Abstract
Within the genus Mycoplasma are species whose cells have terminal organelles, polarized structures associated with cytadherence and gliding motility. Mycoplasma penetrans, found mostly in HIV-infected patients, and Mycoplasma iowae, an economically significant poultry pathogen, are members of the Mycoplasma muris phylogenetic cluster. Both species have terminal organelles that interact with host cells, yet the structures in these species, or any in the M. muris cluster, remain uncharacterized. Time-lapse microcinematography of two strains of M. penetrans, GTU-54-6A1 and HF-2, and two serovars of M. iowae, K and N, show that the terminal organelles of both species play a role in gliding motility, with differences in speed within and between the two species. The strains and serovars also differed in their hemadsorption abilities that positively correlated with differences in motility speeds. No morphological differences were observed between M. penetrans and M. iowae by scanning electron microscopy (SEM). SEM and light microscopy of M. penetrans and M. iowae showed the presence of membranous filaments connecting pairs of dividing cells. Breaking of this filament during cell division was observed for M. penetrans by microcinematography, and this suggests a role for motility during division. The Triton X-100-insoluble fractions of M. penetrans and M. iowae consisted of similar structures that were unique compared to those identified in other mycoplasma species. Like other polarized mycoplasmas, M. penetrans and M. iowae have terminal organelles with cytadherence and gliding functions. The difference in function and morphology of the terminal organelles suggests that mycoplasmas have evolved terminal organelles independently of one another.
INTRODUCTION
Reductive evolution of the bacterial genus Mycoplasma from Gram-positive bacteria has resulted in cell wall-less organisms with both reduced genome size and reduced biosynthetic ability. This genus includes both commensals and pathogens and is associated with vertebrate hosts, including humans. For many pathogenic mycoplasmas, a first step in causing disease is adherence to host cells, often by adhesins associated with a polarized organelle (1). The interactions of mycoplasmas with host cells are diverse and complex. Mycoplasma pneumoniae, a human pathogen, requires a polar terminal organelle for cytadherence and gliding motility (1), which may be required for colonization of host tissue (13). Mycoplasma mobile, a fish pathogen, also has a terminal organelle but with morphology distinct from that of M. pneumoniae (1). Although both species have terminal organelles, the proteins involved in biogenesis and function of these polar structures are completely different. For example, M. pneumoniae adhesins P1 and P30 are located at the tips and/or sides of the terminal organelle (29), whereas the M. mobile adhesin Gli349 is found at the base, or neck, of its terminal organelle (33). The two species also have very different gliding characteristics (11, 25) and distinct internal organizations of their respective polar organelles (3, 23).
Mycoplasma penetrans and Mycoplasma iowae are both found in the Mycoplasma muris cluster within the pneumoniae group. M. penetrans is found commonly in the urogenital tract of HIV-positive individuals (19, 34) but has also been isolated from the blood of a non-HIV-infected individual with antiphospholipid syndrome (35). Previous research has focused on the role of M. penetrans in AIDS. Its lipoproteins are mitogenic (5) and stimulate transcription of the HIV genome in HIV-infected cells in vitro via Toll-like receptors (30), suggesting that M. penetrans may play a role in expediting the progression of AIDS. M. iowae is a poultry pathogen isolated from the respiratory tract of turkeys and chickens (36) and the gastrointestinal tract of turkeys (21). In experimental infections, M. iowae causes embryo death in turkey (36) and chicken eggs (4). M. iowae commercial infections have been identified in turkey poults, resulting in a variety of leg abnormalities (16, 31), which can have a negative impact upon meat production.
Both M. penetrans and M. iowae exhibit polarity, with a terminal polar organelle mediating adherence to human epithelial cells (19) and colonization of intestinal mucosa in turkeys (21), respectively. The proteins involved in the organization and function of the polar organelle remain unknown in both M. penetrans and M. iowae; the M. penetrans genome lacks homologs of genes involved in terminal organelle structure in M. pneumoniae and M. mobile (10, 12, 28). Electron microscopy reveals that both species have two distinct cytoplasmic components. The polar organelles of M. penetrans and M. iowae are densely packed with material of a fine granular structure, compared with the cell body, whose appearance is more typical of bacterial cytoplasm (19, 21). Although it is likely that these organisms utilize a polar organelle to mediate attachment and invade their respective hosts (6), it is unknown whether features typically associated with this structure in other polarized mycoplasmas, including gliding motility and an internal cytoskeleton, are present. Improved understanding of the mechanisms that M. penetrans and M. iowae use to interact with host cells is hampered by the lack of a genetic manipulation system for use in M. penetrans and the lack of a completed genome sequence for M. iowae. Despite the availability of a genome sequence for M. penetrans, it is unclear which proteins are associated with its terminal organelle.
Reasoning that, as in other mycoplasmas, polarity in M. penetrans and M. iowae might be associated with gliding motility and a cytoskeletal structure and that the elements responsible for these properties are likely to be novel, we investigated strains of M. penetrans and M. iowae by detergent treatment, microscopy, and microcinematography. Both M. penetrans and M. iowae were found to be motile but with properties that distinguish both organisms from other mycoplasmas. In addition, they share novel, detergent-insoluble structures consistent with a terminal organelle-associated cytoskeletal element. This study expands the known diversity of mycoplasma cell structure and provides insight into interactions between these two species and their respective host cells.
MATERIALS AND METHODS
Bacterial culture conditions.
Mycoplasma penetrans strains GTU-54-6A1 (18) and HF-2 (35) and Mycoplasma iowae serovars N and K (2) were cultured at 37°C in SP-4 broth (32) or on SP-4 agar plates.
Hemadsorption (HA).
Plates containing mycoplasma colonies were incubated with sheep red blood cells (SRBC) in Alsever's solution (Cleveland Scientific) and washed with phosphate-buffered saline as previously described (8). Colonies were observed and photographed at a ×400 magnification on a Leica DM IRB inverted phase-contrast/epifluorescence microscope.
Time-lapse microcinematography.
M. penetrans and M. iowae cells from frozen, mid-log-phase stocks were filtered through a 0.45-μm-pore-size filter and incubated 3 h at 37°C in glass chamber slides (Nunc) in SP-4 broth supplemented with 3% gelatin. In each analysis, 27 images were captured at a ×1,000 magnification on a Leica DM IRB inverted phase-contrast/epifluorescence microscope at intervals ranging from 0.5 to 1 s. Images were merged and analyzed as previously described (8). Gliding speeds of individual cells were calculated by dividing the distance traveled by the number of frames between which cells moved. For each strain, at least 100 motile cells were analyzed.
SEM.
Cells were prepared for scanning electron microscopy (SEM) as previously described (8). Briefly, they were grown for 6 h to 1 day in SP-4 broth supplemented with 3% gelatin at 37°C. To analyze Triton X-100-insoluble structures, Triton X-100 in 20 mM Tris-HCl (pH 7.5)–150 mM NaCl was added to whole cells to a final concentration of 1%, and the coverslips were incubated for 30 min at 37°C. Coverslips were fixed for 30 min in 1.5% glutaraldehyde, 1% paraformaldehyde, and 0.1 M sodium cacodylate, pH 7.2, rinsed with buffer, and dehydrated through a series of ethanol washes from 25% to 100%. Following dehydration, the coverslips were critical-point dried and then viewed on a Zeiss Supra 35 FEG-VP scanning electron microscope.
Membrane and DNA staining.
To visualize cell membranes, cells were grown overnight in 24-well plates on glass coverslips in SP-4 broth supplemented with 3% gelatin at 37°C. Cells were stained with 3,3′-dihexyloxacarbocyanine iodide (DiOC6; Sigma-Aldrich) for 10 min at a final concentration of 5 μg/ml and then fixed directly in the medium using the same fixative as for SEM. To visualize the nucleoid, cells were grown overnight. The medium and fixative were removed, and 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) solution was added to the coverslips for 30 min in the dark at a final concentration of 1 μg/ml. Cells were visualized using phase-contrast microscopy. DiOC6 and DAPI were visualized by epifluorescence microscopy using rhodamine and DAPI filter sets, respectively.
RESULTS
Gliding speed and HA.
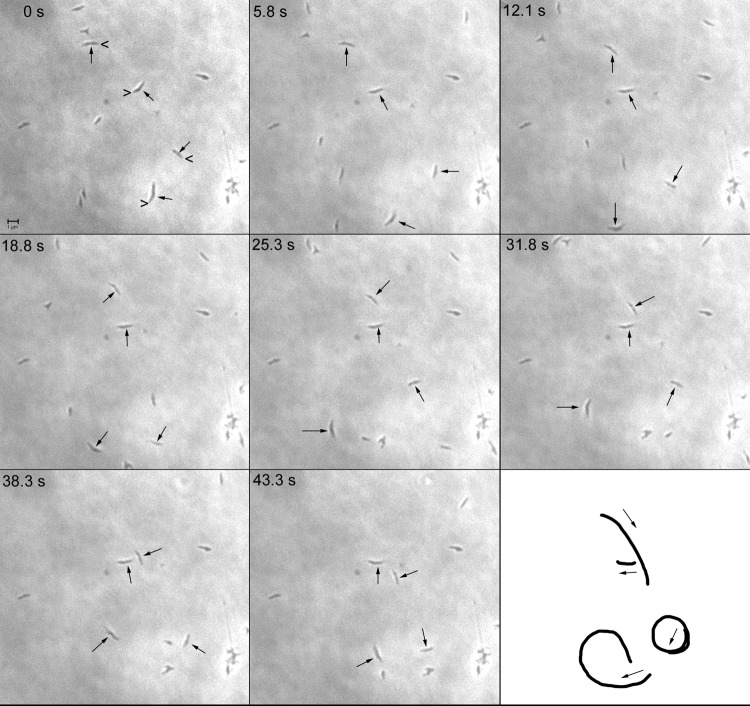
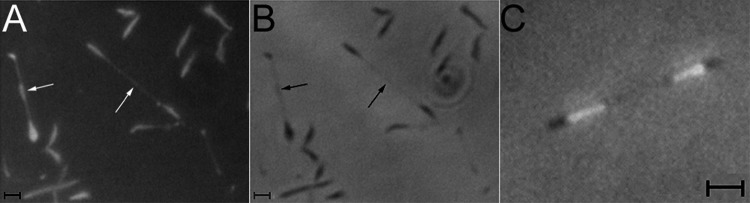
Because M. penetrans and M. iowae attach via a polar structure (19, 21), we tested whether these organisms exhibited gliding motility, which is characteristic of other mycoplasma species bearing similar structures (1). M. penetrans strains GTU-54-6A1 and HF-2 and M. iowae serovars N and K were all found to be motile, albeit at distinctly different speeds (Table 1). Consecutive phase-contrast images of M. penetrans gliding showed that cells moved in a unidirectional manner, led by one end of the cell (Fig. 1). Cells moved in both straight and curved paths but never reversed direction.
Table 1.
Gliding motility parameters and Triton X-100-insoluble structure dimensions of M. penetrans and M. iowae
| Organism | Speed (range [nm/s])a | Cells moving per frame (%) | Length of insoluble structure (nm)a | Greatest width of insoluble structure (nm)a | Width of insoluble structure across narrow portion (nm)a |
|---|---|---|---|---|---|
| M. penetrans strain GTU-54-A1 | 548 ± 217 (151–1,284) | 23 | 449 ± 79 | 187 ± 60 | 127 ± 25 |
| M. penetrans strain HF-2 | 54 ± 18 (25–111) | 30 | ND | ND | ND |
| M. iowae serovar N | 797 ± 427 (200–2,150) | 13 | 442 ± 54 | 237 ± 71 | 109 ± 18 |
| M. iowae serovar K | 270 ± 125 (95–621) | 8 | ND | ND | ND |
Values are means ± standard deviations. ND, not determined.
Fig 1.
Consecutive phase-contrast images of M. penetrans in a chamber slide at intervals of 5 to 6 s at 37°C. Four representative cells are indicated by arrows. In the first frame (0 s), the carets point to the leading ends of gliding cells. In the last frame, the paths of the gliding cells are represented, with arrows indicating the direction of gliding during the observation period. Scale bar, 1 μm.
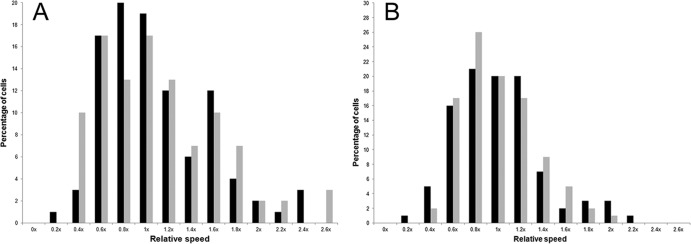
In both species, average gliding speeds were within the range of extremes previously observed for mycoplasma motility, that is, <30 nm/s for Mycoplasma pirum and Mycoplasma insons and >2,500 nm/s for Mycoplasma testudinis and Mycoplasma mobile (9, 14, 26). On average, M. penetrans strain GTU-54-6A1 glided 10 times faster than strain HF-2, and M. iowae serovar N glided 3 times faster than M. iowae serovar K. Unlike other motile species in the pneumoniae group, both M. penetrans and M. iowae exhibited a large range in their average gliding speeds (Table 1; Fig. 2); however, as with M. insons, gliding frequency was low. Motility of M. pneumoniae ceases around the time of cytokinesis (7), raising the possibility that the low percentages of moving M. penetrans and M. iowae cells might be attributable to engagement in the cell division process. Indeed, paired cells were frequently observed (see Fig. 4); individual stationary cells might also have been near cytokinesis. Cells that were clearly dividing (see below) and aggregates or microcolonies of cells were neither analyzed for speed nor enumerated among the total number of cells analyzed.
Fig 2.
Distribution of mycoplasma gliding velocities about the mean. Gliding speeds of the individual motile cells for each strain or serovar were grouped into bins of 0.2× the mean, designated x, for each species. The percentage of cells with speeds in a given range out of the total population of motile cells was plotted against the speed as a fraction of the mean speed for each isolate. (A) Black bars, M. iowae serovar K; gray bars, M. iowae serovar N. (B) M. penetrans. Black bars, strain GTU-54-6A1; gray bars, strain HF-2.
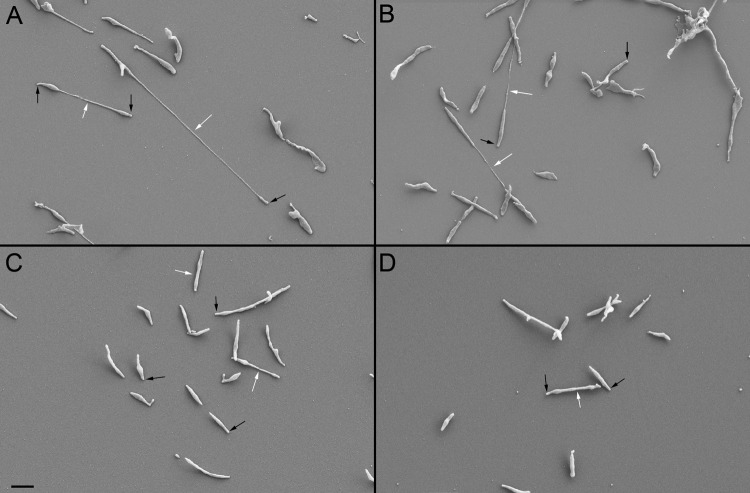
Fig 4.
SEM of M. penetrans and M. iowae cells attached to glass. (A) M. penetrans strain GTU-54-6A1. (B) M. penetrans strain HF-2. (C) M. iowae serovar K. (D) M. iowae serovar N. White arrows, filamentous structures connecting individual cell bodies; black arrows, attachment organelles. Scale bar, 1 μm.
To test whether isolates differed in cytadherence, we performed HA assays on both M. penetrans strains and both M. iowae serovars. Both M. penetrans strains were HA positive, as previously described for strain GTU-54-6A1 (18), but there was a consistent difference in the coverage of colonies by SRBC. GTU-54-6A1 colonies were completely covered by SRBC (Fig. 3A), but HF-2 colonies were more sparsely covered, with most SRBC binding the colony periphery (Fig. 3B). M. iowae serovars N and K were also both HA positive, as previously described (19), and also exhibited differences in SRBC coverage. M. iowae serovar N colonies (Fig. 3C) were completely covered with SRBC, whereas M. iowae serovar K colonies were covered with SRBC only at the colony peripheries (Fig. 3D). Interestingly, for both species it was the faster-gliding variety that exhibited greater coverage by SRBC, but all isolates were nonetheless HA positive.
Fig 3.
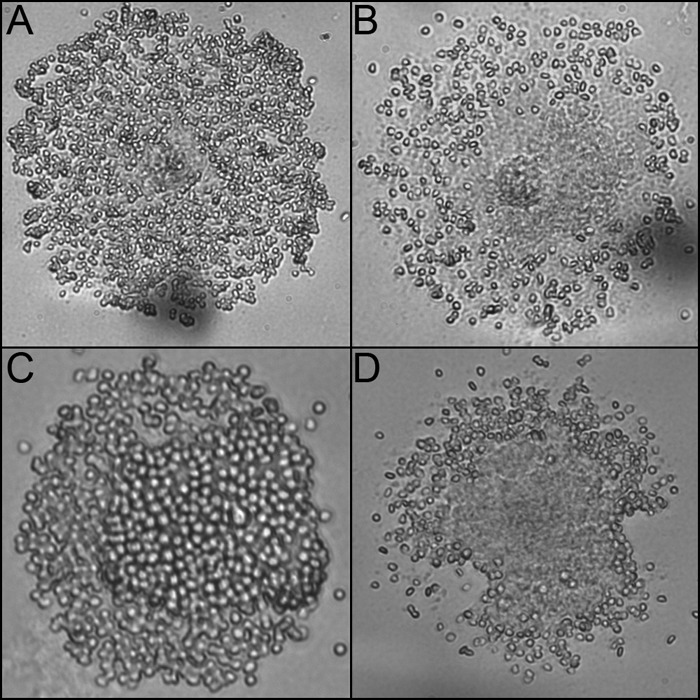
Colony HA of M. penetrans and M. iowae. (A) M. penetrans strain GTU-54-6A1. (B) M. penetrans strain HF-2. (C) M. iowae serovar K. (D) M. iowae serovar N.
Morphology and internal organization of Mycoplasma penetrans and Mycoplasma iowae.
The cell morphologies of glass-attached M. penetrans and M. iowae cells were observed by SEM. M. penetrans (Fig. 4A and B) and M. iowae cells (Fig. 4C and D) had similar appearances. Though pleomorphic, cells consisted of an attachment organelle and a cell body. In both species, paired cells were frequently connected by a filament that was continuous with the cell membrane (data not shown) which varied considerably in length. Unfiltered cells had a propensity to form microcolonies, consistent with the aggregates observed during time-lapse microcinematography.
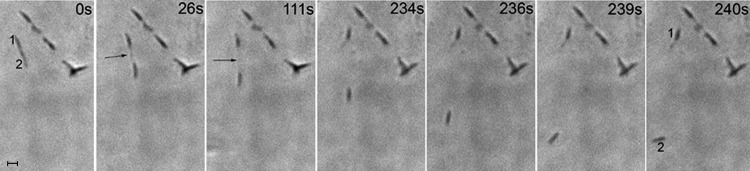
To test whether the filaments connecting cell bodies represented a cytokinesis intermediate, we observed the division of paired M. penetrans strain GTU-54-6A1 cells under the same conditions used for analyzing gliding motility (Fig. 5). The filament routinely increased in length and became thinner as the two cells moved away from each other. Eventually the filament snapped, irrespective of either the passage of a constant amount of time or achievement of a particular length. After division, only one cell glided immediately, whereas the second cell remained stationary for a period of time, as was previously observed for M. pneumoniae (7). Staining of cells with DAPI revealed that the nucleoid was present in the cell body but absent from the terminal organelle and from the filaments connecting paired cells (Fig. 6). The edge of the nucleoid adjacent to the terminal organelle was very flat, suggesting the presence of a physical boundary. The average length of the nucleoid-free space in the region of the terminal organelle was 470 ± 60 nm, and the average width adjacent to the nucleoid was 310 ± 80 nm (n = 32).
Fig 5.
Time-lapse images of dividing M. penetrans strain GTU-54-6A1 cells. Times in the panels after the leftmost panel indicate elapsed time from that starting point. Individual members of each dividing pair are numbered in the first and last panels. Arrows, filamentous structures prior to division. Scale bar, 1 μm.
Fig 6.
Membrane and nucleoid staining in M. penetrans strain GTU-54-6A1. DiOC6 fluorescence (A) and a corresponding phase-contrast image (B) are shown. Arrows, filamentous structures connecting individual cell bodies. (C) White DAPI fluorescence is overlaid onto a phase-contrast image of a pair of dividing cells. Arrowheads, attachment organelles lacking DNA. Scale bar, 1 μm.
Triton X-100-insoluble structures of M. penetrans and M. iowae.
Since M. penetrans and M. iowae are both motile and share similar cell morphologies, we treated cells with Triton X-100 to test for the presence of a cytoskeletal structure such as the structures found in the terminal organelles of M. pneumoniae (20) and M. mobile (23). Treatment of both M. penetrans (Fig. 7A) and M. iowae (Fig. 7B) with 1% Triton X-100 revealed that both species harbored cylindrical or pear-shaped objects, studded by projections, which dominated the images. These structures were indistinguishable between strains within species, although somewhat distinctive between the two species. There was no significant difference in the lengths of the most consistent structures between the two species at 440 to 450 nm (Table 1), corresponding to the length of the nucleoid-free space in the terminal organelle (see above). Because of the shape of the insoluble structures, width measurements were taken at both the widest point and the point at which the structure begins to narrow. For both width measurements, there was a statistically significant difference (one-way analysis of variance [ANOVA], P < 0.05; n = 25) between the two species, with M. iowae exhibiting greater extremes (Table 1). In addition to these predominant structures, both species also contained Triton X-100-insoluble structures that were distinctly shorter or longer, which might represent intermediates in their assembly.
Fig 7.
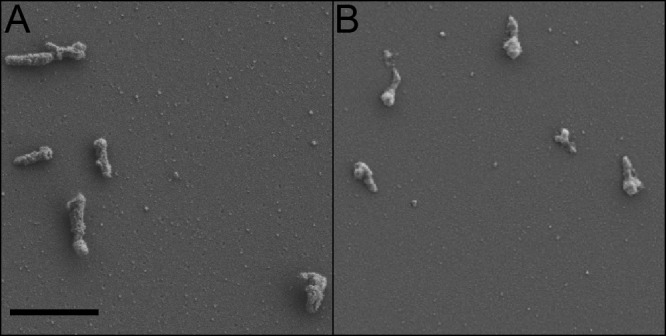
SEM of M. penetrans and M. iowae cells attached to glass, treated with Triton X-100 detergent. (A) M. penetrans strain GTU-54-6A1. (B) M. iowae serovar N. Scale bar, 1 μm.
DISCUSSION
Ultrastructure.
The role of terminal organelles in mediating both cytadherence and gliding motility has been characterized in several members of the Mycoplasma genus (9, 22). M. penetrans and M. iowae are the first motile species identified in the M. muris cluster and increase the number of described motile mycoplasma species to 12, 11 of which have terminal organelles. Despite the close relationship between the M. muris and M. pneumoniae clusters, the overall ultrastructures of the M. iowae and M. penetrans terminal organelles differ substantially from the ultrastructure of M. pneumoniae. The novel Triton X-100-insoluble structures of M. penetrans and M. iowae represent a third type of cytoskeletal organization associated with mycoplasma terminal organelles, with M. pneumoniae and M. mobile representing the first two types. The length of the Triton X-100-insoluble structure of M. penetrans is consistent with that of the nucleoid-free space of the terminal organelle, consistent with a model in which it fills that space. The width of the structure, on the other hand, is less than that of the nucleoid-free space, suggesting either that this structure is at the core of a larger structure whose exterior is solubilized during extraction or that dehydration introduces artifacts that reduce the width. It is likely that the densely packed material within the M. penetrans polar organelle observed in thin sections (19, 24) is actually this Triton X-100-insoluble structure. Similar material is observed in electron micrographs of M. iowae polar organelles (21). The uniform appearance of the densely packed material in these images argues against a second type of structure that was not recovered in our experiments. Further studies are necessary to identify the proteins that comprise both structures as well as their development and function.
Gliding motility.
The gliding characteristics of M. penetrans and M. iowae are distinct from those of other mycoplasma species in particular ways. One is the discontinuity of their movement compared with that of M. mobile cells, which rarely rest during motility (27), and with movement of species of the M. pneumoniae phylogenetic cluster, which rest more frequently than M. mobile (25), but not to the degree observed in M. penetrans and M. iowae. As a measure of this phenomenon, on average, individual M. penetrans strain GTU-54-6A1 cells moved for ∼70% of the observation period (data not shown), whereas Mycoplasma genitalium strain G37 cells moved for ∼90% of the period (J. M. Hatchel and M. F. Balish, unpublished data); in both cases cells that appeared to be impeded were discounted. A second distinctive characteristic is the existence of substantial gliding speed differences among isolates of M. penetrans and M. iowae not observed in other species (data not shown). It is unclear whether the differences observed in gliding speed and coverage of colonies by SRBC in the HA assay have any relationship to the sites from which the organisms were isolated. M. penetrans strain GTU-54-6A1, isolated from the urogenital tract of HIV-infected individuals (19, 34), glides faster than strain HF-2, which was isolated from the respiratory tract of an HIV-negative person with primary antiphospholipid syndrome (35). Additionally, M. iowae serovar N, isolated from the air sac of turkeys (2), glides faster than M. iowae serovar K, isolated from the oviduct of chickens. Further comparison of both strains and serovars among M. penetrans and M. iowae may be helpful in identifying the molecular components associated with adherence and motility in these two species.
Cell division.
In M. penetrans, we observed a clear relationship between motility and cell division. Dividing cells were connected by cell membrane filaments, which increased in length as the distance between both daughter cells increased over time. The straightness of filaments connecting the cell pairs suggests that the filaments experience tension, most likely the result of the gliding-associated forces generated by the terminal organelles of one or both cells of the pair. The lack of DAPI fluorescence in the filaments of paired cells suggests that the filaments are free of DNA and are formed after nucleoid segregation, in agreement with images in which nonfilamentous individual cells appear to have two distinct nucleoids (data not shown). We observed breakage of filaments at the culmination of cell division, with one cell remaining stationary and the other gliding away. This observation is identical to observations of dividing M. pneumoniae cells (7). Like other mycoplasmas, M. penetrans may rely at least in part on the force generated by gliding motility for cell division (17).
Independent evolution of mycoplasma terminal organelles.
Although terminal organelles in M. mobile, the M. pneumoniae cluster, and the M. muris cluster facilitate the same sets of functions, there are significant disparities in overall terminal organelle organization and protein composition in each of these groups. We believe that these data are irreconcilable with a model in which these structures spring from a common evolutionary origin. The observations here regarding M. penetrans and M. iowae not only demonstrate the distinct characteristics of members of the M. muris cluster but also indicate that comparing more Mycoplasma species is critical for gaining a more complete understanding of both the unifying and diverse elements of terminal organelle function and its role in disease caused by any single species.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (Public Health Service grant R15 A1073994) and by the Miami University Doctoral-Undergraduate Opportunities in Scholarship program.
We gratefully acknowledge the following for providing mycoplasma strains: Z. Raviv (The Ohio State University) for M. iowae serovars N and K, M. Davidson (Mollicutes Culture Collection, Purdue University) for M. penetrans strain GTU-54-6A1, and L. Duffy (University of Alabama-Birmingham) for M. penetrans strain HF-2. We thank C. M. Fullem for early work on DAPI staining of M. penetrans. We are also grateful to D. Krause (University of Georgia) for reading the manuscript.
Footnotes
Published ahead of print 23 March 2012
REFERENCES
- 1. Balish MF. 2006. Subcellular structures of mycoplasmas. Front. Biosci. 11:2017–2027 [DOI] [PubMed] [Google Scholar]
- 2. Barber TL, Fabricant J. 1971. A suggested reclassification of avian Mycoplasma serotypes. Avian Dis. 15:125–138 [PubMed] [Google Scholar]
- 3. Biberfeld G, Biberfeld P. 1970. Ultrastructural features of Mycoplasma pneumoniae. J. Bacteriol. 102:855–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bradbury JM, McCarthy JD. 1983. Pathogenicity of Mycoplasma iowae for chick embryos. Avian Pathol. 12:483–496 [DOI] [PubMed] [Google Scholar]
- 5. Feng SH, Lo SC. 1994. Induced mouse spleen B-cell proliferation and secretion of immunoglobulin by lipid-associated membrane proteins of Mycoplasma fermentans incognitus and Mycoplasma penetrans. Infect. Immun. 62:3916–3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giron JA, Lange M, Baseman JB. 1996. Adherence, fibronectin binding and induction of cytoskeleton reorganization in cultured human cells by Mycoplasma penetrans. Infect. Immun. 64:197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hasselbring BM, Jordan JL, Krause RW, Krause DC. 2006. Terminal organelle development in the cell wall-less bacterium Mycoplasma pneumoniae. Proc. Natl. Acad. Sci. U. S. A. 103:16478–16483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hatchel JM, Balish RS, Duley ML, Balish MF. 2006. Ultrastructure and gliding motility of Mycoplasma amphoriforme, a possible human respiratory pathogen. Microbiology 152:2181–2189 [DOI] [PubMed] [Google Scholar]
- 9. Hatchel JM, Balish MF. 2008. Attachment organelle ultrastructure correlates with phylogeny, not gliding motility properties, in Mycoplasma pneumoniae relatives. Microbiology 154:286–295 [DOI] [PubMed] [Google Scholar]
- 10. Himmelreich R, et al. 1996. Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae. Nucleic Acids Res. 22:4420–4449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jaffe JD, Miyata M, Berg HC. 2004. Energetics of gliding motility in Mycoplasma mobile. J. Bacteriol. 186:4254–4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jaffe JD, et al. 2004. The complete genome and proteome of Mycoplasma mobile. Genome Res. 8:1447–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jordan JL, et al. 2007. Protein P200 is dispensable for Mycoplasma pneumoniae hemadsorption but not gliding motility or colonization of differentiated bronchial epithelium. Infect. Immun. 75:518–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirchhoff H, Rosengarten R. 1984. Isolation of motility mycoplasma from fish. J. Gen. Microbiol. 130:2439–2445 [DOI] [PubMed] [Google Scholar]
- 15. Reference deleted. [Google Scholar]
- 16. Ley DH, Marusak RA, Vivas EJ, Barnes HJ, Fletcher OJ. 2010. Mycoplasma iowae associated with chondrodystrophy in commercial turkeys. Avian Pathol. 39:87–93 [DOI] [PubMed] [Google Scholar]
- 17. Lluch-Senar M, Querol E, Piñol J. 2010. Cell division in a minimal bacterium in the absence of ftsZ. Mol. Microbiol. 78:278–289 [DOI] [PubMed] [Google Scholar]
- 18. Lo SC, et al. 1992. Mycoplasma penetrans sp. nov., from the urogenital tract of patients with AIDS. Int. J. Syst. Bacteriol. 42:357–364 [DOI] [PubMed] [Google Scholar]
- 19. Lo SC, et al. 1991. Newly discovered mycoplasma isolated from patients infected with HIV. Lancet 338:1415–1418 [DOI] [PubMed] [Google Scholar]
- 20. Meng KE, Pfister RM. 1980. Intracellular structures of Mycoplasma pneumoniae revealed after membrane removal. J. Bacteriol. 144:390–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mirsalimi SM, Rosendal S, Julian RJ. 1989. Colonization of the intestine of turkey embryos exposed to Mycoplasma iowae. Avian Dis. 33:310–315 [PubMed] [Google Scholar]
- 22. Miyata M, Uenoyama A. 2002. Movement on the cell surface of the gliding bacterium, Mycoplasma mobile, is limited to its head-like structure. FEMS Microbiol. Lett. 215:285–289 [DOI] [PubMed] [Google Scholar]
- 23. Nakane D, Miyata M. 2007. Cytoskeletal “jellyfish” structure of Mycoplasma mobile. Proc. Natl. Acad. Sci. U. S. A. 104:19518–19523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neyrolles O, et al. 1998. Identification of two glycosylated components of Mycoplasma penetrans: a surface-exposed capsular polysaccharide and a glycolipid fraction. Microbiology 144:1247–1255 [DOI] [PubMed] [Google Scholar]
- 25. Radestock U, Bredt W. 1977. Motility of Mycoplasma pneumoniae. J. Bacteriol. 129:1495–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Relich RF, Friedberg AJ, Balish MF. 2009. Novel cellular organization in a gliding mycoplasma, Mycoplasma insons. J. Bacteriol. 191:5312–5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosengarten R, Kirchhoff H. 1987. Gliding motility of Mycoplasma sp. nov. strain 163K. J. Bacteriol. 169:1891–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sasaki Y, et al. 2002. The complete genomic sequence of Mycoplasma penetrans, an intracellular bacterial pathogen in humans. Nucleic Acids Res. 30:5293–5300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seto S, Miyata M. 2003. Attachment organelle formation represented by localization of cytadherence proteins and formation of the electron-dense core in wild-type and mutant strains of Mycoplasma pneumoniae. J. Bacteriol. 185:1082–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shimizu T, Kida Y, Kuwano K. 2004. Lipid-associated membrane proteins of Mycoplasma fermentans and M. penetrans activate human immunodeficiency virus long-terminal repeats through Toll-like receptors. Immunology 113:121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trampel DW, Frederick G., Jr 1994. Outbreak of Mycoplasma iowae infection in commercial turkey poults. Avian Dis. 38:905–909 [PubMed] [Google Scholar]
- 32. Tully JG, Rose DL, Whitcomb RF, Wenzel RP. 1979. Enhanced isolation of Mycoplasma pneumoniae from throat washings with a newly-modified cultured medium. J. Infect. Dis. 139:478–482 [DOI] [PubMed] [Google Scholar]
- 33. Uenoyama A, Kusumoto A, Miyata M. 2004. Identification of a 349-kilodalton protein (Gli349) responsible for cytadherence and glass binding during gliding of Mycoplasma mobile. J. Bacteriol. 186:1537–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang RH, et al. 1992. High frequency of antibodies to Mycoplasma penetrans in HIV-infected patients. Lancet 340:1312–1316 [DOI] [PubMed] [Google Scholar]
- 35. Yañez A, et al. 1999. Mycoplasma penetrans bacteremia and primary antiphospholipid syndrome. Emerg. Infect. Dis. 5:164–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoder HW, Hofstad MS. 1964. Characterization of avian mycoplasma. Avian Dis. 8:481–512 [Google Scholar]