Summary
Inhaled conidia of Aspergillus fumigatus rapidly adhere to pulmonary epithelial cells and other host constituents. Identifying molecular mechanisms underlying A. fumigatus adherence has therefore been the focus of a number of studies aimed at identifying novel therapeutic targets. Early studies of A. fumigatus adherence to host constituents focused on fungal proteins, including RodA and AspF2. None of these proteins however has been found to play a role in virulence in experimental animal models. Recent advances have suggested an important role for fungal carbohydrate components of the cell wall and extracellular matrix in adherence, including sialic acid and mannose residues, and the newly described polysaccharide galactosaminogalactan. Despite these advances, the host cell receptors that are bound by these ligands remain unknown.
Introduction
Adherence of microorganisms to host cells and macromolecules is a key step in the pathogenesis of infectious diseases. Fungi are no exception to this rule, and adherence to host constituents has been identified as an important virulence factor in a number of medically important and plant pathogenic fungi, including the most common opportunistic mold pathogen Aspergillus fumigatus.
In immunocompromised hosts, infection with A. fumigatus is initiated following the inhalation of airborne spores of this ubiquitous fungus. These inhaled conidia then contact airway epithelial cells or pulmonary macrophages where they adhere and can be internalized before undergoing germination and hyphal growth [1–3]. Hyphae continue to grow by apical extension, and interact with pulmonary epithelial cells as they invade deeper tissues. Hyphae of A. fumigatus are also angiotropic, and adhere to and invade the abluminal surface of vascular endothelial cells to gain access to the vascular compartment [4]. Once inside blood vessels, hyphal fragments can be disseminated to distal sites where they adhere to the luminal surface of endothelial cells before traversing them and invading into deep tissues [5]. Since A. fumigatus hyphae induce host cell damage and death, it is likely that the basal lamina within airways and blood vessels are exposed, and that fungal cells can adhere to basement membrane macromolecules such as laminin, collagen and fibronectin.
Surprisingly little is known about the molecular mechanism underlying the adherence of A. fumigatus adherence to host constituents. This review will focus on recent advances in our understanding of the molecular mechanisms underlying A. fumigatus adherence to pulmonary epithelial cells and basement membrane components. Although the focus of much of this work has been invasive aspergillosis, it is likely that these mechanisms also play an important role in the pathogenesis of airway colonization with A. fumigatus in patients with chronic lung disease.
The cell wall of A. fumigatus has been the focus of the majority of adherence studies. A dynamic, complex structure composed of glycans, pigments and proteins, the cell wall is the key site of contact between the organism and host (see Figure 1). While initial attempts to identify adhesion molecules within the cell wall focused predominately on proteins, recent work has implicated glycans as playing an important role in Aspergillus adherence.
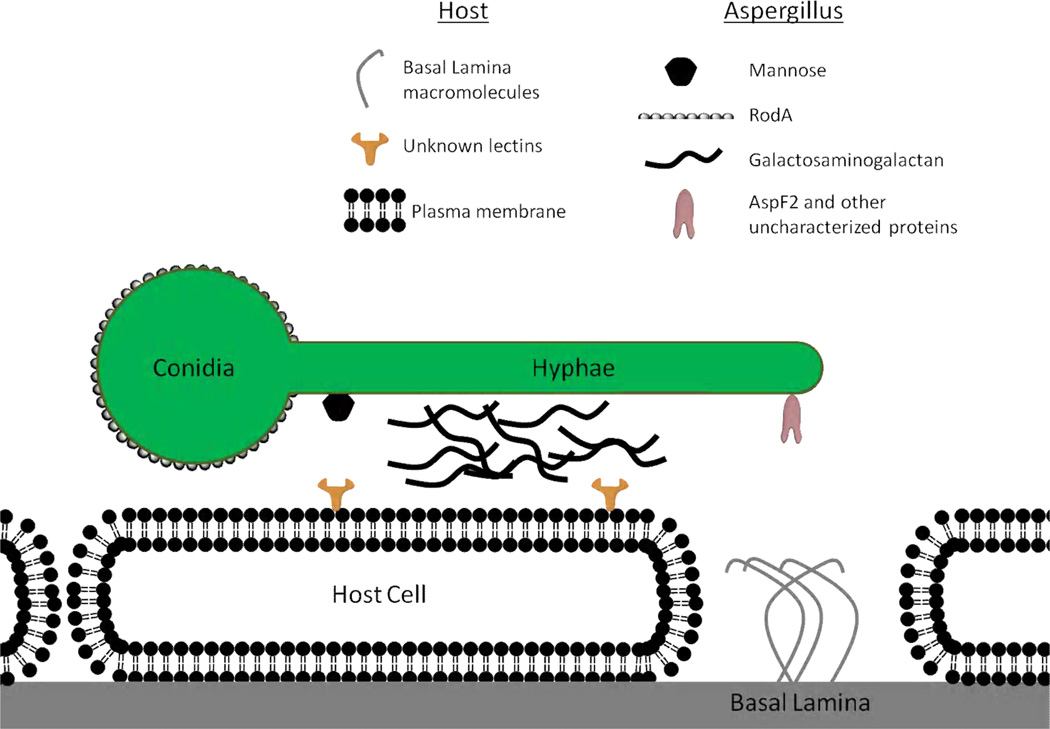
Figure 1.
Model of known and suspected molecules involved in A. fumigatus adherence to host cells and host cell constituents. For the sake of clarity, only single molecules of each type of adhesin and or receptor are represented. Basal laminin molecules include collagen, fibronectin and laminin.
Cell wall proteins as adhesins
The first protein described to mediate adherence of A. fumigatus was the conidial hydrophobin RodA. This protein assembles into a regular array of rodlets on the surface of conidia to render the surface highly hydrophobic as well as prevent recognition of β1,3-glucan by immune cells [6]. Disruption of the rodA gene is associated with a decrease in adherence of conidia to collagen and albumin, though not laminin, fibrinogen or pulmonary epithelial cells [7]. Further, deletion of rodA did not result in an attenuation of A. fumigatus virulence in a murine model of invasive aspergillosis, suggesting that this protein is dispensible for adherence in vivo. The genome of A. fumigatus contains five other genes that are predicted to encode hydrophobins [8]. RodB was also found to be expressed on A. fumigatus conidia, however disruption of the rodB gene had no effect on the conidial rodlet layer as visualized by electron microscopy [9]. Although adherence testing of the mutant was not performed, the ΔrodB mutant strain exhibited normal susceptibility to killing by alveolar macrophages, suggesting that the mutant likely adhered normally to this cell type [9]. The role of the other putatative hydrophobins in adherence or virulence remains unknown.
Other early studies of fungal adherence focused on fungal attachment to the basement membrane components fibronectin and laminin [10–12]. Conidia of A. fumigatus exhibit specific binding to these molecules that can be inhibited when conidia are pretreated with trypsin, suggesting that a fungal protein mediates binding [10–11]. Using affinity purification approaches, several groups have identified polypeptides that bind fibronectin and laminin ranging in size from 23 to 37 kDa, although these were not identified in any further detail in these reports [10–12]. In a subsequent study, one of these laminin binding proteins, (a 37kDa polypeptide) was identified as the Aspergillus allergen AspF2 [13]. Native and recombinant AspF2 protein was found to bind laminin in vitro, although the adherence and virulence of an AspF2-deficient mutant strain has not been evaluated [13]. Therefore, the role of AspF2 and these other laminin binding molecules in adherence and virulence in vivo remains unknown.
Fungal adherence to host tissues has been extensively studied in Candida albicans, the most common fungus causing human disease. In this organism, glycosylphosphatidylinositol (GPI) anchored, serine-threonine rich proteins containing internal tandem repeats have been described as key effectors of fungal adherence [14–15]. To identify similar adhesins in A. fumigatus, Levdansky and colleagues used the software tool EMBOSS ETANDEM to identify A. fumigatus genes containing intragenic repeats, and selected a subset of proteins containing both a putative signal peptide and GPI anchor sequences [16]. Of the 292 proteins identified with tandem repeats, only 10 contained both a putative GPI anchor and N-terminal signal peptide sequence [16]. Deletion of one of these genes cspA (Afu3g08990) was associated with a reduction in the adherence of conidia to extracellular matrix from pulmonary A549 cells but not laminin or polystyrene [16]. As with the ΔrodA mutant strain, deletion of cspA had no effect on virulence [16]. A follow up study from this group identified marked abnormalities in cell wall architecture and altered exposure of cell wall carbohydrates in the absence of cspA [17]. Thus cspA likely plays a role primarily in governing cell wall architecture and its effects on adherence may be mediated through alterations in other cell wall components rather than direct binding to host constituents.
More recently, Upadhyay and colleagues utilized another bioinformatic approach for the identification of A. fumigatus adhesins [18]. A whole proteome analysis was conducted using the software SPAAN, a program for protein sequence based identification of adhesin proteins. Using this approach the authors identified 82 proteins with a 90% or higher probability of encoding an adhesin [18]. From this list, a serine threonine rich protein, CalA (Afu3g09690) was selected for further study. Recombinant CalAp was found to bind to laminin and murine lung and spleen cells in vitro [18]. A calA deletion strain was not constructed. Interestingly, since the publication of this study, the developers of the SPAAN software have published a fungal specific adhesin prediction and bioinformatics portal, Fungal RV [19] which identifies 38 predicted adhesin and adhesin-like proteins in A. fumigatus. A second software system designed to predict fungal adhesins has also been described, FAAPRED [20], but was not applied to A. fumigatus. Validation of the role of any of these other putative adhesins in mediating adherence or virulence has yet to be performed.
Cell wall carbohydrates as adhesins
In addition to proteins, the fungal cell wall is composed of a number of glycans including α- and β-glucans, chitin, galactomannan and galactosaminogalactan [21]. The role of these molecules in mediating A. fumigatus adherence has been highlighted in several recent studies.
One of the earliest suggestions that cell wall carbohydrate components may mediate fungal adherence was by made by Wasylnka and Moore who reported that A. fumigatus conidia bound to the glycosaminoglycan binding domain of fibronectin [22]. Further, negatively charged carbohydrates such as dextran sulphate, chondroitin sulphate, keratin sulphate and heparin, but not neutral sugars, inhibited the binding of conidia to fibronectin and intact basal lamina from pulmonary epithelial cells. Collectively, these data suggest that negatively charged carbohydrate moieties on the conidial surface may mediate binding to host macromolecules [22]. Treatment of conidia with sialidase was found to decrease conidial adherence to fibronectin, suggesting that sialic acid residues mediated adherence to fibronectin and basal lamina constituents. In support of this hypothesis, a higher density of sialic acid residues was found to be present on Aspergillus fumigatus as compared with with other less pathogenic Aspergillus species [23], although no studies specifically testing the contribution of sialic acid residues to virulence have been performed. The actual fungal ligand upon which sialic residues are presented remains unknown, although lectin binding studies suggest that the majority of conidial sialic acids are α2,6-linked to a galactose residue [24].
Galactomannan is a polysaccharide composed of a mannan core linked to short galactofuranose (Galf) chains [25]. Galactomannan is an abundant component of the cell wall where it is bound covalently to β1,3-glucan, and is also secreted into the extracellular matrix. Galactofuranose residues also decorate a number of glycoproteins, and glycolipids. Synthesis of UDP-galactofuranose from UDP-galactopyranose is mediated through the action of UDP galactopyranose mutase [26] (encoded by ugm1 in A. fumigatus). Disruption of ugm1 in A. fumigatus abolished galactofuranose synthesis, and resulted in a strain that is hyperadherent to inert surfaces as well as pulmonary epithelial cells [27]. Similarly in the relatively non-pathogenic species Aspergillus nidulans, disruption of the A. nidulans orthologue of ugm1 (ugmA), resulted in increased adherence to a hydrophilic Si3N4 atomic force microscopic probe [28]. Both groups suggest that Galf residues may mask polar groups during cell wall maturation, and thus modulate adherence. In support of this hypothesis, treatment of the A. fumigatus Δugm1 mutant with mannosidase decreased adherence of germlings to wild-type levels [27]. The authors suggest that mannan residues, exposed in the absence of Galf, are responsible for mediating the hyper-adherence of the Δugm1 mutant strain. The contribution of this adherence to virulence is unclear however, as conflicting results of virulence studies have been reported for mutants deficient in Ugm1. Despite the increase in conidial adherence, Lamarre and colleagues found the Δugm1 strain exhibited normal virulence in a steroid treated murine model of invasive aspergillosis [27] while Schmalhorst et al. reported that a Δugm1 mutant displayed attenuated virulence in a neutropenic murine model of invasive aspergillosis [29]. Although differences in animal models may account for these observations, there were also important phenotypic differences between the two mutant strains in vitro, suggesting that perhaps the genetic background of the parent strain or other undetected mutations may have influenced the virulence phenotypes of these strains. Collectively, these studies suggest that Galf and galactomannan do not function as adhesins but may modulate the expression and exposure of other molecules that mediate adherence.
Extracellular matrix components and adherence
In addition to comprising much of the cell wall, carbohydrates are also a major component of the extracellular matrix secreted by sessile colonies of A. fumigatus. In addition to fungal proteins such as the hydrophobins, the extracellular matrix contains monosaccharides and polysaccharides such as galactomannan, galactosaminogalactan and α-1,3 glucan [8]. ECM production in vivo has been documented at the site of infection in human subjects with aspergilloma, and in experimental murine invasive aspergillosis [30]. In other fungi, the carbohydrates and glycoproteins comprising the extracellular matrix play an important role in fungal adherence to host tissues, often acting as a type of “glue” to anchor the fungi to environmental surfaces or host tissues (reviewed in [31]). Recent studies have begun to reveal a role for components of the A. fumigatus extracellular matrix in mediating adherence.
Galactosaminogalactan (GAG) is a heterogeneous polysaccharide composed of variable repeats of galactose linked to N-acetylgalactosamine. GAG was first identified in cell wall extracts and cultures media of A. fumigatus [21]. Follow up studies have found that GAG is a key component of the extracellular matrix both in vitro and in vivo [30]. In parallel studies, our group identified an A. fumigatus regulatory gene, medA, which governs the production of biofilm formation and adherence to plastic, fibronectin, pulmonary epithelial cells and endothelial cells and a ΔmedA mutant was hypovirulent in a murine model of invasive aspergillosis [32]. Transcriptome and carbohydrate analysis of this strain demonstrated that MedA governs the expression of GAG through the regulation of a novel carbohydrate biosynthetic cluster located on chromosome 3. Disruption of a putative UDP-glucose epimerase gene located within this cluster was associated with a block in GAG synthesis and a near total block in adherence of germlings and hyphae to plastic and pulmonary epithelial cells. GAG deficient strains also exhibited a reduction in their ability to injure and stimulate pulmonary epithelial cells in vitro (Gravelat FN and Sheppard DC. abstract O3, Biofilms in Nosocomial Fungal Infections. Paris, January 2011). Virulence studies to determine the role of GAG mediated adherence in A. fumigatus virulence are ongoing. The host receptor bound by GAG remains unknown.
Conclusion
The available evidence suggests that the adherence of Aspergillus to host constituents is likely mediated by a number of different fungal factors (Figure 1). It is likely that different modes and molecules predominate depending on the stage of fungal development, and the environmental niche of the fungus. An important role is emerging for the extracellular matrix, and in particular galactosaminogalactan, in mediating A. fumigatus adherence. Whether GAG directly engages host receptors, or acts as a substrate for other adhesion factors remains to be determined. A second challenge that lies ahead will be the identification of host receptors that mediate Aspergillus adherence. Host lectins are likely to play an important role as receptors for A. fumigatus glycans, however the identity of these proteins remains elusive.
Highlights.
> Adherence to host constituents is a key early step in the pathogenesis of invasive aspergillosis. > Early studies focused on the identification of fungal proteins as adhesins. > Recently fungal cell wall and secreted glycans have been implicated in adherence. > Galactosaminogalactan, a component of the extracellular matrix mediates adherence.
Acknowledgements
This research was supported by an operating grant from the Canadian Institutes of Health Research (CIHR) and by grant R01AI073829 from the National Institutes of Health, USA. D.C.S. is a CIHR Clinician Scientist, and recipient of a Burroughs Welcome Fund Career Award in the Biomedical Sciences and a Chercheur-Boursier Clinicien Award from the Fonds de la Recherche en Santé du Quebec.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wasylnka JA, Moore MM. Uptake of Aspergillus fumigatus Conidia by phagocytic and nonphagocytic cells in vitro: quantitation using strains expressing green fluorescent protein. Infect Immun. 2002;70:3156–3163. doi: 10.1128/IAI.70.6.3156-3163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wasylnka JA, Moore MM. Aspergillus fumigatus conidia survive and germinate in acidic organelles of A549 epithelial cells. J Cell Sci. 2003;116:1579–1587. doi: 10.1242/jcs.00329. [DOI] [PubMed] [Google Scholar]
- 3.Gomez P, Hackett TL, Moore MM, Knight DA, Tebbutt SJ. Functional genomics of human bronchial epithelial cells directly interacting with conidia of Aspergillus fumigatus. BMC Genomics. 2010;11:358. doi: 10.1186/1471-2164-11-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamai Y, Lossinsky AS, Liu H, Sheppard DC, Filler SG. Polarized response of endothelial cells to invasion by Aspergillus fumigatus. Cell Microbiol. 2009;11:170–182. doi: 10.1111/j.1462-5822.2008.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopes Bezerra LM, Filler SG. Interactions of Aspergillus fumigatus with endothelial cells: internalization, injury, and stimulation of tissue factor activity. Blood. 2004;103:2143–2149. doi: 10.1182/blood-2003-06-2186. [DOI] [PubMed] [Google Scholar]
- 6.Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, Clavaud C, Paris S, Brakhage AA, Kaveri SV, et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460:1117–1121. doi: 10.1038/nature08264. [DOI] [PubMed] [Google Scholar]
- 7.Thau N, Monod M, Crestani B, Rolland C, Tronchin G, Latge JP, Paris S. rodletless mutants of Aspergillus fumigatus. Infect Immun. 1994;62:4380–4388. doi: 10.1128/iai.62.10.4380-4388.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beauvais A, Schmidt C, Guadagnini S, Roux P, Perret E, Henry C, Paris S, Mallet A, Prevost MC, Latge JP. An extracellular matrix glues together the aerial-grown hyphae of Aspergillus fumigatus. Cell Microbiol. 2007;9:1588–1600. doi: 10.1111/j.1462-5822.2007.00895.x. [DOI] [PubMed] [Google Scholar]
- 9.Paris S, Debeaupuis JP, Crameri R, Carey M, Charles F, Prevost MC, Schmitt C, Philippe B, Latge JP. Conidial hydrophobins of Aspergillus fumigatus. Appl Environ Microbiol. 2003;69:1581–1588. doi: 10.1128/AEM.69.3.1581-1588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gil ML, Penalver MC, Lopez-Ribot JL, O'Connor JE, Martinez JP. Binding of extracellular matrix proteins to Aspergillus fumigatus conidia. Infect Immun. 1996;64:5239–5247. doi: 10.1128/iai.64.12.5239-5247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penalver MC, O'Connor JE, Martinez JP, Gil ML. Binding of human fibronectin to Aspergillus fumigatus conidia. Infect Immun. 1996;64:1146–1153. doi: 10.1128/iai.64.4.1146-1153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tronchin G, Esnault K, Renier G, Filmon R, Chabasse D, Bouchara JP. Expression and identification of a laminin-binding protein in Aspergillus fumigatus conidia. Infect Immun. 1997;65:9–15. doi: 10.1128/iai.65.1.9-15.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerjee B, Greenberger PA, Fink JN, Kurup VP. Immunological characterization of Asp f 2, a major allergen from Aspergillus fumigatus associated with allergic bronchopulmonary aspergillosis. Infect Immun. 1998;66:5175–5182. doi: 10.1128/iai.66.11.5175-5182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheppard DC, Yeaman MR, Welch WH, Phan QT, Fu Y, Ibrahim AS, Filler SG, Zhang M, Waring AJ, Edwards JE., Jr Functional and structural diversity in the Als protein family of Candida albicans. J Biol Chem. 2004;279:30480–30489. doi: 10.1074/jbc.M401929200. [DOI] [PubMed] [Google Scholar]
- 15.Staab JF, Bradway SD, Fidel PL, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283:1535–1538. doi: 10.1126/science.283.5407.1535. [DOI] [PubMed] [Google Scholar]
- 16.Levdansky E, Romano J, Shadkchan Y, Sharon H, Verstrepen KJ, Fink GR, Osherov N. Coding tandem repeats generate diversity in Aspergillus fumigatus genes. Eukaryot Cell. 2007;6:1380–1391. doi: 10.1128/EC.00229-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levdansky E, Kashi O, Sharon H, Shadkchan Y, Osherov N. The Aspergillus fumigatus cspA gene encoding a repeat-rich cell wall protein is important for normal conidial cell wall architecture and interaction with host cells. Eukaryot Cell. 2010;9:1403–1415. doi: 10.1128/EC.00126-10. Using a bioinformatics algorithm, in this and the preceding publication the authors identify a novel gpi-anchored cell wall protein and demonstrate it's ability to modulate adherence to host substrates. This is the first successful use of a bioinformatics discovery platform for A. fumigatus adherence molecules.
- 18. Upadhyay SK, Mahajan L, Ramjee S, Singh Y, Basir SF, Madan T. Identification and characterization of a laminin-binding protein of Aspergillus fumigatus: extracellular thaumatin domain protein (AfCalAp) J Med Microbiol. 2009;58:714–722. doi: 10.1099/jmm.0.005991-0. Using a bioinformatics program designed to identify putative adhesins, the authors identify a novel laminin binding protein, although its role in mediating adherence and virulence is not defined.
- 19.Chaudhuri R, Alam Ansari F, Raghunandanan MV, Ramachandran S. FungalRV: Adhesin prediction and immunoinformatics portal for human fungal pathogens. BMC Genomics. 2011;12:192. doi: 10.1186/1471-2164-12-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramana J, Gupta D. FaaPred: a SVM-based prediction method for fungal adhesins and adhesin-like proteins. PLoS One. 2010;5:e9695. doi: 10.1371/journal.pone.0009695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontaine T, Simenel C, Dubreucq G, Adam O, Delepierre M, Lemoine J, Vorgias CE, Diaquin M, Latge JP. Molecular organization of the alkali-insoluble fraction of aspergillus fumigatus cell wall. J Biol Chem. 2000;275:41528. [PubMed] [Google Scholar]
- 22.Wasylnka JA, Moore MM. Adhesion of Aspergillus species to extracellular matrix proteins: evidence for involvement of negatively charged carbohydrates on the conidial surface. Infect Immun. 2000;68:3377–3384. doi: 10.1128/iai.68.6.3377-3384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wasylnka JA, Simmer MI, Moore MM. Differences in sialic acid density in pathogenic and non-pathogenic Aspergillus species. Microbiology. 2001;147:869–877. doi: 10.1099/00221287-147-4-869. [DOI] [PubMed] [Google Scholar]
- 24.Warwas ML, Watson JN, Bennet AJ, Moore MM. Structure and role of sialic acids on the surface of Aspergillus fumigatus conidiospores. Glycobiology. 2007;17:401–410. doi: 10.1093/glycob/cwl085. [DOI] [PubMed] [Google Scholar]
- 25.Latge JP, Kobayashi H, Debeaupuis JP, Diaquin M, Sarfati J, Wieruszeski JM, Parra E, Bouchara JP, Fournet B. Chemical and immunological characterization of the extracellular galactomannan of Aspergillus fumigatus. Infect Immun. 1994;62:5424–5433. doi: 10.1128/iai.62.12.5424-5433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nassau PM, Martin SL, Brown RE, Weston A, Monsey D, McNeil MR, Duncan K. Galactofuranose biosynthesis in Escherichia coli K-12: identification and cloning of UDP-galactopyranose mutase. J Bacteriol. 1996;178:1047–1052. doi: 10.1128/jb.178.4.1047-1052.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lamarre C, Beau R, Balloy V, Fontaine T, Wong Sak Hoi J, Guadagnini S, Berkova N, Chignard M, Beauvais A, Latge JP. Galactofuranose attenuates cellular adhesion of Aspergillus fumigatus. Cell Microbiol. 2009;11:1612–1623. doi: 10.1111/j.1462-5822.2009.01352.x. This study of the role of galactomannan in adherence provides key evidence that fungal cell wall carbohydrates can modulate adherence to host tissues and implicates cell wall mannose as a potential adhesin to host cells.
- 28. Paul BC, El-Ganiny AM, Abbas M, Kaminskyj SG, Dahms TE. Quantifying the importance of galactofuranose in Aspergillus nidulans hyphal wall surface organization by atomic force microscopy. Eukaryot Cell. 2011 doi: 10.1128/EC.00304-10. This study provides the first documented use of atomic force microscopy to quantify adherence phenotypes in fungi and confirms the findings of Lamarre et al.
- 29.Schmalhorst PS, Krappmann S, Vervecken W, Rohde M, Muller M, Braus GH, Contreras R, Braun A, Bakker H, Routier FH. Contribution of galactofuranose to the virulence of the opportunistic pathogen Aspergillus fumigatus. Eukaryot Cell. 2008;7:1268–1277. doi: 10.1128/EC.00109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loussert C, Schmitt C, Prevost MC, Balloy V, Fadel E, Philippe B, Kauffmann-Lacroix C, Latge JP, Beauvais A. In vivo biofilm composition of Aspergillus fumigatus. Cell Microbiol. 2010;12:405–410. doi: 10.1111/j.1462-5822.2009.01409.x. This seminal publication demonstrates the presence of fungal extracellular matrix during infection in vivo and suggests a role for biofilm and adherence in the pathogenesis of invasive aspergillosis
- 31.Epstein L, Nicholson RL. Adhesion and Adhesives of Fungi and Oomycetes. In: Smith AM, editor. Biological Adhesives. Callow JA: Springer-Verlag; 2006. pp. 41–62. [Google Scholar]
- 32. Gravelat FN, Ejzykowicz DE, Chiang LY, Chabot JC, Urb M, Macdonald KD, al-Bader N, Filler SG, Sheppard DC. Aspergillus fumigatus MedA governs adherence, host cell interactions and virulence. Cell Microbiol. 2010;12:473–488. doi: 10.1111/j.1462-5822.2009.01408.x. This study identified and characterized a MedA, regulatory gene, that governs adherence to multiple substrates as well as virulence in experimental invasive aspergillosis. Followup studies have implicated galactosaminogalactan as the key MedA-dependent effector of adherence.