Abstract
Hypertrophic cardiomyopathy (HCM) is frequently caused by mutations in MYBPC3 encoding cardiac myosin-binding protein C (cMyBP-C). The mechanisms leading from gene mutations to the HCM phenotype remain incompletely understood, partially because current mouse models of HCM do not faithfully reflect the human situation and early hypertrophy confounds the interpretation of functional alterations. The goal of this study was to evaluate whether myofilament Ca2+ sensitization and diastolic dysfunction are associated or precede the development of left ventricular hypertrophy (LVH) in HCM. We evaluated the function of skinned and intact cardiac myocytes, as well as the intact heart in a recently developed Mybpc3-targeted knock-in mouse model carrying a point mutation frequently associated with HCM. Compared to wild-type, 10-week old homozygous knock-in mice exhibited i) higher myofilament Ca2+ sensitivity in skinned ventricular trabeculae, ii) lower diastolic sarcomere length, and faster Ca2+ transient decay in intact myocytes, and iii) LVH, reduced fractional shortening, lower E/A and E′/A′, and higher E/E′ ratios by echocardiography and Doppler analysis, suggesting systolic and diastolic dysfunction. In contrast, heterozygous knock-in mice, which mimic the human HCM situation, did not exhibit LVH or systolic dysfunction, but exhibited higher myofilament Ca2+ sensitivity, faster Ca2+ transient decay, and diastolic dysfunction. These data demonstrate that myofilament Ca2+ sensitization and diastolic dysfunction are early phenotypic consequences of Mybpc3 mutations independent of LVH. The accelerated Ca2+ transients point to compensatory mechanisms directed towards normalization of relaxation. We propose that HCM is a model for diastolic heart failure and this mouse model could be valuable in studying mechanisms and treatment modalities.
Abbreviations: cMyBP-C, cardiac myosin-binding protein C; cTnI, cardiac troponin I; CSQ, calsequestrin; HCM, hypertrophic cardiomyopathy; Het, heterozygous Mybpc3-targeted knock-in mice; KI, homozygous Mybpc3-targeted knock-in mice; KO, homozygous Mybpc3-targeted knock-out mice; LVH, left ventricular hypertrophy; max F, maximal Ca2+-activated force; MYBPC3, human cardiac myosin-binding protein C gene; Mybpc3, mouse cardiac myosin-binding protein C gene; NCX, Na+/Ca2+ exchanger; nH, Hill coefficient; pCa50, log of [Ca2+] required for 50% of maximal activation; PKA, cAMP-dependent protein kinase A; PLB, phospholamban; SERCA2, SR-Ca2+ ATPase; SL, sarcomere length; SR, sarcoplasmic reticulum
Keywords: Ca2+ sensitivity, Ca2+ transient, Diastolic dysfunction, Hypertrophy, Mouse model
Highlights
► Absence of left ventricular hypertrophy in heterozygous Mybpc3-targeted knock-in mice. ► Myofilament Ca2+ sensitization in heterozygous Mybpc3-targeted knock-in mice. ► Diastolic dysfunction independent of left ventricular hypertrophy. ► Hypertrophic cardiomyopathy as a model of diastolic heart failure.
1. Introduction
Hypertrophic cardiomyopathy (HCM) is the most common heritable cardiac disorder characterized by left ventricular hypertrophy (LVH), diastolic dysfunction and interstitial fibrosis [1–3]. Affected individuals can be asymptomatic for decades or develop breathlessness, chest pain, congestive heart failure, or sudden death. Over the past 20 years, > 500 different mutations in at least 19 different genes have been shown to cause HCM (for reviews see [4–7]). Most encode proteins of the sarcomere. Among known HCM mutations, those affecting MYBPC3 encoding cardiac myosin binding protein C (cMyBP-C) are one of the most frequent (for reviews, see [8,9]). cMyBP-C is located in the C-zones of the A-band of the sarcomere, and modulates actin–myosin interaction via phosphorylation by different kinases (for reviews, see [9–11]). cMyBP-C is hypophosphorylated in HCM and in human and experimental heart failure [12–14], suggesting a role in diastolic dysfunction under these conditions.
Although human studies have been particularly informative in identifying disease-causing genes, they are limited because of the small number of individuals carrying the same mutation and the difficulty in obtaining myocardial tissue from patients. Accordingly, much of our current knowledge about the molecular pathogenesis has been derived from mouse models of HCM.
We recently developed the first Mybpc3-targeted knock-in mouse model, carrying a point mutation [15], which frequently causes HCM and is associated with a severe phenotype and poor prognosis in humans [16,17]. The genetic architecture of this model closely recapitulates the situation in patients. Whereas homozygous (KI) knock-in mice developed LVH, reduced fractional shortening and interstitial fibrosis, heterozygous (Het) knock-in mice were apparently normal [15]. However, both Het and KI mice are susceptible to stress, which presented with impairment of the ubiquitin–proteasome system [18,19]. The lack of LVH in Het appears to be in line with a common limitation of existing HCM mouse models [11,15,20–23], which develop a disease only in the homozygous state whereas patients with HCM are usually heterozygous for a mutation (rare homozygous cases developed DCM and early death) [16]. However, the absence of LVH in young Het mice mimics the situation in the apparently unaffected patients carrying a disease mutation [24–26], and gave us the chance to study early functional alterations that precede LVH. By extensive phenotypic characterization of Het and KI hearts relative to wild-type (WT), the data now demonstrate that myofilament Ca2+ sensitization and diastolic dysfunction are early phenotypic consequences of the Mybpc3 mutation, independent of LVH.
2. Material and methods
2.1. Animals
The investigation conforms to the guide for the care and use of laboratory animals published by the NIH (Publication No. 85–23, revised 1985). The Mybpc3-targeted Het and KI mice were generated by targeted insertion of a G > A transition on the last nucleotide of exon 6 (Fig. 1) [15], and were maintained on the Black Swiss background.
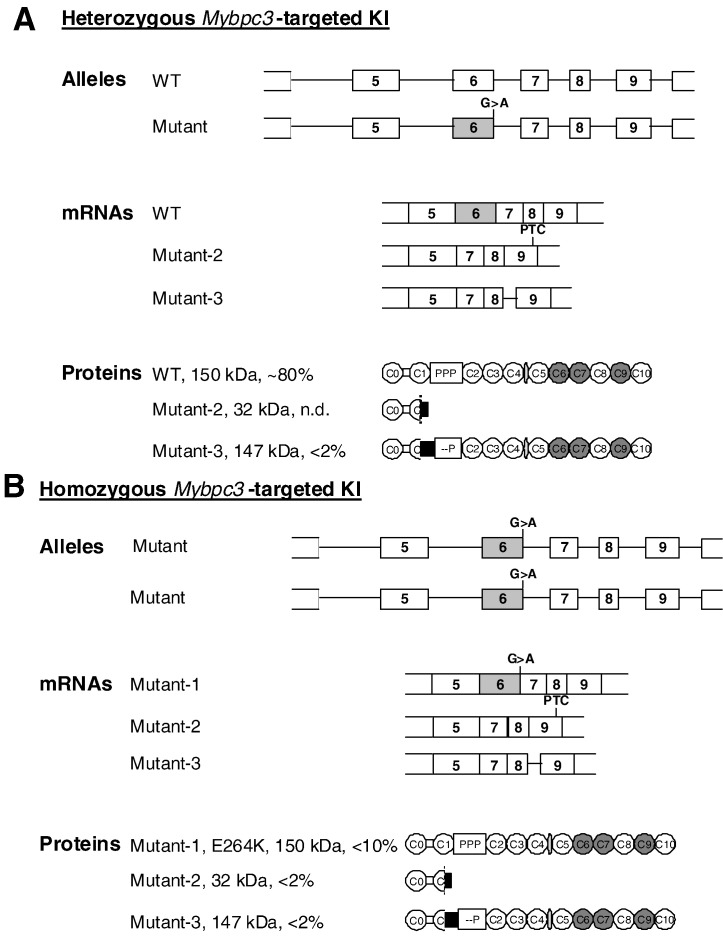
Fig. 1.
Consequences of Mybpc3 targeting in Het and KI mice. A, Schematic structure of alleles, mRNAs and proteins in heterozygous Mybpc3-targeted knock-in (Het) mice. Het-KI have a WT allele and a mutant allele, which contains a G > A transition on the last nucleotide of exon 6. This results in 50% WT mRNA and less than 2% of both mutant-2 (nonsense) and mutant-3 (deletion/insertion) mRNAs resulting from the skipping of exon 6 [15]. Mutant-2 contains a premature termination codon (PTC) in exon 9 and produces a 32 kDa-truncated mutant-2 protein, which has not been detected in Het-KI [15]. Mutant-3 mRNA retains parts of intron 8, which restores the reading frame, and produces a 147-kDa mutant-3 protein, which lacks the Ser-282 phosphorylation site. B, Schematic structure of alleles, mRNAs and proteins in homozygous Mybpc3-targeted knock-in (KI) mice. KI mice have 2 mutant alleles, which contain a G > A transition on the last nucleotide of exon 6. This results in 20% of mutant-1 missense mRNA encoding a full-length mutant-1 E264K protein, as well as in < 5% of mutant-2 and mutant-3 mRNAs and proteins [15].
2.2. Assessment of myofilament function in skinned mouse ventricular trabeculae
Mechanical function of skinned mouse cardiac trabeculae isolated from 7–8-week-old mice was assessed at 18 °C, using equipment and protocols similar to those described in detail previously [27]. Skinned steady-state force–pCa relationship was determined experimentally and fitted to the Hill equation to yield maximal Ca2+-activated force (max F), the log of [Ca2+] required for 50% of maximal activation (pCa50), and the Hill coefficient (nH).
2.3. Sarcomere shortening and Ca2+ transients in adult mouse ventricular myocytes
Adult ventricular myocytes were isolated from 13-week-old mice as previously described [28]. Sarcomere shortening and Ca2+ transients were simultaneously assessed upon field stimulation (0.25–3 Hz with 4-ms-duration, 10 V) using a video-based sarcomere length (SL) detection system (IonOptix corporation) at room temperature. Caffeine (10 mM) was applied to determine sarcoplasmic reticulum (SR) Ca2+ content and the activity of the Na+/Ca2+ exchanger (NCX; [29]).
2.4. Echocardiography
Transthoracic echocardiography was performed using the Vevo 2100 System (VisualSonics, Toronto, Canada) in 10-week-old mice [15]. B-mode recordings were performed using a MS 400 transducer (18–38 MHz) with a frame rate of 230–400 frames/s to assess left ventricular (LV) dimensions. Pulsed-wave Doppler echocardiography was used to measure early (E) and late (A) blood flow velocities through the mitral valve, and Tissue Doppler imaging to measure early (E′) and late (A′) velocities of the mitral annulus [30]. All images were recorded digitally and analysis was performed using the Vevo 2100-software.
2.5. Immunoblot analysis
Immunoblot analysis was carried out as previously described [31,32].
2.6. Statistical analysis
Data are expressed as mean ± SEM. Comparisons were performed using Student's t-test, or using one-way or two-way ANOVA followed by Bonferroni's post-tests. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Higher myofilament Ca2+ sensitivity in Het and KI skinned ventricular trabeculae
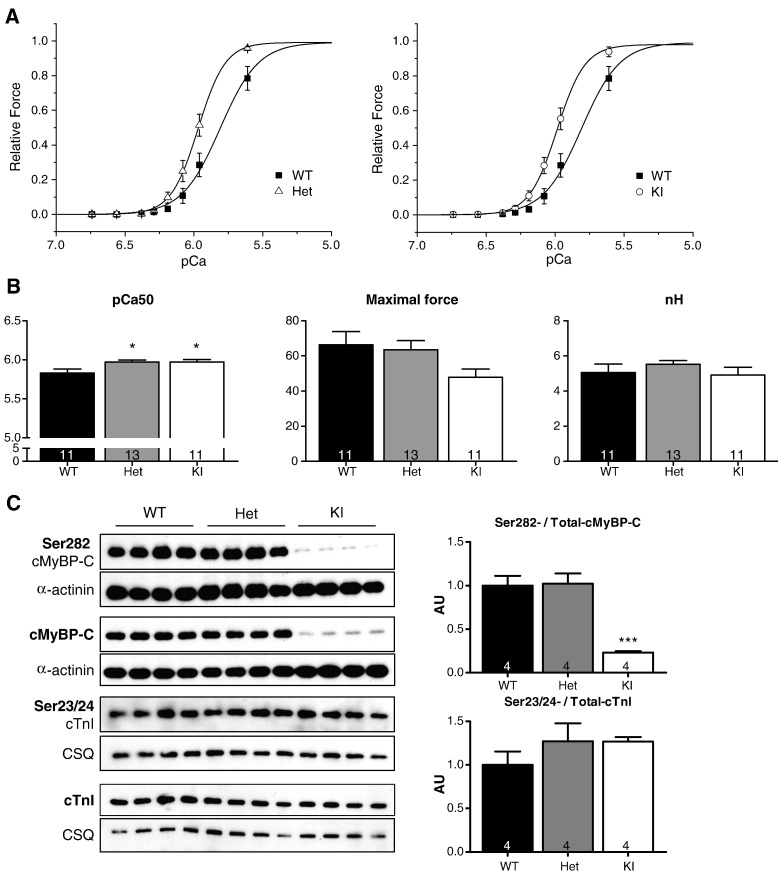
We first assessed the force–pCa relationships in skinned ventricular trabeculae isolated from the 3 groups of mice. Trabeculae were incubated for 30 min with 240 Units of cAMP-dependant protein kinase A (PKA) to eliminate effects of potential phosphorylation differences, which could obscure data interpretation. Both Het and KI skinned trabeculae exhibited higher myofilament Ca2+ sensitivity than WT (Figs. 2A and B), whereas the steepness of the force–pCa relationships and the maximal force (max F) were not affected (Fig. 2B).
Fig. 2.
Ca2+ sensitivity of force development in WT, Het and KI skinned ventricular trabeculae. Trabeculae were incubated for 30 min with 0.1 U/μl PKA. A, The mean force-pCa curves were obtained at a sarcomere length of 2.2 μm. Force values were normalized to the maximum force, measured at pCa 4.5. B, pCa50, maximal force and nH (11–13 trabeculae from 4 hearts per group). C, Western blots stained with the indicated antibodies and bars showing the Ser282-/total-cMyBP-C and Ser23/24-/Total-cTnI protein levels. Values are expressed as mean ± SEM, *P < 0.05 and ***P < 0.01, one-way ANOVA plus Bonferroni post-test. Number of samples is indicated in the bars.
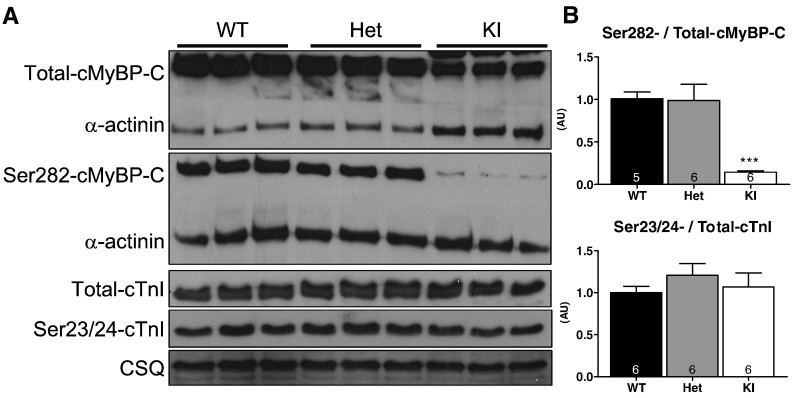
To confirm that sarcomeric proteins were phosphorylated to the same extent in all groups, we performed Western blot analysis of PKA-treated skinned trabeculae. The level of phosphorylated Ser23/24-cTnI did not differ between the groups (Fig. 2C). Phosphorylation of cMyBP-C at the Ser282 site did not differ between Het and WT, but was markedly lower in KI skinned trabeculae despite the expected lower level of total cMyBP-C [15]. This likely corresponds to the fact that < 10% of cMyBP-C mutants in KI are mutant-1, which contains the Ser282 site, and the rest mutant-3, which does not contain this site (Fig. 1) [15].
3.2. Blunted negative force–frequency relationship in Het and KI intact myocytes
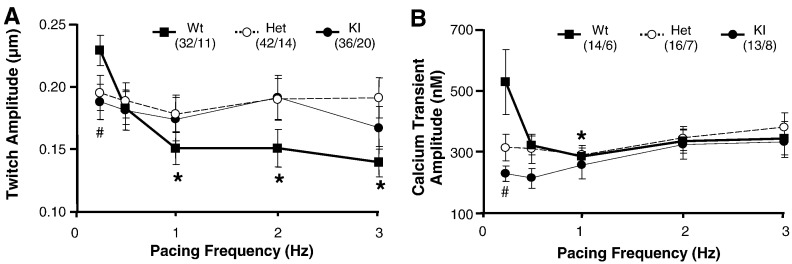
We then evaluated sarcomere shortening and Ca2+ transients in intact cardiac myocytes at different pacing frequencies. As expected for rodents [33], increasing pacing frequency from 0.25 to 1 Hz decreased amplitudes of the twitch and Ca2+ transient in WT myocytes (Figs. 3A and B). Both parameters were lower at 0.25 Hz in KI, and to a lower extent in Het than in WT myocytes, but remained stable over the entire frequency range. The lack of the negative staircase phenomenon suggested abnormal Ca2+ homeostasis in mutant mice.
Fig. 3.
Frequency dependence of sarcomere shortening and Ca2+ transient amplitudes. A) Twitch (sarcomere shortening) amplitude and B) Calcium transient amplitude plotted against pacing frequency. No significant difference in the frequency-amplitude relationships was found between the 3 groups of mice. Each point represents the mean±SEM from n cells and N mice, (n/N is indicated in brackets). *P < 0.05 in WT vs 0.25 Hz, two-way ANOVA plus Bonferroni post-test, and #P < 0.05 in KI vs WT, Student's t-test.
3.3. Faster decay of Ca2+ transients in Het and KI intact myocytes
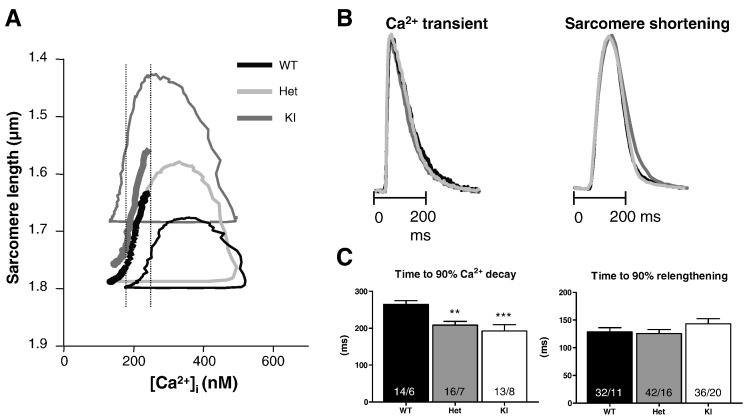
To get a more detailed view of the relation between sarcomere length and cytosolic [Ca2+] ([Ca]i), we assessed phase-plane diagrams as previously described [28]. Results obtained at 2 Hz are shown (Fig. 4). This analysis shows a shift toward lower diastolic SL in KI only. The application of 2,3-butanedione monoxime, which disrupts cross-bridge cycling [34] normalized the diastolic SL in KI (Supplemental Table). Furthermore, in both KI and Het, the area of the loop was larger and the relaxation trajectories were shifted to the left, i.e. relaxation began at lower [Ca2+]i, supporting increased myofilament Ca2+ sensitivity.
Fig. 4.
Recordings of sarcomere shortening and Ca2+ transients at 2 Hz. A, Phase-plane trajectories of SL-[Ca2+]i transients. Loops proceed in a counter-clockwise direction. Bold interrupted curves represent the lower (KI, gray) and upper (WT, black) limits of the 95% confidence interval calculated for the part of the relaxation phase that takes place in a similar [Ca2+]i interval range (illustrated by the vertical dashed bars). The 95% confidence intervals calculated for Het are not shown here, because they overlapped with KI and WT. Averaged phase-plane trajectories were obtained from 14 WT, 16 Het and 13 KI cells, respectively. B, Normalized averaged Ca2+ transient and sarcomere shortening kinetics in WT (n = 14), Het (n = 16) and KI (n = 13) myocytes. C, Time to 90% Ca2+ transient decay and time to 90% relengthening. Bars represent the mean ± SEM. **P < 0.01 and ***P < 0.01 vs WT, one-way ANOVA plus Bonferroni post-test. Number of cells/mice is indicated in the bars.
Whereas the kinetics of sarcomere shortening did not differ in the three groups, Ca2+ transients were shorter in Het and KI myocytes at 2 Hz (Fig. 4B and data not shown). This was also observed at 0.25 Hz (data not shown). This suggests that the elevated myofilament Ca2+ sensitivity is partially compensated by accelerated Ca2+ transient decay, potentially through faster re-uptake of Ca2+ into the SR and/or faster Ca2+ extrusion through NCX. To follow this reasoning, we evaluated SR Ca2+ content and NCX activity by recording caffeine-induced Ca2+ transients (Fig. 5). The amplitude of caffeine-induced Ca2+ transients did not differ between the groups (Fig. 5B), suggesting normal SR Ca2+ content. However, in accordance with faster Ca2+ transients under pacing (Fig. 4), Ca2+ decay was > 2-fold faster in Het and KI than in WT, suggesting higher NCX activity (Fig. 5C).
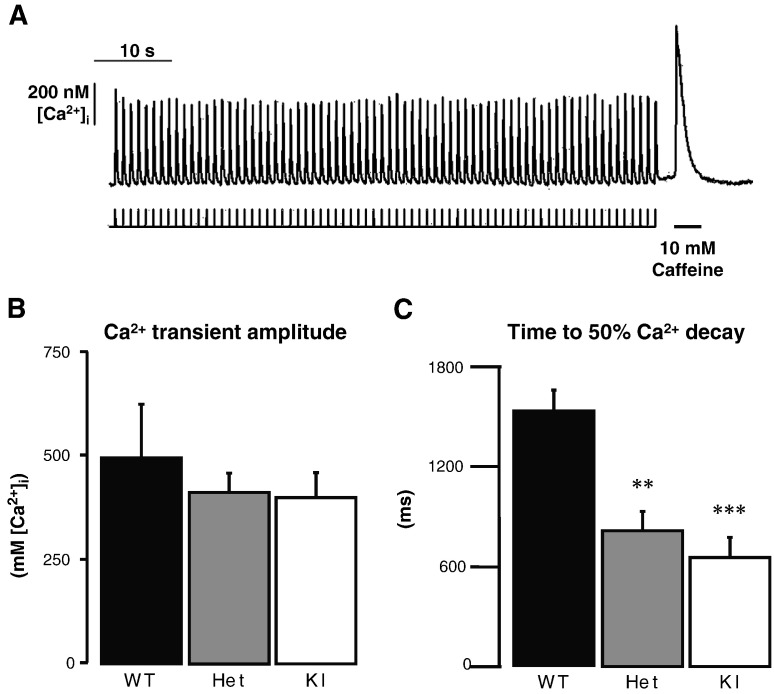
Fig. 5.
Caffeine-induced Ca2+ release response. A, Representative Ca2+ transient recording obtained in a single intact ventricular myocyte isolated from WT mouse. The myocyte was first electrically stimulated at 1 Hz for 1 min to fully fill SR Ca2+ stores. Stimulation was then stopped and 10 mM caffeine was rapidly applied until [Ca2+]i transient amplitude represented less than 50% of the initial peak response. B, Ca2+ transient amplitude. C, Time to 50% Ca2+ decay. Bars represent the mean ± SEM from n/N cells/animals: 14/4 WT, 15/5 Het, and 22/5 KI. **P < 0.01 and ***P < 0.001 vs WT, one-way ANOVA plus Bonferroni post-test.
3.4. Compensatory molecular changes in Het and KI ventricular tissue
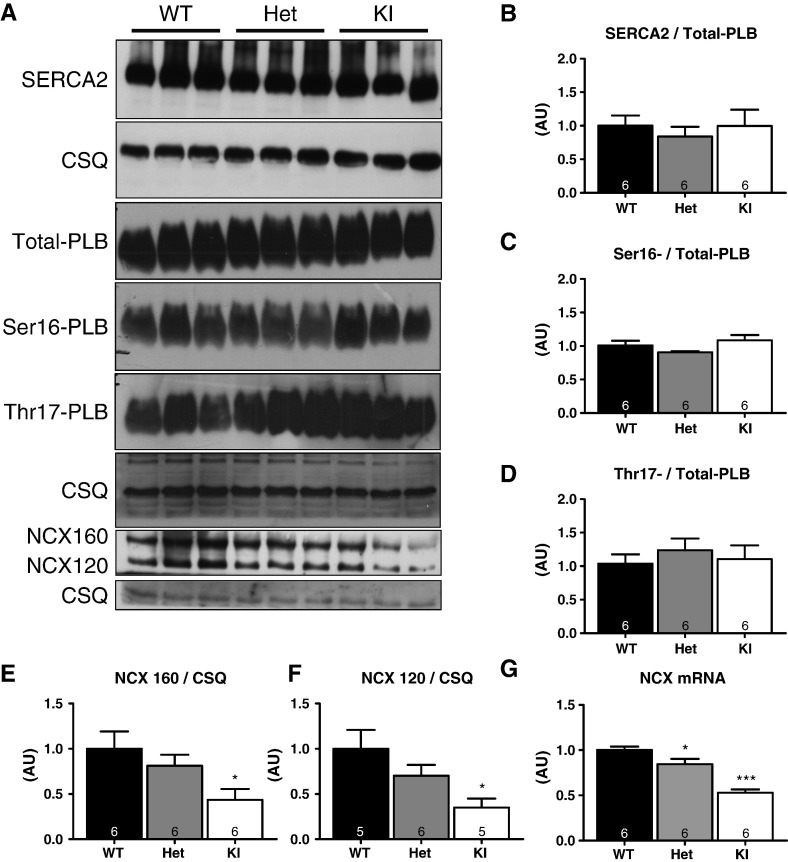
We next analyzed whether the blunted force–frequency relationship and faster Ca2+ transient decay in Het and KI myocytes were associated with changes in the phosphorylation of myofilament (Fig. 6) or Ca2+ handling proteins (Fig. 7) in ventricular tissue. Supporting the data obtained in skinned ventricular myocytes (Fig. 2), the relative Ser282-phosphorylation level was lower in KI ventricular extracts (Fig. 6). No changes in Ser282-phosphorylation were observed in Het. Ser23/24-phosphorylation of cardiac troponin I (cTnI) also did not differ between the groups (Fig. 6). Levels of SR-Ca2+-ATPase (SERCA2), total-phospholamban (PLB) and SERCA2/PLB ratio as well as phosphorylation of PLB (at Ser16 and Thr17; Fig. 7) also did not differ. Surprisingly, however, the concentrations of NCX1 at both apparent molecular weights (160 and 120 kDa) and of the corresponding mRNA were lower in KI and, to a lesser extent in Het than in WT mice (Fig. 7).
Fig. 6.
Determination of sarcomeric protein phosphorylation in ventricular tissue from 10-week-old WT, Het and KI mice. A) Representative Western blots stained with antibodies directed against total and Ser282-phosphorylated cardiac myosin-binding protein-C (total-cMyBP-C and Ser282-cMyBP-C), α-actinin, total and Ser23/24-phosphorylated cardiac troponin I (total-cTnI and Ser23/14-cTnI), and calsequestrin (CSQ); B) Ser282-/Total cMyBP-C levels and Ser23/24-/Total-cTnI levels. ***P < 0.001 vs WT, one-way ANOVA plus Bonferroni post-test. Number of mice is indicated in the bars.
Fig. 7.
Determination of Ca2+ handling protein or mRNA levels in ventricular tissue from 10-week-old WT, Het and KI mice. A) Representative Western blots stained with antibodies directed against SR-Ca2+-ATPase (SERCA2), total phospholamban (Total-PLB), Ser16-phosphorylated phospholamban (Ser16-PLB), Thr17-phosphorylated phospholamban (Thr17-PLB), Na+/Ca2+ exchanger (NCX1), and calsequestrin (CSQ). B) Level of SERCA2/PLB normalized to CSQ level. C) Ser16-/Total-PLB levels; D) Thr17-/Total-PLB levels. E, Protein level of the 160-kDa-NCX isoform. F, Protein levels of the 120-kDa-NCX isoform. G, NCX mRNA levels normalized to CSQ. *P < 0.05, and ***P < 0.001 vs WT, one-way ANOVA with Bonferonni post-test. Number of mice is indicated in the bars.
3.5. Diastolic dysfunction in Het and KI mice
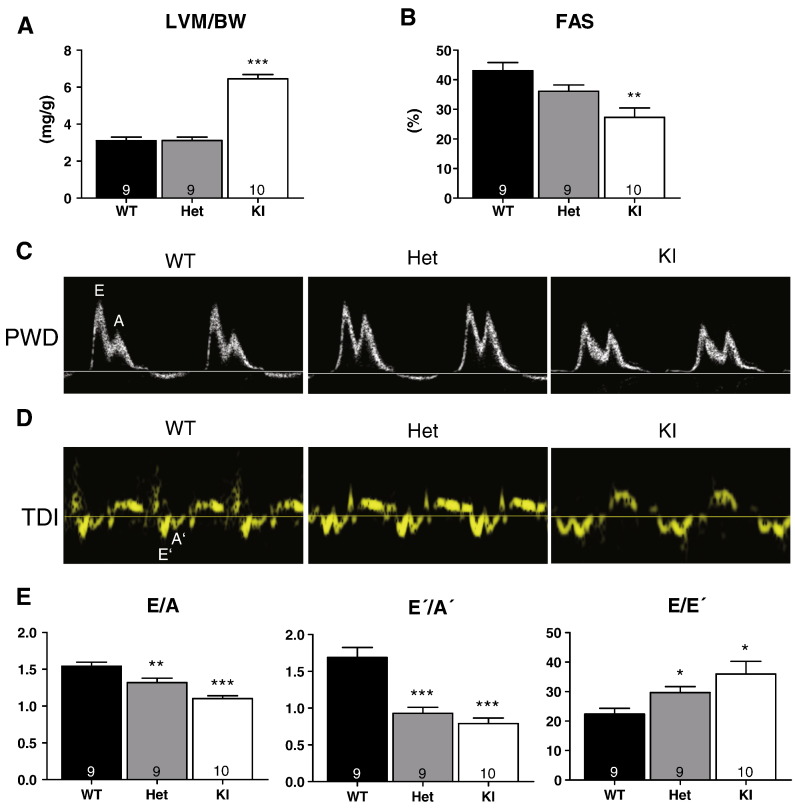
The results in skinned ventricular trabeculae and intact myocytes suggested that the increased Ca2+ sensitivity in Het and KI is partially compensated by accelerated Ca2+ transients, promoting relaxation. To evaluate how these alterations translate into whole heart function, we evaluated cardiac function in 10-week-old WT, Het and KI mice by echocardiography. The left ventricular mass-to-body-weight ratio was higher and the fractional area shortening smaller in KI than in WT mice, but both parameters were not altered in Het mice (Figs. 8A and B). Using conventional pulsed-wave Doppler echocardiography, peak early (E) and late (A) blood flow velocities through the mitral valve were measured (Fig. 8 C). From the tissue Doppler imaging measured at the septal corner of the mitral annulus, peak early (E′) and late (A′) diastolic velocities were measured (Fig. 8D). In WT mice, E/A and E′/A′ ratios were > 1.5 (Fig. 7E). In Het and KI mice, E/A and E′/A′ ratios were lower that in WT (Fig. 8E). Conversely, E/E′ ratio was higher in Het and KI mice than in WT (Fig. 8E). These data strongly suggest diastolic dysfunction in Het and KI mice.
Fig. 8.
Transthoracic echocardiography and Doppler data. Analyses were performed in 10-week-old WT, Het and KI mice. Conventional echocardiography measurements were obtained from B-mode. A, left ventricular mass-to-body-weight ratio (LVM/BW) and B, fractional area shortening (FAS). C, Representative pulsed-wave Doppler (PWD) recordings of the mitral inflow in WT, Het and KI mice. PWD provides mitral inflow velocity patterns, from which early (E) and late (A) diastolic velocities, as well as the E/A ratio were derived. D, Representative tissue Doppler imaging (TDI) in WT, Het and KI mice. The E′ wave corresponds to the motion of the mitral annulus during early diastolic filling of the left ventricle, and the A′ wave originates from atrial systole during late filling. E, Bars represent diastolic function parameters E/A, E′/A′ and E′E′. Data are expressed as mean ± SEM with *P < 0.05, **P < 0.01 and ***P < 001 vs WT, one-way ANOVA plus Bonferroni post-test. Number of animals is indicated in the bars.
4. Discussion
The present study evaluated in heterozygous and homozygous Mybpc3-targeted knock-in mice whether alterations in the function of skinned ventricular muscle preparations and intact ventricular myocytes in vitro as well as the intact heart in vivo precede the development of LVH, and may therefore represent primary changes, rather than secondary responses, in HCM. The major findings of this study were as follows. Compared to WT, KI mice exhibited i) higher myofilament Ca2+ sensitivity in skinned trabeculae, ii) lower diastolic SL and faster Ca2+ transient decay in intact myocytes, and iii) LVH, systolic and diastolic dysfunction in vivo. Het mice, whose genotype closely mimics the situation in human HCM, exhibited higher myofilament Ca2+ sensitivity in skinned trabeculae, faster Ca2+ transient decay in intact myocytes, and diastolic dysfunction in vivo. These alterations were observed in the absence of LVH or systolic dysfunction in Het mice, providing the first evidence in a disease-relevant gene-targeted mouse model of HCM that myofilament Ca2+ sensitization and diastolic dysfunction are primary phenotypic consequences of the Mybpc3 mutation.
The data in homozygous KI mice essentially support and extend earlier data in homozygous KO mice, demonstrating higher Ca2+ sensitivity in skinned myocytes [35], as well as lower diastolic SL and altered phase-plane diagrams in intact myocytes [28]. The shift to lower SL as well as the larger area of the [Ca2+]i-SL phase-plane diagram in KI recapitulates the effect of alkalosis or pharmacological Ca2+ sensitizers [36,37]. This suggests that they are the consequences of a primary increase in myofilament Ca2+ sensitivity. Taken together, the data support the concept that cMyBP-C normally prevents residual crossbridge cycling at low cytosolic Ca2+ concentrations in diastole and thereby promotes diastolic relaxation [28,38]. Lack of cMyBP-C in KO or its marked reduction in KI [15] makes myofilaments hyperreactive to low [Ca2+] and causes the cellular and skinned muscle phenotypes observed.
One of the problems of experiments in homozygous KI and KO mice is the presence of LVH, even in very young animals [15,21,22]. Thus, it is difficult to deduce whether the observed changes are primary consequences of cMyBP-C deficiency and/or secondary to cardiac myocyte hypertrophy, which is known to be associated with contraction slowing and altered Ca2+ cycling (for review, see [11]). The most interesting data are therefore the findings in Het mice. At 10 weeks of age, Het mice exhibited normal ventricular weight and normal systolic heart function. Thus, similar to many HCM patients at young age, they appeared unaffected at first sight. However, a more detailed analysis revealed several abnormalities. (1) Skinned muscles and intact myocytes from Het mice exhibited increased Ca2+ sensitivity. (2) Most importantly, from a clinical point of view, Het mice exhibited diastolic dysfunction as evidenced by reduced E/A and E′/A′ratios.
In both Het and KI mice, we found evidence for at least one mechanism of compensation. Myocytes from both Het and KI mice exhibited accelerated Ca2+ transient decay. The exact reason for the increased velocity of Ca2+ removal from the cytosol is unknown, but SERCA2/PLB protein and phosphorylation levels were shown to be unaffected, making enhanced rates of Ca2+ uptake into the SR unlikely. Unaltered SR Ca2+ content also argues against a change at the SR level. Since the major other Ca2+ extrusion route is NCX, the finding of decreased NCX mRNA and protein levels came as a surprise. In human systolic heart failure Ca2+ transients are prolonged [39], NCX levels are elevated [40], and intracellular [Na+] is increased [41]. This is associated and likely causally related to a reversal of the positive force–frequency relationship in human heart to a flat or negative one [42]. The changes observed in Het and KI myocytes point in the opposite direction (shorter Ca2+ transients, NCX down, negative force–frequency relationship reversed to a flat one), suggesting that Het and KI myocytes may have decreased intracellular [Na+], facilitating Ca2+ extrusion via the NCX. Clearly more work is needed to substantiate this hypothesis.
The present data have implications for the understanding and potentially treatment of diastolic dysfunction in HCM and beyond. First, in accordance with recent human data [43,44] and in mice carrying a Myh6 mutation [45] they clearly indicate that diastolic dysfunction can exist without LVH and is thus a primary change in HCM due to MYBPC3 mutations. Second, they indicate that diastolic dysfunction cannot only be due to increased extracellular matrix/passive stiffness [46] and changes in titin isoforms [47] and/or phosphorylation state [48,49], but also due to residual actin–myosin interactions in diastole that are the consequence of increased myofilament Ca2+ sensitivity. Thus, the data suggest that HCM is a model for diastolic heart failure and mouse models such as the one studied here could be valuable in studying mechanisms and treatment modalities of diastolic heart failure. Finally, the endogenous compensatory mechanism identified here (acceleration of Ca2+ transients) could have therapeutic consequences, because it predicts that drugs interfering with that mechanism may have beneficial or adverse effects. Thus, drugs with a desensitizing effect on myofilament Ca2+ sensitivity (e.g. ranolazine [50]) should have beneficial effects, while those that increase intracellular [Na+] or [Ca2+], such as digitalis glycosides, should clearly be avoided. Studies are ongoing to directly test these hypotheses.
The following are the supplementary materials related to this article.
Supplementary materials.
Resting sarcomere length (SL) in WT, Het and KI myocytes.
Source of fundings
This work was supported by the Institut de la Santé et de la Recherche Médicale (PNRMC-A04048DS), the Association Française contre les Myopathies (AFM-9471), the sixth and seventh Framework Programs of the European Union (Marie Curie EXT-014051; Health-F2-2009-241577-Big-Heart project), the Deutsche Forschungsgemeinschaft (FOR-604/1-2), the Centre National de la Recherche Scientifique, the British Heart Foundation (BHF grant PG/07/056/23150) and by the German Center for Cardiovascular Research (Deutsches Zentrum für Herz-Kreislauf-Forschung, DZHK).
Disclosure statement
None.
Acknowledgments
We thank Mathias Gautel (King's College London) and Sakthivel Sadayappan (Chicago Loyola University) for antibodies directed against cMyBP-C.
References
- 1.Maron B.J., Gardin J.M., Flack J.M., Gidding S.S., Kurosaki T.T., Bild D.E. Prevalence of hypertrophic cardiomyopathy in a general population of young adults: echocardiographic analysis of 4111 subjects in the CARDIA study. Circulation. 1995;92:785–789. doi: 10.1161/01.cir.92.4.785. [DOI] [PubMed] [Google Scholar]
- 2.Gersh B.J., Maron B.J., Bonow R.O., Dearani J.A., Fifer M.A., Link M.S. ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58 doi: 10.1016/j.jacc.2011.10.825. e261–60. [DOI] [PubMed] [Google Scholar]
- 3.Elliott P., Andersson B., Arbustini E., Bilinska Z., Cecchi F., Charron P. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 4.Watkins H., Ashrafian H., Redwood C. Inherited cardiomyopathies. N Engl J Med. 2011;364:1643–1656. doi: 10.1056/NEJMra0902923. [DOI] [PubMed] [Google Scholar]
- 5.Seidman C.E., Seidman J.G. Identifying sarcomere gene mutations in hypertrophic cardiomyopathy: a personal history. Circ Res. 2011;108:743–750. doi: 10.1161/CIRCRESAHA.110.223834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrier L., Schlossarek S., Willis M.S., Eschenhagen T. The ubiquitin-proteasome system and nonsense-mediated mRNA decay in hypertrophic cardiomyopathy. Cardiovasc Res. 2010;85:330–338. doi: 10.1093/cvr/cvp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedrich F.W., Carrier L. Genetics of hypertrophic and dilated cardiomyopathy. Curr Pharm Biotechnol. Jan 20 2012 doi: 10.2174/138920112804583041. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8.Harris S.P., Lyons R.G., Bezold K.L. In the thick of it: HCM-causing mutations in myosin binding proteins of the thick filament. Circ Res. 2011;108:751–764. doi: 10.1161/CIRCRESAHA.110.231670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlossarek S., Mearini G., Carrier L. Cardiac myosin-binding protein C in hypertrophic cardiomyopathy: mechanisms and therapeutic opportunities. J Mol Cell Cardiol. 2011;50:613–620. doi: 10.1016/j.yjmcc.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Carrier L. Cardiac myosin-binding protein C in the heart. Arch Mal Coeur Vaiss. 2007;100:238–243. [PubMed] [Google Scholar]
- 11.Barefield D., Sadayappan S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol. 2010;48:866–875. doi: 10.1016/j.yjmcc.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Armouche A., Pohlmann L., Schlossarek S., Starbatty J., Yeh Y.H., Nattel S. Decreased phosphorylation levels of cardiac myosin-binding protein-C in human and experimental heart failure. J Mol Cell Cardiol. 2007;43:223–229. doi: 10.1016/j.yjmcc.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 13.van Dijk S.J., Dooijes D., Dos Remedios C., Michels M., Lamers J.M., Winegrad S. Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy. haploinsufficiency, deranged phosphorylation, and cardiomyocyte dysfunction. Circulation. 2009;119:1473–1483. doi: 10.1161/CIRCULATIONAHA.108.838672. [DOI] [PubMed] [Google Scholar]
- 14.van Dijk S.J., Paalberends E.R., Najafi A., Michels M., Sadayappan S., Carrier L. Contractile dysfunction irrespective of the mutant protein in human hypertrophic cardiomyopathy with normal systolic function. Circ Heart Fail. 2012;5:36–46. doi: 10.1161/CIRCHEARTFAILURE.111.963702. [DOI] [PubMed] [Google Scholar]
- 15.Vignier N., Schlossarek S., Fraysse B., Mearini G., Kramer E., Pointu H. Nonsense-mediated mRNA decay and ubiquitin-proteasome system regulate cardiac myosin-binding protein C mutant levels in cardiomyopathic mice. Circ Res. 2009;105:239–248. doi: 10.1161/CIRCRESAHA.109.201251. [DOI] [PubMed] [Google Scholar]
- 16.Richard P., Charron P., Carrier L., Ledeuil C., Cheav T., Pichereau C. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations and implications for molecular diagnosis strategy. Circulation. 2003;107:2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- 17.Olivotto I., Girolami F., Ackerman M.J., Nistri S., Bos J.M., Zachara E. Myofilament protein gene mutation screening and outcome of patients with hypertrophic cardiomyopathy. Mayo Clin Proc. 2008;83:630–638. doi: 10.4065/83.6.630. [DOI] [PubMed] [Google Scholar]
- 18.Schlossarek S., Englmann D.R., Sultan K.R., Sauer M., Eschenhagen T., Carrier L. Defective proteolytic systems in Mybpc3-targeted mice with cardiac hypertrophy. Basic Res Cardiol. 2012;107:1–13. doi: 10.1007/s00395-011-0235-3. [DOI] [PubMed] [Google Scholar]
- 19.Schlossarek S., Schuermann F., Geertz B., Mearini G., Eschenhagen T., Carrier L. Adrenergic stress reveals septal hypertrophy and proteasome impairment in heterozygous Mybpc3-targeted knock-in mice. J Muscle Res Cell Motil. Nov 11 2011 doi: 10.1007/s10974-011-9273-6. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.McConnell B.K., Fatkin D., Semsarian C., Jones K.A., Georgakopoulos D., Maguire C.T. Comparison of two murine models of familial hypertrophic cardiomyopathy. Circ Res. 2001;88:383–389. doi: 10.1161/01.res.88.4.383. [DOI] [PubMed] [Google Scholar]
- 21.Harris S.P., Bartley C.R., Hacker T.A., McDonald K.S., Douglas P.S., Greaser M.L. Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ Res. 2002;90:594–601. doi: 10.1161/01.res.0000012222.70819.64. [DOI] [PubMed] [Google Scholar]
- 22.Carrier L., Knoell R., Vignier N., Keller D.I., Bausero P., Prudhon B. Asymmetric septal hypertrophy in heterozygous cMyBP-C null mice. Cardiovasc Res. 2004;63:293–304. doi: 10.1016/j.cardiores.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Geisterfer-Lowrance A.A., Christe M., Conner D.A., Ingwall J.S., Schoen F.J., Seidman C.E. A mouse model of familial hypertrophic cardiomyopathy. Science. 1996;272:731–734. doi: 10.1126/science.272.5262.731. [DOI] [PubMed] [Google Scholar]
- 24.Charron P., Carrier L., Dubourg O., Tesson F., Desnos M., Richard P. Penetrance of familial hypertrophic cardiomyopathy. Genet Couns. 1997;8:107–114. [PubMed] [Google Scholar]
- 25.Charron P., Dubourg O., Desnos M., Isnard R., Hagege A., Bonne G. Genotype-phenotype correlations in familial hypertrophic cardiomyopathy. A comparison between mutations in the cardiac protein-C and the beta-myosin heavy chain genes. Eur Heart J. 1998;19:139–145. doi: 10.1053/euhj.1997.0575. [DOI] [PubMed] [Google Scholar]
- 26.Niimura H., Bachinski L.L., Sangwatanaroj S., Watkins H., Chudley A.E., McKenna W. Mutations in the gene for cardiac myosin-binding protein C and late-onset familial hypertrophic cardiomyopathy. N Engl J Med. 1998;338:1248–1257. doi: 10.1056/NEJM199804303381802. [DOI] [PubMed] [Google Scholar]
- 27.Bardswell S.C., Cuello F., Rowland A.J., Sadayappan S., Robbins J., Gautel M. Distinct sarcomeric substrates are responsible for protein kinase D-mediated regulation of cardiac myofilament Ca2 + sensitivity and cross-bridge cycling. J Biol Chem. 2010;285:5674–5682. doi: 10.1074/jbc.M109.066456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pohlmann L., Kroger I., Vignier N., Schlossarek S., Kramer E., Coirault C. Cardiac myosin-binding protein C is required for complete relaxation in intact myocytes. Circ Res. 2007;101:928–938. doi: 10.1161/CIRCRESAHA.107.158774. [DOI] [PubMed] [Google Scholar]
- 29.Loughrey C.M., Seidler T., Miller S.L., Prestle J., MacEachern K.E., Reynolds D.F. Over-expression of FK506-binding protein FKBP12.6 alters excitation-contraction coupling in adult rabbit cardiomyocytes. J Physiol. 2004;556:919–934. doi: 10.1113/jphysiol.2003.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schumacher A., Khojeini E., Larson D. ECHO parameters of diastolic dysfunction. Perfusion. 2008;23:291–296. doi: 10.1177/0267659109102485. [DOI] [PubMed] [Google Scholar]
- 31.Mearini G., Gedicke C., Schlossarek S., Witt C.C., Kramer E., Cao P. Atrogin-1 and MuRF1 regulate cardiac MyBP-C levels via different mechanisms. Cardiovasc Res. 2010;85:357–366. doi: 10.1093/cvr/cvp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haworth R.S., Cuello F., Herron T.J., Franzen G., Kentish J.C., Gautel M. Protein kinase D is a novel mediator of cardiac troponin I phosphorylation and regulates myofilament function. Circ Res. 2004;95:1091–1099. doi: 10.1161/01.RES.0000149299.34793.3c. [DOI] [PubMed] [Google Scholar]
- 33.Endoh M. Force-frequency relationship in intact mammalian ventricular myocardium: physiological and pathophysiological relevance. Eur J Pharmacol. 2004;500:73–86. doi: 10.1016/j.ejphar.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Ostap E.M. 2,3-Butanedione monoxime (BDM) as a myosin inhibitor. J Muscle Res Cell Motil. 2002;23:305–308. doi: 10.1023/a:1022047102064. [DOI] [PubMed] [Google Scholar]
- 35.Cazorla O., Szilagyi S., Vignier N., Salazar G., Kramer E., Vassort G. Length and protein kinase A modulations of myocytes in cardiac myosin binding protein C-deficient mice. Cardiovasc Res. 2006;69:370–380. doi: 10.1016/j.cardiores.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Wolska B.M., Solaro R.J. Method for isolation of adult mouse cardiac myocytes for studies of contraction and microfluorimetry. Am J Physiol. 1996;271:1250–1255. doi: 10.1152/ajpheart.1996.271.3.H1250. [DOI] [PubMed] [Google Scholar]
- 37.Spurgeon H.A., duBell W.H., Stern M.D., Sollott S.J., Ziman B.D., Silverman H.S. Cytosolic calcium and myofilaments in single rat cardiac myocytes achieve a dynamic equilibrium during twitch relaxation. J Physiol. 1992;447:83–102. doi: 10.1113/jphysiol.1992.sp018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colson B.A., Bekyarova T., Fitzsimons D.P., Irving T.C., Moss R.L. Radial displacement of myosin cross-bridges in mouse myocardium due to ablation of myosin binding protein-C. J Mol Biol. 2007;367:36–41. doi: 10.1016/j.jmb.2006.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beuckelmann D.J., Erdmann E. Ca(2 +)-currents and intracellular [Ca2 +]i-transients in single ventricular myocytes isolated from terminally failing human myocardium. Basic Res Cardiol. 1992;87(Suppl. 1):235–243. doi: 10.1007/978-3-642-72474-9_19. [DOI] [PubMed] [Google Scholar]
- 40.Studer R., Reinecke H., Bilger J., Eschenhagen T., Bohm M., Hasenfuss G. Gene expression of the cardiac Na(+)–Ca2 + exchanger in end-stage human heart failure. Circ Res. 1994;75:443–453. doi: 10.1161/01.res.75.3.443. [DOI] [PubMed] [Google Scholar]
- 41.Pieske B., Maier L.S., Piacentino V., III, Weisser J., Hasenfuss G., Houser S. Rate dependence of [Na +]i and contractility in nonfailing and failing human myocardium. Circulation. 2002;106:447–453. doi: 10.1161/01.cir.0000023042.50192.f4. [DOI] [PubMed] [Google Scholar]
- 42.Mulieri L.A., Hasenfuss G., Leavitt B., Allen P.D., Alpert N.R. Altered myocardial force-frequency relation in human heart failure. Circulation. 1992;85:1743–1750. doi: 10.1161/01.cir.85.5.1743. [DOI] [PubMed] [Google Scholar]
- 43.Michels M., Soliman O.I., Kofflard M.J., Hoedemaekers Y.M., Dooijes D., Majoor-Krakauer D. Diastolic abnormalities as the first feature of hypertrophic cardiomyopathy in Dutch myosin-binding protein C founder mutations. JACC Cardiovasc Imaging. 2009;2:58–64. doi: 10.1016/j.jcmg.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Ho C.Y., Carlsen C., Thune J.J., Havndrup O., Bundgaard H., Farrohi F. Echocardiographic strain imaging to assess early and late consequences of sarcomere mutations in hypertrophic cardiomyopathy. Circ Cardiovasc Genet. 2009;2:314–321. doi: 10.1161/CIRCGENETICS.109.862128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Georgakopoulos D., Christie M.E., Giewat M., Seidman C.M., Seidman J.G., Kass D.A. The pathogenesis of familial hypertrophic cardiomyopathy: early and elvolving effects from an a-cardaic myosin heavy chain missense mutation. Nat Med. 1999;5:327–330. doi: 10.1038/6549. [DOI] [PubMed] [Google Scholar]
- 46.Borlaug B.A., Kass D.A. Mechanisms of diastolic dysfunction in heart failure. Trends Cardiovasc Med. 2006;16:273–279. doi: 10.1016/j.tcm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Borbely A., Falcao-Pires I., van Heerebeek L., Hamdani N., Edes I., Gavina C. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–786. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 48.Kruger M., Kotter S., Grutzner A., Lang P., Andresen C., Redfield M.M. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104:87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 49.Hudson B., Hidalgo C., Saripalli C., Granzier H. Hyperphosphorylation of mouse cardiac titin contributes to transverse aortic constriction-induced diastolic dysfunction. Circ Res. 2011;8:858–866. doi: 10.1161/CIRCRESAHA.111.246819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lovelock J.D., Monasky M.M., Jeong E.M., Lardin H.A., Liu H. Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ Res. 2012;110:841–850. doi: 10.1161/CIRCRESAHA.111.258251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.
Resting sarcomere length (SL) in WT, Het and KI myocytes.