMed] [Google Scholar]
- 49. Vaughan JA, Scheller LF, Wirtz RA, Azad AF. 1999. Infectivity of Plasmodium berghei sporozoites delivered by intravenous inoculation versus mosquito bite: implications for sporozoite vaccine trials. Infect. Immun. 67:4285–4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. VSN International 2003. GenStat for Windows, 7th ed VSN International Ltd., Hemel Hempstead, United Kingdom [Google Scholar]
- 51. Wilson RJ, McGregor IA. 1973. Immunoglobulin characteristics of antibodies to malarial S-antigens in man. Immunology 25:385–398 [PMC free article] [PubMed] [Google Scholar]
- 52. Xu J, et al. 2001. Genomewide screen and identification of gene-gene interactions for asthma-susceptibility loci in three U.S. populations: collaborative study on the genetics of asthma. Am. J. Hum. Genet. 68:1437–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zeidner NS, Higgs S, Happ CM, Beaty BJ, Miller BR. 1999. Mosquito feeding modulates Th1 and Th2 cytokines in flavivirus susceptible mice: an effect mimicked by injection of sialokinins, but not demonstrated in flavivirus resistant mice. Parasite Immunol. 21:35–44 [DOI] [PubMed] [Google Scholar]
- 54. Zhang Y, et al. 2003. Positional cloning of a quantitative trait locus on chromosome 13q14 that influences immunoglobulin E levels and asthma. Nat. Genet. 34:181–186 [DOI] [PubMed] [Google Scholar]
- 55. Zhu J, Paul WE. 2008. CD4 T cells: fates, functions, and faults. Blood 112:1557–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]


