Abstract
Repetitive transcranial magnetic stimulation (rTMS) has been applied with variable success to terminate the seizures of epilepsia partialis continua. The rationale for using this technique to suppress ongoing seizures is the capacity of rTMS to interrupt ongoing neuronal activity, and to produce a lasting decrease in cortical excitability with low-frequency (≤1 Hz) stimulation. We report a case of epilepsia partialis continua in a child with Rasmussen’s encephalitis, in whom seizures were transiently suppressed by 1-Hz rTMS delivered in nine daily 30-minute sessions. In this case, total ictal time was significantly reduced during stimulation, but the daily baseline seizure rate remained unchanged. Notably, the detection and quantification of this short-lived improvement were enabled by recording EEG continuously during the rTMS session. Thus, we present this case to illustrate a potential utility of combined continuous EEG recording and rTMS in seizure treatment.
Keywords: Transcranial magnetic stimulation, Epilepsia partialis continua, Electroencephalography, Rasmussen’s encephalitis
1. Introduction
We describe a case of short-lived suppression of epilepsia partialis continua (EPC) in a patient with Rasmussen’s encephalitis achieved with 1-Hz repetitive transcranial magnetic stimulation (rTMS) combined with continuous EEG recording.
Repetitive transcranial magnetic stimulation is a method for noninvasive focal cortical stimulation that is based on Faraday’s principle of electromagnetic induction, where small intracranial electrical currents are generated by a powerful fluctuating extracranial magnetic field. rTMS can disrupt neuronal function and, thus, can interfere with ongoing seizures [1]. Additionally, low-frequency (≤1 Hz) rTMS can lead to lasting reductions in cortical excitability, likely through mechanisms similar to those of long-term depression (LTD) that are induced by direct electrical stimulation at low frequencies, and to lasting seizure suppression after several daily sessions [2].
In instances where low-frequency rTMS has been applied to treat the ongoing seizures of EPC, only post-treatment outcomes (continued or suppressed seizures) have been reported [1,3]. The electrographic state of the patient’s seizures during low-frequency rTMS has not been described. Here, we present a case where the transient effects of rTMS on EPC were documented with EEG recordings acquired simultaneously with rTMS.
2. Case report
Our patient presented at age 14 with throat clearing, anarthria, drooling, and movement of his tongue to the left. One month later, he developed left facial twitching. These episodes lasted 1 to 2 minutes and, within 4 months, increased in frequency to once every few minutes. Electro-graphically the seizures were apparent as rhythmic spikes and sharp waves seen broadly in the right parasagittal chain, although at times most prominently in the right central (C4) lead. Often, the nearly constant activity spread to involve both hemispheres.
The patient’s workup included serial brain MRI scans that revealed, over approximately 6 months, progressive atrophy and increasing T2 hyperintensity in the right hemisphere consistent with Rasmussen’s encephalitis. Subsequently, this diagnosis was confirmed by biopsy. An extensive infectious and metabolic workup was unrevealing. His past medical history was notable for hemophilia A, vitiligo, and attention deficit disorder. His birth history and developmental histories were normal. His father had been diagnosed with Yamaguchi syndrome resulting in cardiomegaly, and his mother was a hemophilia A carrier. There was no family history of seizures.
Treatment of our patient’s seizures with conventional anticonvulsants was not successful. Among the medications tried at therapeutic doses were fosphenytoin, oxcarbazepine, levetiracetam, valproate, diazepam, and lorazepam. Treatment with intravenous immunoglobulin and corticosteroids was not successful either.
3. Materials and methods
As our patient continued to have intractable seizures, after obtaining written informed consent from the family, we offered a therapeutic trial using low-frequency rTMS to attempt seizure suppression. We applied 1-Hz rTMS with a MindCare MagPro X100 stimulator and Fig.-8 Cool-B65 Coil (Tonica, Farum, Denmark) positioned over the seizure focus as identified by scalp EEG (right central, C4). To position the coil against the scalp, the C4 lead was removed during stimulation. rTMS was delivered at 100% motor threshold stimulus intensity, in daily trains of 1800 pulses (30 minutes), for 9 consecutive days.
Conventional EEG with 10–20 international electrode configuration was recorded continuously during stimulation (BioLogic, Chicago, IL, USA). We used MRI-compatible plastic cup EEG electrodes (Ives EEG Solutions, ON, Canada) to avoid potential injury to the scalp from heating of disc electrodes [4].
The data were analyzed post hoc by visual inspection. To minimize interscorer bias, the EEG was reviewed by two clinical neurophysiologists (M.T. and D.D.C.) who were not directly associated with the stimulation. Because of the prominent rTMS artifact (Fig. 1), scorers were not blinded as to the treatment phase. Timing of each seizure onset and termination was determined by consensus between the two scorers. Seizure durations were summed to calculate the total ictal time during 30 minutes of stimulation on each of the 9 days. These values were compared with 30-minute EEG segments immediately before and after the daily rTMS session. The averages of daily values before, during, and after stimulation were compared with one-way ANOVA. As the C4 lead was removed during stimulation, information from the same lead was also suppressed during the post hoc analysis.
Fig. 1.
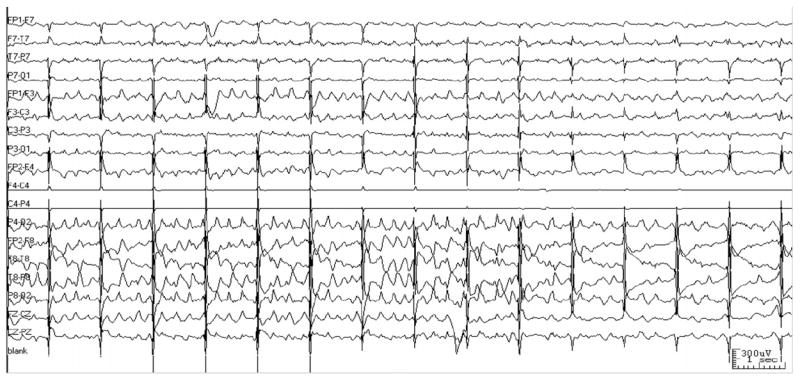
Minimal rTMS artifact on EEG. This 15-second tracing shows a seizure terminating during rTMS. The C4 lead has been removed for rTMS coil placement. Note that the 1-Hz rTMS artifact (vertical lines) does not obscure the EEG, and enables accurate assessment of seizure duration.
4. Results
Our patient tolerated the daily rTMS sessions well without side effects. Apart from the prominent brief rTMS artifact (<100 ms) associated with each individual TMS pulse, the EEG recording was relatively artifact free during the rTMS sessions (Fig. 1). In this case, characteristic rhythmic sharp waves, associated with seizure onset and termination, were readily distinguished from the background EEG, which was poorly organized with slowing and infrequent individual interictal spikes and sharp waves over the right hemisphere.
We found a short-lived response to 1-Hz rTMS. Clinical and electrographic seizure suppression was apparent during the stimulation sessions. Clinically, the patient had improved articulation and reduced frequency of clonic hemifacial and tongue movements. On EEG, total ictal time was reduced during the 30-minute rTMS sessions relative to baseline (Fig. 2A) on each day of stimulation. The daily baseline, however, did not significantly change with time (Fig. 2B).
Fig. 2.
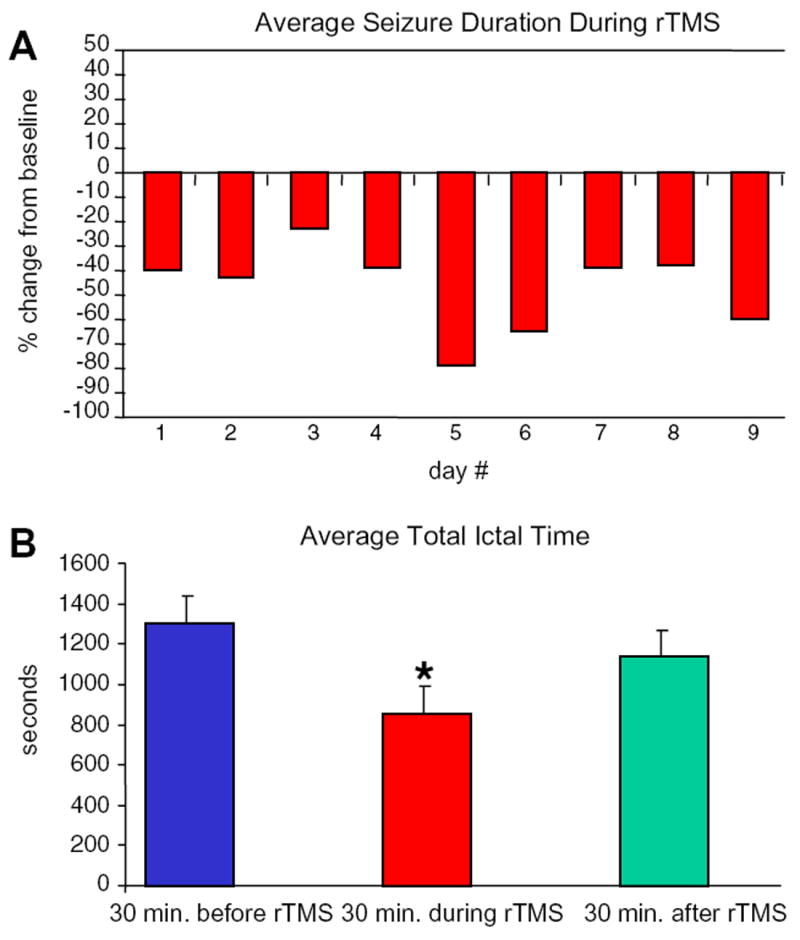
Daily change in (A) average seizure duration and (B) average total ictal time. Thirty-minute EEG segments before, during, and after 1-Hz rTMS were compared. Seizures (n = 219) were significantly shortened during treatment (one-way ANOVA, P < 0.01); however, the daily baseline was essentially unchanged.
5. Discussion
In this case, coupling of real-time EEG acquisition and rTMS enabled us to clearly document and quantify seizure suppression by 1-Hz rTMS delivered over the seizure focus. Although the desired outcome of a lasting anticonvulsive response was not achieved, the temporary anticonvulsive effect of rTMS is nevertheless encouraging as it suggests several plausible extensions of the current findings for future trials. For instance, repeated or prolonged application of rTMS may be beneficial when a transient effect is identified. Additionally, the mild effect seen by stimulating a focal area may be augmented by stimulating a broader region. Further, the transient benefit after 1-Hz rTMS may indicate that repeated applications of rTMS, or replacement of rTMS with durable neurostimulation such as by implanted stimulating electrodes, may be useful to maintain seizure suppression in similar cases.
We note that real-time and post hoc inspection of the EEG did not show evidence of spike or seizure provocation by 1-Hz rTMS delivered over an active seizure focus. Additionally baseline seizures were not exacerbated by the rTMS. Our patient otherwise tolerated rTMS without side effects. This observation is in accordance with published reports demonstrating the relative safety of rTMS in patients with epilepsy, including those with EPC [1,4-6].
From the described case, we suggest that explorations of antiseizure rTMS effect would benefit from EEG recording during the stimulation to identify real-time ameliorative anticonvulsive effects or exclude undesired side effects such as seizure provocation.
Acknowledgments
A.R. acknowledges the Siegel Family Fund for Epilepsy Research. A.P.L. acknowledges K24 RR018875.
References
- 1.Misawa S, Kuwabara S, Shibuya K, Mamada K, Hattori T. Low-frequency transcranial magnetic stimulation for epilepsia partialis continua due to cortical dysplasia. J Neurol Sci. 2005;234:37–9. doi: 10.1016/j.jns.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 2.Fregni F, Otachi P, Valle A, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Ann Neurol. 2006;60:447–55. doi: 10.1002/ana.20950. [DOI] [PubMed] [Google Scholar]
- 3.Pascual-Leone A, Dhuna A, Roth BJ, Cohen L, Hallett M. Risk of burns during rapid-rate magnetic stimulation in presence of electrodes. Lancet. 1990;336:1195–6. doi: 10.1016/0140-6736(90)92815-y. [DOI] [PubMed] [Google Scholar]
- 4.Morales OG, Henry ME, Nobler MS, Wassermann EM, Lisanby SH. Electroconvulsive therapy and repetitive transcranial magnetic stimulation in children and adolescents: a review and report of two cases of epilepsia partialis continua. Child Adolesc Psychiatr Clin North Am. 2005;14:193–210. doi: 10.1016/j.chc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Graff-Guerrero A, Gonzales-Olvera J, Ruiz-Garcia M, Avila-Ordonez U, Vaugier V, Garcia-Reyna JC. rTMS reduces focal brain hyperper-fusion in two patients with EPC. Acta Neurol Scand. 2004;109:290–6. doi: 10.1046/j.1600-0404.2003.00222.x. [DOI] [PubMed] [Google Scholar]
- 6.Bae EH, Schrader LM, Machii K, Alonso AM, Riviello JJ, Pascual-Leone A. Safety and tolerability of repetitive transcranial magnetic stimulation in patients with epilepsy: a review of the literature. Epilepsy Behav. 2007;10:521–8. doi: 10.1016/j.yebeh.2007.03.004. [DOI] [PubMed] [Google Scholar]


